Abstract
Resistance and tolerance to Meloidogyne graminicola infection of introgressed rice genotypes derived from crosses between M. graminicola-resistant Oryza glaberrima genotype CG14 and -susceptible O. sativa genotype IR64 were evaluated in an indoor growth chamber and outdoor raised beds. None of the 44 introgressed genotypes: 24 first backcross F2 generation (BC1F2) and 20 first backcross F3 generation (BC1F3) evaluated did express the same level of resistance as the resistant O. glaberrima reference genotypes included in the experiments for comparison. Lower nematode multiplication factor on the BC1F3 genotypes suggests that M. graminicola resistance trait segregated among the 3rd generation progeny of the backcross population. The majority of the introgressed genotypes were susceptible and sensitive to M. graminicola infection, some genotypes were susceptible but tolerant and few were both resistant and tolerant to nematode infection. Several genotypes with resistance and/or tolerance to M. graminicola were identified that could either be further developed into advanced breeding lines to produce resistant and/or tolerant cultivars or in the short-term developed into M. graminicola-resistant and/or –tolerant cultivars for use by resource-poor farmers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The rice root-knot nematode, Meloidogyne graminicola Golden and Birchfield, 1965, is considered one of the most important pathogen of rice in South and Southeast Asia and the major causal agent of yield loss in tropical aerobic rice (De Waele and Elsen 2007; Kreye et al. 2009; Jain et al. 2012; De Waele et al. 2013; Mantelin et al. 2017). Rice roots infected with this nematode develop galls, especially at the root tips where they are typical hook-like, while inside the root the permanent feeding sites (giant cells) induced by the nematodes disorganise the vascular cylinder affecting the transport and absorption of water and nutrients. Infected rice plants in a wide range of rice-based agro-ecosystems, including irrigated and rainfed rice, lowland and upland rice, and deepwater rice, showed considerable yield losses (Bridge and Page 1982; Arayarungsarit 1987; Plowright and Bridge 1990; Netscher and Erlan 1993; Tandingan et al. 1996; Soriano et al. 2000; Soriano and Reversat 2003; Padgham et al. 2004; Sharma-Poudyal et al. 2004; Win et al. 2015).
The options to manage M. graminicola are scarce because most practices, such as flooding, crop rotation and nematicides, have serious drawbacks limiting their use in rice fields (Mantelin et al. 2017). The use of rice varieties with resistance and/or tolerance to M. graminicola is considered a promising alternative for the management of this pathogen. Resistance to M. graminicola has been identified in Oryza longistaminata A. Chev. and Roehrich (Soriano et al. 1999), Oryza glaberrima Steud. (Plowright et al. 1999; Soriano et al. 1999; Cabasan et al. 2012) and Oryza sativa L. (Jena and Rao 1977; Yik and Birchfield 1979; Sharma-Poudyal et al. 2004; Sabir and Gaur 2004; Prasad et al. 2006; Jena et al. 2012; Ravindra et al. 2015; Dimpka et al. 2015). However, few of these O. sativa genotypes are truly resistant (Bridge et al. 2005) and the majority of the germplasm is susceptible to M. graminicola.
Oryza glaberrima, originally domesticated in West Africa, is considered an important genetic resource to develop rice genotypes suitable for resource-poor farmers who are suffering from low yield due to multiple abiotic and biotic stresses in rice fields (Khush 1997; Futakuchi and Sié 2009). However, in contrast with O. sativa, O. glaberrima yield is low due to grain shattering and poor resistance to lodging (Linares 2002). The development of introgressed genotypes between O. glaberrima and O. sativa offers an opportunity to exploit the useful traits present in both rice species (Ghesquière et al. 1997). Although the transfer of useful genes from O. glaberrima into O. sativa is constrained by sterility in the early progenies of crosses (Second 1982), fertile progenies can be produced by backcrossing with the O. sativa parents (Jones et al. 1997a).
So far, efforts to introgress the resistance to M. graminicola from O. glaberrima into O. sativa has not been successful, as the interspecific progenies do not express the same degree of resistance observed in O. glaberrima. Plowright et al. (1999) identified four less susceptible progenies out of 14 progenies from a cross between O. glaberrima (CG14) and O. sativa (tropical japonica genotype WAB56-104) based on the low number of M. graminicola females/root system. In crosses of the O. glaberrima genotype TOG5681 and upland rice O. sativa indica genotype IR55423-01, Bimpong et al. (2010) identified two genotypes as resistant out of 15 introgressed progenies screened in outdoor raised beds and one genotype screened in a phytotron, based on the number of second-stage juveniles (J2)/root system.
Crossings carried out at the International Rice Research Institute (IRRI) in the Philippines have succeeded in producing fertile interspecific progenies from crosses between O. glaberrima genotype CG14 and O. sativa genotype IR64 through backcrossing the F1 hybrids to the O. sativa parent. Backcross breeding aims to introgress one or more genes of interest from a donor parent into an elite crop genotype. The genotypes CG14 and IR64 were selected on the basis of their useful traits. CG14, an upland genotype of O. glaberrima, has weed competitiveness (Jones et al. 1997b), resistance to M. graminicola (Plowright et al. 1999; Cabasan et al. 2012), drought (Jones et al. 1997b) and water lodging (Futakuchi et al. 2001), adaptability to acidic soils with low phosphorus availability (Sahrawat et al. 2000) and strong resistance to iron toxicity (Sahrawat and Sika 2002). Resistance to multiple abiotic and biotic constraints is a highly desirable characteristic for rice cultivated by resource-poor farmers who are usually confronted with multiple problems in the fields. IR64 is an O. sativa indica rice genotype which is widely grown in irrigated lowland areas in tropical Asia and has been popular for many farmers because of its good yield potential and good eating quality (Khush 1987). However, it is only moderately susceptible to drought with drastic yield reduction when drought occurs around flowering (Wade et al. 1999).
The objective of this study was to evaluate the resistance and tolerance to M. graminicola of BC1F2 and BC1F3 backcrossed progenies of crosses between M. graminicola-resistant O. glaberrima genotype (CG14) and M. graminicola-susceptible O. sativa genotype (IR64).
Materials and Methods
Nematode inoculum
The M. graminicola isolate used in this study was obtained from naturally infected O. sativa plants (unidentified cultivar) collected in Batangas, Philippines, established and maintained on the susceptible and sensitive O. sativa upland genotype UPLRi-5, in pots in the glasshouse at 28±2°C under upland conditions. The nematode inoculum for the indoor growth chamber experiment was obtained by extraction of J2 from galled roots 24 h after incubation in a mist chamber (Seinhorst 1950); while for the raised beds experiment, galled roots were washed free of soil and cut into 1-cm-pieces. The initial population density in the soil was established by determining the number of J2 per g of infected roots inoculated in the known volume of sterilized soil.
Plant materials
Forty-four introgressed genotypes (24 BC1F2 and 20 BC1F3 genotypes) were randomly selected from the backcross populations. The introgressed genotypes were developed from a cross between O. glaberrima CG14 (IRGC 96717) and O. sativa IR64. Seeds from a single F1 plant were backcrossed to IR64 to produce the F1 generation of the first backcross (BC1F1) population. Random selection of introgressed genotypes was from 2nd (F2) and 3rd (F3) generations, which are partially and highly fertile, respectively. These populations were available as part of the wide hybridisation program of the Plant Breeding, Genetics and Biotechnology Division of IRRI. The O. glaberrima-resistant genotypes CG14, TOG5674 and TOG5675, and the O. sativa-susceptible genotypes IR64 and UPLRi-5 were included in the experiments as reference genotypes for comparison.
Host phenotype evaluation in an indoor growth chamber (IGC)
This experiment was conducted in an indoor growth chamber (IGC) at 26-29 °C (night-day temperatures) with a 12 h photoperiod and 70 % relative humidity. Seeds were pre-germinated in Petri dishes at room temperature. The 5-days-old seedlings were transplanted into 2.6-cm-diameter x 21-cm-high polyvinyl chloride (PVC) tubes filled with 150 g of a heat-sterilised sandy-loam soil (52 % sand, 21 % loam, 27 % clay; pH 6.3; 0.14 % N, 1.06 % C). The soil was saturated (100 % of the soil volume filled with water) at planting and at field capacity (50 % of the soil volume filled with water) during nematode inoculation. The bottom of each tube was closed with a 0.25 mm mesh stainless steel sieve. The PVC tube was lined inside with a sheet of transparent polyethylene, slightly projected over the top of the PVC tube, to easily pull the cylinder of soil out of the tube without damaging the roots at the end of the experiment. Eight two-week-old plants from each introgressed genotype were inoculated with 75 M. graminicola J2. Nematode inoculation was repeated 2 days later to obtain a final pathogen pressure of 1 J2/g soil or 150 J2/tube. One day after nematode inoculation, the plants were watered at field capacity simulating upland conditions during the experiment and fertilized 3 times per week with Hoagland’s nutrient solution. The pots were arranged in a completely randomized block design.
At 60 days after seed germination, i.e. 46 days after nematode inoculation, the plants were removed from the tubes and the root systems washed carefully and rated for galls according to a scale of 0-5 (Hussey and Janssen 2002). Fresh root system weights were recorded. The roots were cut into 1-cm-sections and placed in a mist chamber for 14 days to determine the final nematode population (Pf; Seinhorst 1950). The nematode multiplication factor (Mf) was calculated as Pf/initial population density (Pi = 150 J2). The Mf of each introgressed rice genotype was compared with that of the susceptible and resistant reference genotypes. Classification of the host phenotype as resistant, partially resistant, susceptible or inconclusive was according to the criteria used by (Dochez et al. 2005, Table 1). Resistance/susceptibility on the one hand and tolerance/sensitivity on the other hand are independent, relative qualities of a host plant based on comparison between genotypes. A host plant may either suppress (resistance) or allow (susceptibility) nematode development and reproduction; it may suffer either little injury (tolerance), even when heavily infected with nematodes, or much injury (sensitivity), even when relatively lightly infected with nematodes (Bos and Parlevliet 1995).
Host phenotype evaluation in an outdoor raised bed (ORB)
Concrete raised beds (3.6 m long x 1.08 m wide x 0.14 m deep) were filled with 600 kg of a heat-sterilised sandy-loam soil. For the M. graminicola-infected treatment, 450 g of finely chopped infected roots of the susceptible genotype UPLRi-5 were distributed evenly at 8 cm above the bed bottom and covered with sterilised soil. The Pi was equivalent to 2 J2/g soil. Non infested beds were included as control. The rice genotypes (5-days-old seedlings) were planted in rows, spaced 10 x 20 cm, in a split-plot arrangement in a randomized complete block design with eight replicates/genotype. Plants were watered occasionally as needed to maintain a field capacity water regime simulating upland conditions throughout the growing season and fertilised 3 times at planting, and at 30 and 60 days after planting (DAP) at a rate of 90-60-60 kg/ha of NPK. Insecticide was sprayed when needed to protect the plants from plant hopper infestation.
The number of tillers/plant was counted at 30 DAP. Shoot height was measured at maturity; at harvest, the number of panicles/plant and number of spikelets/panicle were counted and fresh root and shoot weights recorded. The % filled grains/panicle, weight of 100 grains/plant and filled grain weight/plant (adjusted to 14% moisture content) were also recorded. The yield/plant was measured based on the weight of the filled grains/plant, not including the unfilled and partially filled grains.
In the uninoculated treatment, the introgressed rice genotypes and the resistant O. glaberrima reference genotypes matured at 100 DAP and plants were harvested while the susceptible O. sativa reference genotypes matured and were harvested at 110 DAP. In the inoculated treatment, the introgressed rice genotypes started to mature from 110 DAP onwards and were harvested at 118 DAP. The resistant O. glaberrima reference genotypes matured and were harvested at 104 DAP while the susceptible O. sativa at 118 DAP. Yield data was collected as soon as the plant matures to avoid grain shattering and tiller lodging which are typical for O. glaberrima genotypes and some of the introgressed genotypes. Plants were uprooted according to the maturity days of the genotypes in order to obtain the yield data and determine the tolerance/sensitivity of the genotypes to nematode infection.
Assessment of root galling severity and Mf were performed as referred before. Introgressed genotypes of the two different generations were evaluated in two batches, the first with the BC1F2 and the second with the BC1F3 populations. The same set of reference genotypes was included in the two batches and both were conducted during the dry season.
Statistical analysis
Statistical analyses were performed using STATISTICA 10.0 software. Data were subjected to log(x+1) transformation prior to analysis to meet the assumptions of analysis of variance (ANOVA), i.e. normality and homogeneity of variances. One-way ANOVA was used to analyse significant differences between rice genotypes screened in the indoor growth chamber experiment. When a significant effect was found, mean comparison was done using Tukey´s HSD test (P < 0.05) and Dunnett´s test was used to compare the mean of the rice genotype to the mean of the susceptible and resistant reference rice genotypes. A factorial split-plot ANOVA was used to examine the effect of nematode inoculation (compared with uninoculated plants) on rice genotypes grown in the ORB experiments. In the case of absence of interaction between the two factors (rice genotype and nematode infection) for a specific vegetative growth or yield-contributing trait, the factor level means (M. graminicola inoculated and uninoculated plants) were compared by Tukey’s HSD test and presented for all rice genotypes together. In the case of interaction between the two factors, individual comparisons were made between inoculated and uninoculated plants with the LSD t-test (P<0.05) and presented for each rice genotype separately.
Results
Host phenotype evaluation in an IGC
At 46 days after nematode inoculation, the fresh root weights of BC1F3 genotypes were 25 % lower compared to BC1F2 genotypes but no significant differences in J2/g roots, J2/root system, Mf and root galling were observed (Table 2). None of the 24 BC1F2 genotypes was classified as resistant to M. graminicola. Among the BC1F3 genotypes significant differences in numbers of J2 were observed. One out of the 20 BC1F3 genotypes was classified as resistant (IR87226-110-18-B) to M. graminicola and two (IR87226-106-6-B and IR87226-107-2) as partially resistant. The severity of root galling at 60 days after germination of the BC1F2 genotypes (on average 3.5) was higher compared to the BC1F3 genotypes (2.7) and comparable to the susceptible reference genotypes IR64 and UPLRi-5.
Host phenotype evaluation in an ORB
At plant maturity, the BC1F2 genotypes had about the same number of J2/g roots, J2/root system and root galling index compared to the susceptible reference genotypes, and about 15 and 9 times more (P<0.05) J2/g roots and J2/root system, respectively, compared to the resistant reference genotypes (Table 3). None of the BC1F2 genotypes was classified as resistant to M. graminicola.
No interaction between the BC1F2 genotypes and M. graminicola infection was observed for six out of the nine parameters examined. Hence, the effect of M. graminicola on these vegetative growth and yield-contributing parameters was measured for all introgressed genotypes combined (Supplement Table 1). Meloidogyne graminicola infection significantly (P<0.05) affected all these parameters, except number of panicles/plant and weight of 100 grains/plant. The highest (P<0.05) % reductions were observed in the number of spikelets/panicle and tillers/plant (-36 and -35.5 %, respectively). In about 75 % of the BC1F2 genotypes, M. graminicola infection significantly reduced the fresh shoot weight, % filled grains/panicle and filled grain weight/plant (Table 4). The highest reductions observed were 82.7% for fresh shoot weight, 68.3 % for % filled grains/panicle and 81.4 % for filled grain weight/plant for genotypes IR87226-41, IR87226-44 and IR87226-41, respectively. IR87226-63 and IR87226-35 appear to be tolerant: their yield decreased only 26 and 32.3 %, respectively, infected by as many as 63,750 and 61,564 J2/root system, respectively (on average) despite similar nematode levels to susceptible IR64.
At plant maturity, the BC1F3 genotypes had about the same number of J2/g roots compared to susceptible genotype UPLRi-5 but lower compared to IR64 (Table 5). The number of J2/root system of the BC1F3 genotypes was not significantly lower compared to both susceptible genotypes UPLRi-5 and IR64. The root galling index was comparable with both susceptible genotypes UPLRi-5 and IR64. Two of the 20 BC1F3 genotypes were classified as resistant (IR87226-110-15-B and IR87226-110-18-B) to M. graminicola, although they had, on average, 5.5 and 2.9 more J2/g roots, and 2.9 and 2.8 more J2/root system, respectively, compared to resistant genotype CG14.
No interaction between the 20 BC1F3 genotypes and M. graminicola infection was observed for the nine plant parameters analysed. Hence, the effect of M. graminicola on these vegetative growth and yield-contributing parameters was measured for all introgressed genotypes combined (Supplement Table 2). Meloidogyne graminicola infection significantly affected the number of tillers/plant, % filled grain/panicle and filled grain weight/plant. The highest (P<0.05) % reductions were observed for filled grain weight/plant and number of tillers/plant (44.6 and 30.4 %, respectively).
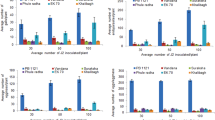
BC1F3 genotypes showed a reduction in yield when inoculated with M. graminicola (Fig. 1). IR87226-106-3-B and IR87226-104-19-B had the highest reduction in yield with 76 and 67.7 %, respectively while IR87226-104-15-B, IR87226-110-23-B and IR87226-106-1-B had the lowest yield reduction (5.3, 16.9 and 17.1 %, respectively). IR87226-104-11-B also had the lowest yield reduction but also the genotype with the lowest yield, while IR87226-111-10 had the highest yield in both uninfected and infected treatments. Susceptible genotype IR64 had the second-highest yield only when uninoculated and its yield was reduced significantly (P<0.05) in the presence of M. graminicola (58.1 %). Resistant genotype TOG5674 showed no reduction in yield when infected with M. graminicola. IR87226-110-15-B and IR87226-111-12 appear to be highly sensitive genotypes: their yield decreased by 64.2 and 54.5 %, respectively, infected with 12,851 and 27,316 J2/root system, respectively. In contrast, IR87226-104-15-B and IR87226-106-1-B appear to be tolerant: their yield decreased with only 5.3 and 17.1 %, respectively, infected by as many as 44,893 and 42,040 J2/root system, respectively.
Discussion
The number of J2/g roots and J2/root system on the BC1F2 and BC1F3 genotypes were lower than on the two susceptible reference genotypes. However, the Mf of the BC1F3 genotypes was lower compared to the BC1F2 genotypes. This difference suggests that the M. graminicola resistance trait segregated among the F3 generation progeny of the backcross population. In the IGC experiment low root galling severity observed in the BC1F3 genotypes can be explained by the presence of less nematodes in the roots. When defense response mechanisms were present, plant resistant to M. graminicola have a hypersensitive response in the early stage of infection resulting to a failure of feeding site establishment and a late sensitive response after induction of giant cells resulting to less number of nematodes that could reproduce in the roots; as observed in the resistant O. glaberrima CG14 (Cabasan et al. 2014). However, in the ORB experiment, the severity of root galling at plant maturity of the BC1F2 plants was comparable to the BC1F3 plants and susceptible reference genotypes. The difference in root galling severity between the two experiments could be due to the time of the experiments: raised beds experiment (110 days) could generate more nematodes (6 vs 3 life cycles) than the IGC experiment (60 days; Fernandez et al. 2013).
Resistance and tolerance of plants to nematode infection may differ between experiments due to differences in experimental conditions, type of inoculum or inoculum pressure. However inclusion of the same reference genotypes in both experiments will minimize this variation. Genotypes IR87226-106-6 and IR87226-107-2 were classified as partially resistant in the IGC experiment and susceptible in the ORB experiment. In contrast, the genotype IR87226-110-15-B was classified as susceptible in the IGC experiment and as resistant in the ORB experiment. This inconsistency in host phenotype also suggests segregation for M. graminicola resistance among F3 plants and, also, that the F3 plants are more heterozygous. Only the genotype IR87226-110-18-B was classified as resistant in both experiments.
Variability in sensitivity/tolerance to M. graminicola was observed within and between the two introgressed genotype populations. BC1F2 populations grown in M. graminicola-infested soil were more sensitive to nematode infection with a reduction on average of the yield/plant of 65 % vs 44.6 % in the BC1F3 populations. Higher yields in BC1F3 genotypes could be a result of an accumulation of alleles that favor the yield or a reduction of alleles for an undesirable trait, or both.
Some of the introgressed genotypes were both susceptible and sensitive to M. graminicola infection while some others were susceptible but tolerant. Resistance and tolerance to a nematode species can be independent attributes of a plant species (Trudgill 1991). The results of our study suggest that resistance and tolerance to M. graminicola infection are either independently or simultaneously expressed.
In our study, BC1F2 and BC1F3 genotypes identified as resistant or partially resistant to M. graminicola infection appeared to be tolerant, less sensitive or hypersensitive in terms of yield reduction when infected with M. graminicola. The BC1F2 genotypes IR87226-89 and IR87226-103 classified as susceptible (inconclusive in the ORB experiment) were both hypersensitive to nematode infection resulting in a high yield loss (61 %). The BC1F3 genotype IR87226-105-10-B was also classified as susceptible (inconclusive in the ORB experiment) and the yield decreased by 45 %. In contrast, the yield of the BC1F2 genotype IR87226-98, classified as susceptible in the IGC and as inconclusive in the ORB experiment, was only reduced by 20 %. IR87226-110-15-B was resistant (inconclusive in the IGC experiment) but hypersensitive to nematode infection resulting in a yield loss of 64 % while IR87226-110-18-B was resistant resulting in a yield loss of 25 %.
Although the majority of the susceptible BC1F2 and BC1F3 genotypes were sensitive to M. graminicola infection, some genotypes simultaneously expressed resistance and tolerance to nematode infection. This also suggests that resistance and tolerance to M. graminicola in rice may be expressed or inherited simultaneously or independently (Boerma and Hussey 1992; Barker 1993; Davis and May 2003). This variability in host phenotype indicates that numerous genes for tolerance are likely to be involved. Similar to nematode resistance, the trait for nematode tolerance may be quantitative in nature and controlled by more than one gene (Shrestha et al. 2007).
In conclusion, our results demonstrate the potential of genotypes derived of crosses between the resistant O. glaberrima genotype CG14 and the susceptible O. sativa genotype IR64 to improve resistance in O. sativa to M. graminicola. Promising genotypes with resistance and/or tolerance to M. graminicola infection were identified that could be further developed into advanced breeding lines and ultimately resistant and/or tolerant cultivars. Although it would require several years to develop a new rice genotype with superior phenotypes for nematode resistance and/or tolerance (Boerma and Hussey 1992), available resistant and tolerant rice genotypes could already alleviate the problem caused by M. graminicola and prevent yield reduction caused by this important nematode species (De Waele et al. 2013). This will in turn increase food security and cash income of farmers. Molecular technologies could enhance the efficiency of the breeding programs. Data on the genetic basis of resistance to M. graminicola in rice are limited. The identification of molecular markers that are closely associated with M. graminicola resistance and tolerance quantitative trait loci is currently underway.
References
Arayarungsarit L (1987) Yield ability of rice varieties in fields infested with root-knot nematode. International Rice Research Notes 12:14
Barker KR (1993) Resistance/tolerance and related concepts/terminology in plant nematology. Plant Disease 77:111–113
Bimpong IK, Carpena AL, Mendioro MS, Fernandez L, Ramos J, Reversat G, Brar DS (2010) Evaluation of Oryza sativa x O. glaberrima derived progenies for resistance to root-knot nematode and identification of introgressed alien chromosome segments using SSR markers. African Journal of Biotechnology 9:3988–3997
Boerma HG, Hussey RS (1992) Breeding plants for resistance to nematodes. Journal of Nematology 24:242–252
Bos L, Parlevliet JE (1995) Concepts and terminology on plant/pest relationships: toward consensus in plant pathology and crop protection. Annual Review of Phytopathology 33:69–102
Bridge J, Page SLJ (1982) The rice root-knot nematode, Meloidogyne graminicola, on deep water rice (Oryza sativa subsp. indica). Revue de Nématologie 5:225–232
Bridge J, Plowright RA, Peng D (2005) Nematode parasites of rice. In: Luc M, Sikora R, Bridge J (eds) Plant parasitic nematodes in subtropical and tropical agriculture. CAB International, London, pp 87–130
Cabasan MTN, Kumar A, De Waele D (2012) Comparison of migration, penetration, development and reproduction of Meloidogyne graminicola on susceptible and resistant rice genotypes. Nematology 14:405–415
Cabasan MTN, Kumar A, Bellafiore S, De Waele D (2014) Histopathology of the rice root-knot nematode Meloidogyne graminicola on Oryza sativa and O. glaberrima. Nematology 16:73–81
Davis RF, May OL (2003) Relationships between tolerance and resistance to Meloidogyne incognita in cotton. Journal of Nematology 35:411–416
De Waele D, Elsen A (2007) Challenges in tropical plant nematology. Annual Review of Phytopathology 45:457–485
De Waele D, Das K, Zhao D, Tiwari RKS, Shrivastava DK, Vera-Cruz C, Kumar A (2013) Host response of rice genotypes to the rice root-knot nematode (Meloidogyne graminicola) under aerobic soil conditions. Archives of Phytopathology and Plant Protection 46:670–681
Dimpka SON, Lahari Z, Shrestha R, Douglas A, Gheysen G, Price AH (2015) A genome-wide association study of a global rice panel reveals resistance to Oryza sativa to root-knot nematodes. Journal of Experimental Biology 67:1191–1200
Dochez C, Whyte J, Tenkouano A, Ortiz R, De Waele D (2005) Response of East African highland bananas and hybrids to Radopholus similis. Nematology 7:655–666
Fernandez L, Cabasan MTN, De Waele D (2013) Life cycle of the rice root-knot nematode Meloidogyne graminicola at different temperatures under non-flooded and flooded conditions. Archives of Phytopathology and Plant Protection 47:1042–1049
Futakuchi K, Sié M (2009) Better exploitation of African rice (Oryza glaberrima Steud.) in varietal development for resource poor farmers in West and Central Africa. Agricultural Journal 4:96–102
Futakuchi K, Jones MP, Ishi R (2001) Physiological and morphological mechanism of submergence resistance in African rice (Oryza glaberrima Steud.) Japanese Journal of Tropical Agriculture 45:8–14
Ghesquière A, Séquier J, Second G, Lorieux M (1997) First steps towards a rational use of African rice, Oryza glaberrima, in rice breeding through a ‘contig line’ concept. Euphytica 96:31–39
Hussey H, Janssen GJW (2002) Root-knot nematode: Meloidogyne species. In: Starr JL, Cook R, Bridge J (eds) Plant resistance to parasitic nematodes. CAB International, Wallingford, pp 43–70
Jain RK, Khan MR, Kumar V (2012) Rice root-knot nematode (Meloidogyne graminicola) infestation in rice. Archives of Phytopathology and Plant Protection 45:635–645
Jena RN, Rao YS (1977) Nature of resistance in rice (Oryza sativa L.) to the root knot nematode (Meloidogyne graminicola) II. Mechanism of resistance. Proceedings of the Indian Academy of Science 86:31–38
Jena M, Mohanty SK, Panda RS, Bose LK, Behera L, Sahu SC (2012) Two breeding lines of rice resistant to the rice root-knot nematode. Nematologia Mediterranea 40:207–208
Jones MP, Dingkuhn M, Aluko GK, Semon M (1997a) Interspecifc Oryza sativa L. x O. glaberrima Steud. progenies in upland rice improvement. Euphytica 92:237–246
Jones MP, Mande S, Aluko K (1997b) Diversity and potential of Oryza glaberrima Steud. upland rice breeding. Breeding Science 47:395–398
Khush GS (1987) Rice breeding: past, present and future. Journal of Genetics 66:195–216
Khush GS (1997) Origin, dispersal, cultivation and variation of rice. Plant Molecular Biology 35:25–34
Kreye C, Bouman BAM, Reversat G, Fernandez L, Vera Cruz C, Elazegui F, Faronilo JE, Llorca L (2009) Biotic and abiotic causes of yield failure in tropical aerobic rice. Field Crops Research 112:97–106
Linares OF (2002) African rice (Oryza glaberrima): history and future potential. Proceedings of the National Academy of Sciences of the United States of America 99:16360–16365
Mantelin S, Bellafiore S, Kyndt T (2017) Meloidogyne graminicola: a major threat to rice agriculture. Molecular Plant Pathology 18:3–15
Netscher C, Erlan X (1993) A root-knot nematode, Meloidogyne graminicola, parasitic on rice in Indonesia. Afro-Asian Journal of Nematology 3:90–95
Padgham JL, Duxbury JM, Mazid AM, Abawi GS, Hossain M (2004) Yield loss caused by Meloidogyne graminicola on lowland rainfed rice in Bangladesh. Journal of Nematology 36:42–48
Plowright R, Bridge J (1990) Effects of Meloidogyne graminicola (Nematoda) on the establishment, growth and yield of rice cv. IR36. Nematologica 36:81–89
Plowright RA, Coyne DL, Nash P, Jones MP (1999) Resistance to the rice nematodes Heterodera sacchari, Meloidogyne graminicola and M. incognita in Oryza glaberrima and O. glaberrima x O. sativa interspecific hybrids. Nematology 1:745–751
Prasad JS, Vijayakumar CHM, Sankar M, Varaprasad KS, Prasad MS, Rao YK (2006) Root-knot nematode resistance in advanced back cross populations of rice developed for water stress conditions. Nematologia Mediterranea 34:3–8
Ravindra H, Sehgal M, Narasimhamurthy HB, Imran Khan HS, Shruthi SA (2015) Evaluation of rice landraces against rice root-knot nematode, Meloidogyne graminicola. African Journal of Microbiology Research 9:1128–1131
Sabir N, Gaur HS (2004) Reaction of rice varieties against geographically different populations of Meloidogyne graminicola and M. triticoryzae. Annals of Plant Protection Sciences 12:384–387
Sahrawat KL, Sika M (2002) Comparative tolerance of Oryza sativa and O. glaberrima rice cultivars for iron toxicity in West Africa. International Rice Research Notes 27:30–31
Sahrawat KL, Jones MP, Diatta S (2000) The role of tolerant genotypes and plant nutrients in the management of acid soil infertility in upland rice. In: Management and conservation of tropical soils for sustainable crop production, Proceedings of a Consultants Meeting, International Atomic Energy Agency. Vienna, Austria, pp 29–43.
Second G (1982) Origin of the genic diversity of cultivated rice (Oryza spp.): study of the polymorphism scored at 40 isozyme loci. Japanese Journal of Genetics 57:25–57
Seinhorst JW (1950) De betekenis van de toestand van de grond voor het optreden van aantasting door het stengelaaltje (Ditylenchus clipsaci (Kühn) Filipjev). Tijdschr. Plantenziekten 56:289–348
Sharma-Poudyal D, Pokharel RR, Shrestha SM, Khatri-chhetri GB (2004) Evaluation of common Nepalese rice cultivars against rice root-knot nematode. Nepal Agricultural Research Journal 5:33–36
Shrestha R, Uzzo F, Wilson MJ, Price AH (2007) Physiological and genetic mapping study of tolerance to root-knot nematode in rice. New Phytologist 176:665–672
Soriano IR, Reversat G (2003) Management of Meloidogyne graminicola and yield of upland rice in South-Luzon, Philippines. Nematology 5:879–884
Soriano IR, Schmit V, Brar DS, Prot J-C, Reversat G (1999) Resistance to rice root-knot nematode Meloidogyne graminicola identified in Oryza longistaminata and O. glaberrima. Nematology 1:395–398
Soriano IR, Prot J, Matias DM (2000) Expression of tolerance for Meloidogyne graminicola in rice cultivars as affected by soil type and flooding. Journal of Nematology 32:309–317
Tandingan IC, Prot J-C, Davide RG (1996) Influence of water management on tolerance of rice cultivars for Meloidogyne graminicola. Fundamental and Applied Nematology 19:189–192
Trudgill DL (1991) Resistance to and tolerance of plant parasitic nematodes in plants. Annual Review of Phytopathology 29:167–192
Wade LJ, McLaren CG, Quintana L, Harnpichitvitaya D, Rajatasereekul S, Sarawgi AK, Kumar A, Ahmed HU, Sarwoto SAK, Rodriquez R, Siopongco J, Sarkarung S (1999) Genotype by environment interactions across diverse rainfed lowland rice environments. Field Crops Research 64:35–50
Win PP, Kyi PP, Maung ZTZ, Myint YY, DeWaele D (2015) Comparison of the damage potential and yield loss of the rice root-knot nematode, Meloidogyne graminicola, on lowland and upland rice varieties from Myanmar. Russian Journal of Nematology 23:53–72
Yik CP, Birchfield W (1979) Host studies and reactions of cultivars to Meloidogyne graminicola. Phytopathology 69:497–499
Acknowledgements
This research was supported by a Flemish Interuniversity Council (VLIR-UOS) Ph.D. scholarship to MTN Cabasan. The authors would like to thank the Plant Breeding, Genetics and Biotechnology Division (PBGB) and Crop and Environmental Sciences Division (CESD) of IRRI for the seeds, equipment, facilities and assistance in conducting the experiments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Section Editor: Diana Fernandez
Electronic supplementary material
ESM 1
(DOCX 22 kb)
Rights and permissions
About this article
Cite this article
Cabasan, M.T.N., Kumar, A. & De Waele, D. Evaluation of resistance and tolerance of rice genotypes from crosses of Oryza glaberrima and O. sativa to the rice root-knot nematode, Meloidogyne graminicola. Trop. plant pathol. 43, 230–241 (2018). https://doi.org/10.1007/s40858-018-0210-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40858-018-0210-8