Abstract
Molecular phylogenetic analysis, morphology and pathogenicity to citrus fruit were used to study two isolates of Elsinoë australis associated with scab-like symptoms on a fruit of Citrus australasica (finger lime) and Simmondsia chinensis (jojoba) in Australia. In addition to being associated with finger lime, the isolate from finger lime could cause scab symptoms on C. × aurantium cv. Murcott tangor in pathogenicity tests, but could not cause scab symptoms on the other orange, mandarin, lemon or grapefruit tested. Pathogenicity tests also support previous studies showing the isolate from jojoba could not produce symptoms on fruit of C. natsudaidai. Based on the findings of this study, two novel pathotypes of E. australis are designated from Australia; namely the Finger Lime (FL) pathotype associated with finger lime, and the Jojoba Black Scab (JBS) pathotype associated with black scab of jojoba. The significance of these novel E. australis pathotypes on market access and biosecurity issues for citrus are briefly discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Raciborski (1900) introduced Elsinoë for an ascomycete that formed scabs (Geschwülste) on plant tissues and produced asci irregularly in a pseudoparenchyma, with E. canavaliae from Java, Indonesia as the type species. The name Elsinoë was taken from the heroine in the play Iridion, written by the Polish dramatist and poet Zygmunt Krasiński in 1836 (Saccardo and Sydow 1902). Jenkins and Bitancourt (1941) provided an amended description of Elsinoë and its anamorphic state Sphaceloma (de Bary 1974). To date there are 48 species of Elsinoë, 14 of which have been recorded in Australia (Kirk et al. 2008).
Citrus spp. are some of the more commercially important hosts to Elsinoë spp., where the fungi cause raised, corky lesions on the fruit and/or foliage of certain Citrus spp. and cultivars (Timmer 2000). To date two species, E. fawcettii Bitancourt and Jenkins and E. australis Bitancourt and Jenkins, have been described from Citrus spp. Within each species several pathotypes have been described based primarily on differential host ranges. The E. fawcettii pathotypes include the Tryon’s and Lemon pathotypes, which are the only pathotypes known to occur in Australia, and primarily infect C. limon (L.) Burm.f. (lemon) (Timmer et al. 1996). Exotic to Australia are the Florida Broad Host Range (FBHR), Florida Narrow Host Range (FNHR) (Timmer et al. 1996), Jinguel, the Satsuma, Rough lemon, Grapefruit, Clementine (SRGC), and three additional cryptic pathotypes from Korea (Hyun et al. 2009), as well as three cryptic pathotypes from Florida (Wang et al. 2009). Recently, eight E. fawcettii pathotypes have been reported from China (Hou et al. 2014). The E. australis pathotypes include the well-established Sweet Orange Scab (SOS) from South America (Bitancourt and Jenkins 1937) and Natsudaidai from Korea (Hyun et al. 2001). Recently, a cryptic Natsudaidai-like pathotype was reported from Texas, USA (Kunta et al. 2013). However, the pathogenicity results and phylogenetic analyses are not entirely consistent with those reported by Hyun et al. (2009) for the Natsudaidai pathotype, indicating that the isolates from Texas may represent a novel pathotype. Another Natsudaidai-like pathotype has also been reported as causing black scab disease of Simmondsia chinensis (Link) Schneider (jojoba) plants in Australia (Ash et al. 2012).
The diverse and confusing nature of the Elsinoë spp. associated with Citrus spp. prompted an examination of Elsinoë spp. in Australia, which focused on two isolates of interest. The first isolate (DAR 77387 (BRIP 54681)) is that reported by Ash et al. (2012) from black scab disease on leaves and stems of jojoba collected in New South Wales (NSW) in 2006. This isolate was identified as a putative new E. australis pathotype, and was a new record for Australia and the host plant family. This identification was based on similarities to the internal transcribed spacer (ITS) region sequences of E. australis isolates from GenBank. The second isolate (BRIP 52616) was isolated from scab-like symptoms on the fruit of C. australasica F. Mueller (finger lime) in Queensland (Qld) in 2009 (Fig. 1). Finger lime is a species endemic to the forests along the coastal border region of NSW and Qld, Australia (Clarke and Prakash 2001). This isolate was also identified as E. australis based on the ITS sequence. However, Hyun et al. (2009) used molecular phylogenetic analyses based on the ITS region and partial region of the translation elongation factor 1-α gene (TEF) to give a better resolution between the E. australis pathotypes on Citrus spp.
Prior to these recent discoveries in Australia, E. australis had only been reported from Citrus spp., and most prominently as scab on fruit of mainly sweet oranges (C. sinensis L. Osbeck) in South America (the SOS pathotype) (Bitancourt and Jenkins 1937), and only on the fruit of C. natsudaidai Hayata in Korea (the Natsudaidai pathotype) (Hyun et al. 2001). The aim of this study was to further characterise the Australian isolates from finger lime and jojoba using molecular phylogeny, morphology and pathogenicity. The significance of these results to the citrus industry is discussed.
2 Materials and Methods
2.1 Isolates and Morphological Analysis
The isolates examined in this study are shown in Table 1. The isolate of Elsinoë sp. from finger lime was obtained using the methods of Hyun et al. (2001). Isolates from jojoba and finger lime were deposited in the Plant Pathology Herbarium (BRIP; Department of Agriculture, Fisheries and Forestry, Dutton Park, Queensland, Australia). The cultures were described after 4 weeks incubation in the dark on potato dextrose agar (PDA), or from microcolonies in Fries media (Whiteside 1975). Fungal structures were mounted on glass slides in lactic acid (100 % v/v) for microscopic examination after 28 days of incubation. At least 20 measurements of selected structures were made and the values were expressed as minimum - maximum. Images were captured with a Leica DFC 500 camera attached to a Leica DM5500B compound microscope with Nomarski differential interference contrast. Colony colours were recorded using the names in Rayner (1970).
2.2 DNA Extraction, PCR Amplification, Sequencing and Data Analysis
Mycelia from each isolate were placed in a 2.0 mL safe-lock tube (Eppendorf South Pacific). Then 0.5 mm diameter glass beads (Daintree Scientific) were added and the mycelia was lysed using the Tissue Lyser (Qiagen) for 2 min at 30 Hz/s. Genomic DNA was extracted from this mixture using the Gentra Puregene kit (Qiagen), following the manufacturer’s instructions. PCR amplification was conducted using the Phusion High Fidelity PCR Master Mix (New England Biolabs), which consisted of 12.5 μl of 2 × Master Mix, 0.5 μl each of 10 mM of forward and reverse primers, and 25 ng of DNA template. The ITS region was amplified with primers ITS1 and ITS4 (White et al. 1990), and the TEF was amplified with primers elongation-1-F and elongation-1-R (Hyun et al. 2009). Temperature cycling of the samples was performed in a Bio-Rad C1000 thermal cycler (Bio-Rad) under the following conditions: 95 °C for 2 min, 30 cycles at 95 °C for 30 s, 55 °C (for ITS) or 58 °C (for TEF) for 30 s, 72 °C for 1 min, followed by a 5 min final extension at 72 °C. The PCR products were purified using the QIAquick PCR Purification Kit (Qiagen) and were then sequenced by Macrogen Inc. using the AB 3730xl DNA Analyser (Applied Biosystems).
The ITS and TEF sequences from the Australian isolates were assembled using Vector NTI Advance (Invitrogen) and aligned against published sequences from GenBank (Table 1). The ITS and TEF sequences were initially aligned separately using MAFFT v. 6 (http://mafft.cbrc.jp/alignment/server/index.html) and the alignments checked by eye using MEGA 5.2.1 (Tamura et al. 2011). The ITS and TEF data were combined and a maximum likelihood (ML) tree was constructed using RAXML v. 8.0.0 (Stamatakis 2006) using the general time reversible (GTR) model of evolution (GTRMIX setting) and 1000 bootstrap replications. Bayesian Markov chain Monte Carlo (MCMC) analysis [MrBayes v. 3.1, Ronquist and Huelsenbeck (2003)] was performed with the combined ITS and TEF data. Four chains were run: one cold and three heated with the temperature parameter of the analysis set at 0.25. Data were partitioned into ITS and TEF with the Jukes-Cantor model of evolution. The ML tree constructed using RAXML was used as a starting tree for each analysis. The analysis was run for 1 × 108 generations with tree sampling every 100 generations. The analysis was stopped when the standard deviation of split frequencies dropped below 0.03. The resulting consensus tree displaying posterior probabilities was viewed using FigTree v. 1.4.0.
2.3 Pathogenicity and Koch’s Postulates
Pathogenicity tests were conducted on fruits from various Citrus spp., with the exception of finger lime, which were conducted on leaves due to an absence of fruit (Table 2). Previously characterised isolates of the E. fawcettii FNHR pathotype from Florida, and the E. australis SOS pathotype from South America, were included as positive controls (Timmer et al. 1996; Hyun et al. 2009). A culture of the E. australis Natsudaidai pathotype could not be obtained. All experiments were conducted in a high-security quarantine facility located at the Ecosciences Precinct, Qld, Australia. Detached stems with attached fruit were maintained in vitro as follows to best simulate natural conditions. Fruit-bearing stems of up to 25 cm long were cut from trees, then surface sterilised by immersion in 0.5 % sodium hypochlorite for 2 min, 70 % ethanol for 2 min, followed by three rinses in sterile distilled water (Timmer et al. 1996). After surface sterilisation, all leaves from the lower half of the stem were removed, and a fresh 45° cut made at the base of the stem. The cut end was immediately immersed in a sterile 250 mL flask containing sufficient autoclaved cut flower preservative solution (Chrysal Clear Universal, Pokon & Chrysal International BV) to cover the cut. The stem was then held in place with sterile cotton wool and aluminium foil. The stems were maintained in sealed plastic containers to maintain high humidity. Two separate inoculation experiments were conducted due to the different times susceptible host material were available. Experiment 1 was undertaken with fruit of C. natsudaidai only, whereby fruit-bearing stems were obtained from field trees growing in a region of southern NSW where scab does not occur. The stems were harvested and then delivered under cool and humid conditions to the quarantine facility. The E. australis isolate from jojoba was only included in experiment 1 to confirm its non-pathogenicity to C. natsudaidai fruit, as per Ash et al. (2012). Jojoba plants were not available to confirm the Koch’s postulates demonstrated by Ash et al. (2012). Experiment 2 was conducted with a wider host range, using fruit from glasshouse-raised host trees free from any previous Elsinoë spp. infection.
In both experiments, conidia of the isolates were produced according to Whiteside (1975). Cultures were grown on PDA for 7 days at 25 °C under a 12 h cycle of black light and darkness. About 5 mm2 of mycelium was scraped from the leading edge of the colony, then crushed in a petri dish using a sterile spatula. The dish was then flooded by pipetting 5–6 mL of modified liquid Fries medium and incubated for 24 h at 25 °C under a 12 h cycle of black light and darkness. After incubation, the Fries medium was decanted and the remaining micro-colonies rinsed three times in sterile distilled water. The dishes were then flooded with 5–6 mL of filter-sterilised pond water and incubated for another 24 h as above. The resulting conidial suspension was decanted into a sterile measuring cylinder and adjusted to 1 × 106 conidia per mL. In experiment 1, sporulation of DAR 70041 was poor, so the suspension was amended to 2 × 105 colony forming units per mL by adding hyphal fragments from a colony growing on PDA.
Inoculation of fruit in both experiments was performed by first marking the point of inoculation with a permanent marker. The fruit were inoculated by pipetting a 10 μL droplet of conidial suspension or water. To replicate field conditions, fruit were inoculated a second time after 5 days with a further 30 μL of conidial suspension or water. For each isolate a minimum of three individual fruit from each host were inoculated. Leaves of C. australasica were inoculated in the same manner. Following inoculation, the stems were maintained at high humidity at ca. 24 °C and exposed to ambient light conditions. The stems were incubated for 3 weeks, or until the fruit abscised. Fruit that abscised were observed under a dissecting microscope to observe any symptom development, and then further incubated in a sealed plastic bag containing a filter paper saturated in sterile distilled water to maintain high humidity until the 3 weeks incubation period was complete, or until the fruit began to show signs of senescence. After incubation, the fruit were inspected for any symptom development for a final time. In experiment 1, the fruit were surface sterilised by immersion for 1 min in 70 % ethanol before attempting to re-isolate the inoculated fungus. In experiment 2, the fruit were surface sterilised by immersion for 1 min in 70 % ethanol and then 1 min in 1 % sodium hypochlorite followed by rinsing in sterile water (Hyun et al. 2001). Tissue from the point of inoculation was plated onto streptomycin amended PDA to complete Koch’s postulates.
3 Results
3.1 Morphology
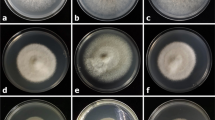
Colonies of the JBS pathotype on PDA after 4 weeks at 25 °C in the dark grew to 1.5–2.5 cm diameter, were velutinous, convoluted, sulcate, with up to 5 radial ridges near the margin, and ca. 4 mm high in the centre (Fig. 2a–d). Colonies were Fuscous Black, margin shallowly lobed to irregularly crenate; reverse Violet Slate, stellate, and soluble pigment was not produced. Conidiomata, composed mostly of yellowish brown textura angularis that separated readily under pressure. Conidiophores formed on the surface of the colony, scattered, branched, 0–4 septate, pale brown, lageniform to cylindrical, and 10–40 × 3–6 μm. Conidiogenous cells were polyphialidic, with 1–4 integrated loci, hyaline to pale brown, 5–15 × 3–4 μm, cylindrical to irregular, and nodose to digitate at the apex. Conidia were hyaline, aseptate, cylindrical, 2–6 × 1–1.5 μm, and sometimes apparent en masse as pale brown ooze on the surface of the colony.
a–d. Jojoba black scab (JBS) pathotype of Elsinoë australis (BRIP 54681). a. Culture after 4 wk at 25° C in the dark on PDA; b. Conidiomatum (arrowed); c. Conidia; d. Conidiophores with polyphialidic conidiogenous cells (arrowed). e–g. Finger lime (FL) pathotype of E. australis (BRIP 52616). e. Culture after 4 wk at 25°C in the dark on PDA; f. Conidiomatum (arrowed); G. Conidium (left arrow) and conidiophores (right arrow). Scale bars: a, e = 1 cm; b = 1 mm; c–d, g = 10 µm; f = 100 µm
Colonies of the FL pathotype on PDA after 4 weeks at 25 °C in the dark grew to 2–3 cm diameter, were convoluted with a scant covering of aerial mycelium becoming waxy and glistening without aerial mycelium at the margin, ca. 4 mm high in the centre, cinnamon to buff at the margins with irregular rosy buff to rosy vinaceous patches of varying hues across most of the colony (Fig. 2e–g). The colony margin was shallowly lobed, reverse buff at the margin becoming brown vinaceous towards the centre. Soluble pigment was not produced. In culture in Fries medium conidiophores formed on the surface of agar pieces, were hyaline, branched or unbranched, 10–80 × 2–5 μm. Conidiogenous cells were terminal and intercalary in the conidiophore, polyphialidic with 1–3 integrated apical loci, hyaline, 8–15 × 3–5 μm, ampulliform to lageniform. Conidia were hyaline, aseptate, cylindrical to ellipsoidal, 4–7 × 2–4 μm.
3.2 Phylogeny
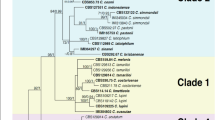
The combined ITS and TEF alignment contained 977 characters, of which 100 were parsimony informative, 103 were variable, and 873 conserved. In the case of the Texas isolates, the data available from GenBank did not include sites 1–247, 558–596, and 939–977 of the alignment, which accounts for missing data at 3 of the 16 variable sites specific to the isolates of E. australis. The Bayesian MCMC analysis revealed a clear separation of the E. fawcettii isolates from the remaining isolates with posterior probability value (PP) of 1.0 (Fig. 3). Within the E. fawcettii group, the Jinguel pathotype formed a subclade with posterior probability of 0.87. Within the E. australis clade, isolates from the SOS, FL and JBS pathotypes clustered separately from the Natsudaidai pathotype and Texas isolates with PP of 0.85. Within this clade, the SOS and JBS pathotypes clustered separately from the FL pathotype with PP of 0.95 and 0.97, respectively. Less well-resolved were the Natsudaidai pathotype and the Texas isolates, amongst which there were polytomies and a significant division within some of the isolates from Texas.
3.3 Pathogenicity and Koch’s Postulates
In both experiments and in all inoculation combinations, the points of inoculation remained wet for at least 48 h. The majority of fruit in both experiments then remained attached to the stems for 5–10 days, with some remaining for 17 days. In no cases did inoculation with water produce any symptoms of disease.
In experiment 1, none of the isolates tested produced any symptoms of citrus scab on fruit of C. natsudaidai (Table 2). Superficial stroma was seen growing on the fruit surface. When this stroma was removed with adhesive tape, the fruit tissue below was free of any symptoms. When attempts were made to culture the inoculated isolate from the inoculation point, all were re-isolated with the exception of the Tryon’s isolate. In one case, after surface sterilisation by immersion in 70 % ethanol, some of the superficial stroma was cultured separately from any plant tissue, and still grew. This indicated that the fungus was able to survive the ethanol treatment in this state. Consequently, the more aggressive surface sterilisation method described above was adopted in experiment 2 to ensure that re-isolation of the fungus was not the result of superficial mycelium.
In experiment 2, typical scab symptoms were observed on fruit in the SOS × sweet orange (C. sinensis cv. Valencia) inoculation, the FNHR × grapefruit (C. × paradisi cv. Henderson) and Tryon’s × lemon (C. limon cv. Eureka lemon) inoculations, as expected for these positive control pathotype × host combinations (Table 2). The symptoms that developed in these cases were typical of young scab symptoms, whereby lesions appeared as conspicuous outgrowths ranging in colour from salmon buff, flesh ochre to bright shades of pink or red (Winston 1923). The FL × Murcott (C. × aurantium cv. Murcott tangor) and SOS × Murcott inoculations produced very similar symptoms, although the scab symptoms were less advanced than seen in the ‘sweet orange scab’ × sweet orange inoculation. In some isolate × host inoculations, spotting symptoms atypical of scab were observed and designated as “spotting but no clearly identifiable scab lesions” (Table 2). These atypical spotting symptoms were small (<1 mm), slightly sunken, light coloured lesions, or small dark spots, similar to the spotting described by Hyun et al. (2009) and are not considered to be a typical scab host/pathogen reaction. In nearly all cases the inoculated isolate could be re-isolated from the inoculation point, regardless of symptom status and despite the use of the more aggressive surface sterilization protocol in experiment 2. Recovery rates were higher from fruit showing typical scab symptoms.
4 Discussion
In this study we describe two novel E. australis pathotypes from Australia; the Finger Lime (FL) pathotype from finger lime, and the Jojoba Black Scab (JBS) pathotype from jojoba. The JBS pathotype is the name proposed for the putative pathotype first described by Ash et al. (2012), and is supported on the basis of the previous study, and by the additional morphology, phylogeny and pathogenicity we have provided. The JBS pathotype is only known to occur on jojoba, and represents the only Elsinoë sp. described from the host family Buxacae. In contrast, Elsinoë spp. are well known on hosts in the family Rutaceae (Timmer et al. 1996), with the FL pathotype naturally associated with finger lime and artificially able to infect fruit of Murcott. The FL pathotype has only ever been recovered from a single finger lime fruit in Australia, despite repeated sampling at the original site of collection. The FL pathotype was isolated from scab-like symptoms on fruit, but previous scab-like symptoms on finger lime fruit have been associated with the E. fawcettii Tryon’s pathotype (Timmer et al. 1996). The JBS and FL pathotypes have not been reported outside of Australia, and both pathotypes are pathologically and phylogenetically distinct from established E. australis and E. fawcettii pathotypes exotic to Australia. Morphology within the previously described E. australis and E. fawcettii pathotypes remain indistinct, as previously observed (Timmer et al. 1996).
The combined ITS and TEF phylogeny illustrates polytomies within E. australis that reflect the existing pathotypes (Natsudaidai and SOS), the novel pathotypes described in this study (FL and JBS), and the isolates from Texas. The resolution of E. australis phylogeny is limited by two issues. Firstly, the published sequences on GenBank for the Texas isolates are incomplete relative to the sequences available for the isolates characterised by Hyun et al. (2009); the benchmark study for the phylogeny and pathogenicity of Elsinoë spp. associated with Citrus spp. This leaves the status of three polymorphic sites within the ITS and TEF loci unknown for the isolates from Texas. Secondly, only ITS and TEF sequences are publicly available for all E. australis and E. fawcettii pathotypes. The phylogeny would be better resolved through additional gene sequence data. For example, recent phylogenetic analysis of Phyllosticta spp. in relation to citrus black spot disease utilized actin and glyceraldehyde-3-phosphate dehydrogenase gene sequences in addition to ITS and TEF (Glienke et al. 2011). However, access to cultures in order to generate additional sequence data is as geographically limited as the pathotypes and species are themselves, and a coordinated international effort will be required to generate and analyse additional sequence information.
The JBS pathotype did not produce any symptoms on fruit of C. natsudaidai, as previously reported (Ash et al. 2012). There is currently no evidence to suggest the JBS pathotype is of consequence to citrus. The FL pathotype did not produce scab symptoms on the defining hosts of the previously described E. australis pathotypes, namely sweet orange for the SOS pathotype and C. natsudaidai for the Natsudaidai pathotype (Hyun et al. 2001; Bitancourt and Jenkins 1937). However, the FL pathotype did produce symptoms on the fruit of Murcott. While the FL pathotype has the potential to impact the production of finger lime and Murcott in Australia, the rarity of this fungus in the field indicates any significant impact is unlikely. Even so, additional studies are needed to determine the potential impact of the FL pathotype on commercial production. Such studies should include pathogenicity testing of the FL pathotype on a wider range of host fruit and leaves, as well as on fruit of finger lime.
Internationally, reports of novel cryptic Elsinoë spp. pathotypes have become more frequent in recent years, particularly in association with Citrus spp. (Kunta et al. 2013; Wang et al. 2009; Ash et al. 2012; Hou et al. 2014). Often these reports indicate more complex variations in phylogeny, pathogenicity and symptomology than observed in past studies of the Elsinoë spp. associated with Citrus spp. (Timmer et al. 1996; Tan et al. 1996; Hyun et al. 2001). A possible explanation for the apparently cryptic nature of these Elsinoë spp. pathotypes may be the ability of the fungi to survive and reproduce without producing classic scab symptoms. For example, E. australis has been associated with wind-scarred tissue and other similarly cryptic symptoms on citrus fruit in Texas, USA (Kunta et al. 2013). In the current study, it was possible to culture the Elsinoë spp. from inoculated, asymptomatic and surface sterilised fruit tissue, indicating its ability to penetrate the citrus fruit rind. Both these observations support the possibility of saprophytic or endophytic growth. Better comprehension of these fungi and their host interactions is clearly required.
Elsinoë spp. on Citrus spp. have biosecurity implications (Broadbent 1995). The E. fawcettii pathotypes in Australia predominantly infect lemon fruit (Timmer et al. 1996). Scab in Australia is only occasionally problematic on lemons grown in the higher rainfall areas of the north coast of NSW and coastal Qld. Scab is absent from the drier, inland production regions in South Australia, Victoria and southern NSW (Broadbent 1995). Should a species or pathotype with a wider host range establish in Australia, such as the E. fawcettii FBHR pathotype (Timmer et al. 1996), then the wider production base of mandarins, oranges, and grapefruit in the higher rainfall areas will be at risk. Commercially traded fresh citrus fruit are unlikely to act as a pathway for Elsinoë spp. or pathotypes such as the E. australis FL pathotype, for several reasons, (i) the FL pathotype is extremely rare on finger lime and has never been reported on Murcott in the field; (ii) only asymptomatic fruit are packed for export, and asymptomatic fruit are not considered a pathway (USDA APHIS PPQ 2010); and (iii) the standard postharvest practices used in Australia such as high pressure washing (Cunningham 2002), brushing (Taverner and Cunningham 1999), and sodium ortho-phenylphenate tetrahydrate treatment (Taverner 2012) are likely to further reduce the pathway potential.
References
Ash GJ, Stodart B, Hyun JW (2012) Black scab of jojoba (Simmondsia chinensis) in Australia caused by a putative new pathotype of Elsinoë australis. Plant Dis 96:629–634
Bitancourt AA, Jenkins AE (1937) Sweet orange fruit scab caused by Elsinoë australis. J Agric Res 54:1–18
Broadbent P (1995) Quarantine in relation to Australian citrus imports and exports. Australas Plant Pathol 24:145–156
Clarke K, Prakash N (2001) Floral morphology and embryology of two Australian species of Citrus (Rutaceae). Aust J Bot 49:199–207
Cunningham N (2002) Sooty mould cleaners and high pressure washing. Packer Newsl 67:1–3
de Bary A (1974) Über den sogenannten Brenner der Reben. Ann der Oenologie 4:165–167
Glienke C, Pereira OL, Stringari D, Fabris J, Kava-Cordeiro V, Galli-Terasawa L, Cunnington J, Shivas RG, Groenewald JZ, Crous PW (2011) Endophytic and pathogenic Phyllosticta species, with reference to those associated with Citrus Black Spot. Persoonia 26:47–56
Hou X, Huang F, Zhang T, Xu J, Hyde DK, Ll H (2014) Pathotypes and genetic diversity of Chinese collections of Elsinoë fawcettii causing citrus scab. J Integr Agric 13:1293–1302
Hyun JW, Timmer LW, Lee S-C, Yun S-H, Ko S-W, Kim KS (2001) Pathological characterization and molecular analysis of Elsinoë isolates causing scab diseases of citrus in Jeju Island in Korea. Plant Dis 85:1013–1017
Hyun JW, Yi SH, Mackenzie SJ, Timmer LW, Kim KS, Kang SK, Kwon HM, Lim HC (2009) Pathotypes and genetic relationship of worldwide collections of Elsinoë spp. causing scab diseases of citrus. Phytopathology 99:721–728
Jenkins AE, Bitancourt AA (1941) Revised descriptions of the genera Elsinoë and Sphaceloma. Mycologia 33:338–340
Kirk PM, Cannon PF, Minter DW, Stalper JA (2008) Dictionary of the Fungi, 10th edn. CABI International, Surrey
Kunta M, Rascoe J, de Sa P, Timmer LW, Palm ME, da Graca JV, Mangan RL, Mangan RL, Malik N, Salas B, Satpute A, Setamou M, Skaria M (2013) Sweet orange scab with a new scab disease “syndrome” of citrus in the USA associated with Elsinoë australis. Trop Plant Pathol 38:203–212
Raciborski M (1900) Parasitische Algen und Pilze Java’s. Batavia, Staatsdruckerei
Rayner RW (1970) A mycological colour chart. Kew, England. Commonwealth Mycological Institute
Ronquist FJ, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Saccardo PA, Sydow P (1902) Sylloge Fungorum omnium hucusque cognitorum. Digessit P.A. Saccardo 16:804
Stamatakis A (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Tan MK, Timmer LW, Broadbent P, Priest M, Cain P (1996) Differentiation by molecular analysis of Elsinoë spp. causing scab diseases of citrus and its epidemiological implications. Phytopathology 86:1039–1044
Taverner P (2012) Sodium o-phenylphenate tetrahydrate (SOPP). Packer Newsl 103:3–5
Taverner P, Cunningham N (1999) Sanitation survey of Riverland packingsheds. Packer Newsl 56:1–5
Timmer LW (2000) Scab diseases. In: Timmer LW, Garnsey SM, Graham JH (eds) Compendium of citrus diseases. APS Press, St Paul, pp 31–32
Timmer LW, Priest M, Broadbent P, Tan MK (1996) Morphological and pathological characterization of species of Elsinoë causing scab diseases of citrus. Phytopathology 86:1032–1038
USDA APHIS PPQ (2010) The significance of Citrus spp. fruit as a pathway for the introduction or spread of Elsinoë australis, the organism that causes Sweet orange scab disease. Raleigh, NC, USA. Center for Plant Health Science and Technology, Plant Epidemiology and Risk Analysis Laboratory
Wang L, Liao H, Bau H, Chung K (2009) Characterization of pathogenic variants of Elsinoë fawcettii of citrus implies the presence of new pathotypes and cryptic species in Florida. Can J Plant Pathol 31:28–37
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols a guide to methods and applications. USA. Academic Press, San Diego, pp 315–322
Whiteside JO (1975) Biological characteristics of Elsinoë fawcettii pertaining to the epidemiology of sour orange scab. Phytopathology 65:1170–1175
Winston JR (1923) Citrus scab: its cause and control. Bulletin 118. Beltsville, MD, USA. United States Department of Agriculture
Acknowledgments
This research was partially funded by Horticulture Australia Limited using the citrus industry levy and matched funds from the Australian Government, and The University of Queensland, and the Queensland Department of Agriculture, Fisheries and Forestry. We wish to thank Dr. Mui Keng Tan and Dr. Nerida Donovan (NSW DPI) for providing the ‘jojoba black scab’ isolates. Dr. Jae-Wook Hyun (Rural Development Administration, National Institute of Horticultural & Herbal Science, Korea) for providing isolates of E. australis and E. fawcettii. Dr. Graeme Sanderson (NSW DPI) for providing the shoots of C. natsudaidai. Dr. Nerida Donovan (NSW DPI), Sylvia Jelinek (NSW DPI) and Tim Hermann (AusCitrus) for supplying various host plants. Dr. Jay Anderson (formerly DAFF Qld), Dr. Dean Beasley (DAFF Qld) and Cecilia O’Dwyer (UQ) for technical assistance, and Pat Barkley for advice on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Section Editor: Meike Piepenbring
Rights and permissions
About this article
Cite this article
Miles, A.K., Tan, Y.P., Shivas, R.G. et al. Novel Pathotypes of Elsinoë australis Associated with Citrus australasica and Simmondsia chinensis in Australia. Trop. plant pathol. 40, 26–34 (2015). https://doi.org/10.1007/s40858-015-0005-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40858-015-0005-0