Abstract
The effect of oxygen on interfacial phenomena between slag and copper melt was investigated at 1400 °C. The removal of impurities (iron and silicon) in the copper melt during oxygen blowing was confirmed. Also, it was revealed that composition change of the slag occurs due to the oxygen potential in the slag depending on the oxygen injection method. In addition, over-blowing of oxygen after removing impurities is a factor that significantly changes the surface tension of molten copper and slag. Over blown oxygen increases the instability of the interface by reducing interfacial tension between slag and liquid copper. Due to lowered interfacial tension and increased instability, copper droplets can be mixed into slag phase which induces copper loss. Oxygen blowing and/or bubbling is involved in chemical and weak physical reactions in the gas-slag-metal multiphase system, which should be carefully controlled because it is significant factor affecting the recovery rate of the metal.
Graphical Abstract
Overall reactions and slag/metal interfacial phenomena in impurity removal regime and over-blowing regime for (a) bottom blowing and (b) top blowing conditions.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Traditionally, various pyrometallurgical refining processes of scrap or ore using reactions between the gas, slag, and metal have been developed to produce target metals with a desirable composition. In addition, several special treatment procedures are also used to produce high grade ingots. For instance, in the high-grade copper production, in order to manufacture the electrolytic copper, an electro-refining process is performed after converting and slag cleaning, followed by the fire refining process [1, 2]. However, some critical impurities may not be removed in the converting process. In particular, iron adversely affects the cathode and acts as a factor in deteriorating the quality of the electrolyte solution [3]. Therefore, it is necessary to thoroughly remove such impurities in the converting process to result in an economical yield. The relevant issues must be taken into account both in the primary smelting and converting processes using the copper concentrates and in the smelting process of secondary sources such as e-waste and/or black copper scrap. To achieve this goal, as mentioned above, the oxidative impurities are removed through the oxygen blowing at high temperatures.
After most of the removal process is complete, residual oxygen also reacts with the liquid metal. A physicochemical effect of gas on liquid metals has been reported by several researchers under various conditions [4,5,6,7,8,9]. It is widely known that the release of droplets into the slag layer has the effect of improving heat and mass transfer in the gas-slag-metal multiphase reactions. However, if metal droplets are excessively entrapped in slag, it needs to be properly treated to recover metals such as slag cleaning process. However, this process also consumes energy and operation time, it is necessary to properly control the formation of metal droplets and this control is possible through quantitative understanding of the mechanism for droplet formation in the gas blowing system.
The mechanism of droplet formation by gas blowing has been previously summarized in detail [5]. Gas blowing creates an impingement zone at the slag/metal interface where droplets form intensively. It has been found that droplets are intensively released and circulated by turbulence in this zone. From the viewpoint of the physical effect of bottom bubbling, it was revealed that rising gas bubbles can induce metal droplet entrapment in slag [5,6,7,8]. Depending on the size of the metal droplets, the formation mechanism changes somewhat but what has been commonly found is that metal entrapment occurs due to splashing by gas injection and turbulence at the impingement zone. In addition, it was found that the droplet number distribution by gas injection changes around the impingement zone [6].
Many researchers have revealed that top blowing and bottom bubbling of gas physically causes metal entrapment. However, studies on gas impingement at the slag/metal interface have mainly been conducted using inert gases, so it is ambiguous to represent the process involving the chemical effect of oxygen used in the converting process, which is complexly involved with the physical effect including agitation of the melt. In the case of oxygen, which chemically reacts with metal, it may bring other phenomena combined with physical effects including metal entrapment and droplet formation.
Several studies on the effect of oxygen adsorption on liquid metal have been carried out in various systems. The representative effect of oxygen on liquid metal is the change of surface tension. It is well known by the Langmuir isotherm model that the surface tension of liquid metal changes based on the oxygen potential. In studies by Belton [10], and Gallois and Lupis [11], the effect of the adsorption of oxygen on the surface tension of liquid metal is well explained. The oxygen saturated area can be changed by controlling the oxygen potential in the experimental atmosphere [12, 13].
Also, it was found that the surface tension can be effectively reduced at high temperatures over a wide oxygen partial pressure range in the case of liquid copper [14]. In the case where the oxygen partial pressure was sufficiently low, temperature is a dominant variable on the surface tension of liquid copper [15]. This tendency is reversed when the oxygen partial pressure is sufficiently high. In other words, in a condition where oxygen is continuously injected, oxygen seems to have a dominant effect on the surface tension of liquid metal. If the surface tension of the molten metal changes due to these factors, it is expected that the formation behavior of droplets protruding from the surface of the molten metal will also change.
When oxygen reacts with metal surface, the effective amount of oxygen that is substantially adsorbed onto the surface to lower the surface tension should be less than the solubility under the given conditions. In this regard, the oxygen solubility of liquid copper was determined under various conditions. It was reported that the oxygen solubility is about 10,000 ppm or more [16, 17]. Therefore, it can be assumed that only within this range, oxygen can be adsorbed on the surface of pure liquid copper and effectively affect the surface tension.
In the condition where the surface tension of the melt changes due to oxygen, the slag/metal interface appears to be unstable. As suggested by Good [18], interfacial tension occurs between the two immiscible liquids. The related phenomena of the interface are the Kelvin–Helmholtz instability and Marangoni flow [19]. Interfacial instability due to change of interfacial tension between slag and liquid metal can induce droplets by oscillation dampening at the interface. When this oscillation by specific momentum occurs at the slag/metal interface, it can form a wavy shaped interface and tearing tip [20]. When this phenomenon occurs, surface reactants such as oxygen are expected to have a great influence.
Clearly, it seems that the slag/metal interface state is related to droplet formation phenomena. Representatively, Cramb et al. [21, 22] also suggested that the ejection of metal droplets into slag is induced by spontaneous emulsification, which is a result of the instability of the phase interface of the two liquids resulting from the difference of the fluid flow velocities between liquid iron and slag. Another researcher proposed that the surface state also appears to have a great influence on droplet behavior in slag [23]. It was found that the formation and distribution behavior such as flotation or spreading of droplets is greatly influenced by the interfacial tension between the slag and metal. In other words, the difference of the flow velocities between fluids in the horizontal and normal direction of the system can cause droplets to be generated.
Despite numerous papers published about surface tension as well as the droplet formation mechanism and behavior, there is little data of the quantitative relationship between droplet formation and surface tension and/or oxygen potential available for the situation where the surface state of the melt continuously changes as oxygen is steadily injected, which causes the physical and chemical gas-slag-metal complex reactions. In establishing a quantitative relationship between the surface tension and droplet entrapment in slag, there have been partial considerations of the droplet and surface tension [9], even though this seems to have caused errors in the analysis because of some measurement limitations.
The present research investigates the effect of the interfacial state on emulsification phenomenon that can occur when oxygen is over-injected through the process of removing impurities in crude copper melt and changes of the accompanying physicochemical phenomena. Through this study, the understanding of complex phenomena due to the physical and chemical effects of gas injection in gas-slag-metal reactions can be improved. In addition, it will be helpful to provide the insight to solve the problems related to the productivity in the recycling process of secondary copper scrap (i.e., black copper) as well as in the primary copper smelting process.
Experimental Procedure
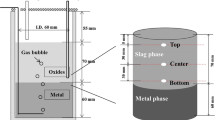
Five experiments were carried out using a high frequency induction furnace for 60 min except for the “No Slag” condition performed only for 30 min, as schematically shown in Fig. 1a with additional attached equipment. Metal and slag were initially put in a fused magnesia crucible (OD: Outer Diameter, ID: Inner Diameter, and HT: Height, ɸOD: 60 mm, ɸID: 50 mm, HT: 120 mm) with a graphite heater (ɸOD: 80 mm, ɸID: 65 mm, HT: 120 mm) for efficient heating. The magnesia crucible was surrounded by a commercial purity alumina insulation board (ɸOD: 104 mm, ɸID: 87 mm, HT: 180 mm). The oxygen blowing method is shown in Fig. 1b. In the case of top blowing, oxygen was blown from the slag surface and for bottom blowing, an alumina lance (ɸOD: 10 mm, ɸID: 5 mm) was used to inject oxygen 5 mm from the bottom of the vessel.
A quartz reaction chamber (ɸOD: 120 mm, ɸID: 114 mm, HT: 400 mm) was present in the high frequency induction furnace. Before induction heating, the reaction chamber was evacuated using a rotary vane pump to remove air and some impurities. The flow rate of argon gas (99.9% purity) was controlled by using a mass flow controller to fix the flow rate at 300 ml min−1. The argon gas was blown through Drierite® to remove undesirable impurities and another chamber filled with silica gel to eliminate moisture. Also, by passing through a magnesium turnings furnace at 500 °C during the experiment, the undesirable oxygen in the inert gas were chemically removed.
The experimental temperature was 1400 °C, which was maintained to within ± 2 °C using a proportional integral differential controller (GR-100) and a B-type (Pt–30wt%Rh/Pt–6wt%Rh) thermocouple covered with an alumina. After stabilization of the experimental temperature, a metal sample (0 min) was collected by suction using a quartz tube with a specific radius (ɸOD: 6 mm, ɸID: 4 mm) and then quenched by ice water. Prepared master slag chips of a specific size (1–2 mm diameter) were added for 10 s by using a quartz tube (ɸOD: 12 mm, ɸID: 10 mm) to the surface of the molten crude copper. After slag addition, oxygen gas (99.9% purity) was blown through the alumina lance (ɸOD: 10 mm, ɸID: 5 mm) with a flow of 500 ml min−1 at the start of the experiment for 60 min. Small amounts of metal and slag were continuously collected at specific time intervals for 60 min. Slag samples were collected by slightly dipping the tip of an iron rod into the slag layer, followed by quenching in ice water. The collected metal and slag samples were stored after the elimination of moisture and residuals. The initial (\(t = 0{\text{ min}})\) and final (\(t = 30 \,{\text{or}}\, 60\,{\text{min}})\) compositions of the metal and slag as well as the oxygen injection method employed in each experiment are shown in Table 1, in which the Run ID for all experiments were named based on the initial slag conditions. Also, Table 1 shows a slight increase in Al2O3 content even though Al2O3 was not added in the initial slag (No Slag and CSM runs), which was caused by a dissolution of alumina lance during experiments.
After the experiment was complete, final samples including refractory were cooled in argon gas for 10 min. Slag and slag/refractory interface samples were collected for the characterization using a field emission scanning electron microscope (FE-SEM) imaging. Taken at intervals, the metal samples were polished using a hand grinder and sand paper to peel off the oxide on the sample surface and then cut into pieces with a constant weight to prevent analysis error. The contaminants remaining on the surface of the metal were removed by ultrasonic cleaning for 10 min by using anhydrous ethanol. The oxygen content in the metal sample was determined by a combustion analyzer (TC-300, LECO). Also, the quantitative composition of the metal was determined by using inductively coupled plasma spectroscopy (ICP-AES, SPECTRO ARCOS).
Slag samples used for X-ray diffraction analysis (XRD, Rigaku), titration method, and ICP-AES analysis were crushed and properly mixed by using a mortar. These samples were sieved to under 100 μm. The slag titration analysis employed a KS-E-3016 standard to determine the content of acid soluble Fe2+ ions (FeO). About 0.1 g of the slag sample was used and analyzed by adding about four drops of hydrofluoric acid to dissolve silica in solution. The solid phase in slag was detected by XRD. For SEM imaging of slag, slag samples that were not crushed yet were produced in a mold form by cold mounting and the surface of mold was ground using sand paper. Metal droplets and oxides in the slag were observed by using FE-SEM and energy-dispersive X-ray spectroscopy (EDS) (Nova Nano SEM 450, FEI).
Results and Discussion
Phenomena by Oxygen Blowing: Impurity Removal and Oxygen Potential Changes
As oxygen is injected into the liquid copper, impurities with high affinity with oxygen such as iron and silicon in the melt begin to be removed by oxidation reaction as shown in Fig. 2. At the beginning of the reaction, iron is hardly removed because of the presence of silicon in the melt, which can be easily understood through the thermodynamic estimation. The equation for the removal reaction of silicon and iron in liquid copper is shown in Eqs. (1) and (2) respectively, based on thermodynamic data from the literature [24, 25].
In this period, i.e., impurity removal regime, silicon and iron competitively react with blown oxygen. The phenomenon that silicon is primarily removed rather than iron can be predicted by Eqs. (1) and (2). The process of competitive oxidation of several elements occurs in the basic oxygen furnace (BOF) during oxygen steelmaking process [26,27,28,29]. Silicon is completely removed within 10 to 15 min after oxygen is blown, followed by iron removal from 15 to 45 min depending on the slag composition and/or mass of crude copper melt.
As the impurities in the melt are oxidized, iron and copper contents in the slag increase as shown in Fig. 3. It is noticeable that the amount of copper incorporated into the slag significantly increases after the most of iron in the liquid copper has been oxidized. From the point at which the copper content drastically increases over than approx. 10 wt% due to a continuous oxygen blowing is defined as oxygen over-blowing regime. Hence, it is presumed that the transition from the impurity removal regime to the oxygen over-blowing regime occurred in conjunction with the change of the slag/metal interfacial state, which will be discussed in detail in the following section.
In the oxygen over-blowing regime, several crystalline phases are formed in the slag, as shown in Fig. 4. It can be seen that copper exists in the slag as metallic particle or oxide phase such as Cu2O. Because gas flow rate was fixed as 500 ml min−1, under the same condition for the amount of oxygen blowing, the copper speciation in the slag seems to be dependent on the mass of copper melt: the lower the total mass of molten copper, the more the copper is oxidized when oxygen is blown at a given condition. More specifically, the composition of the slag or copper phases are affected by the O2 gas to copper mass ratio. Also, when the oxygen blowing pattern is varied, the slag composition is significantly affected. For instance, the magnetite is formed only in the top blowing (T) condition. In a study by Naito et al. [30], in the case of top blowing, it can be seen that injected gas is not sufficiently mixed with the liquid metal and is pushed into the slag or air layer. Therefore, it is reasonable to assume that the Fe3O4 formation is determined by the oxygen potential of the slag. In general, Fe2O3 and Fe3O4 are known to increase the melting point and viscosity of the slag [26,27,28].
Depending on oxygen potential in slag, the difference in the copper phase in the slag appears to be clear. In the CaO–SiO2–MgO–Al2O3 (B) system, both metallic and oxidic copper phases are observed. This implies that copper droplets can be oxidized by the oxygen potential of the surrounding slag. In the work of Bellemans et al. [31], it was pointed out that slag can be a medium such as reduction or oxidation at the slag/droplet interface. In addition, it is revealed by De Wilde et al. [32] that the copper in liquid slag can exist as oxide, metal droplets, or a mixed state depending on the oxygen potential of the system or the presence of reaction sites such as spinel compound.
Obviously, the ratio of Fe3+ (ferric) to Fe2+ (ferrous) in the slag also seems to be strongly influenced by the oxygen blowing method as shown in Fig. 5. When oxygen is continuously injected, most of the impurities are removed and the Fe3+/Fe2+ ratio increases, which seems to originate from the over-blowing of oxygen. In the bottom blowing case, it can be confirmed that the Fe3+/Fe2+ ratio is maintained at a relatively constant value even though oxygen is continuously injected after most of the impurities are removed. In the case of top blowing, the oxygen potential in the slag is higher than that of bottom blowing condition. Therefore, the formation of Fe2O3 in the slag is promoted by increasing the driving force of the ‘2(FeO) + 0.5O2(g) = (Fe2O3) reaction. In general, Fe2O3 in the slag serves to decrease surface tension of the slag. The surface tension of the slag will be discussed in detail in the next section.
The high oxygen potential of slag promotes oxidation of the elements in slag. The effect of the oxygen blowing method on the slag composition is shown in Fig. 6. The phase diagrams were constructed using FactSage (v.7.3 with FToxid database), which is a commercial thermochemical computing software. In the impurity removal regime, the composition seems to similarly change regardless of the oxygen blowing method. After most of the impurities are removed, in the oxygen over-blowing regime, the oxygen potential gradient between the slag and metal occurs due to the fixed position of oxygen lance. In the case of top blowing with a high oxygen potential loaded on the slag, the driving force for the generation of magnetite (spinel) increases.
This phenomenon is in good agreement with the composition change tendency that can occur due to the increase of the oxygen potential in the slag by top blowing in BOF process, as previously revealed by several researchers [26,27,28]. In the present study, even after impurities such as silicon and iron in liquid copper are almost removed, the oxygen blown seems to increase the proportion of ferric oxide (Fe3+), resulting in the formation of magnetite. In general, magnetite provides the increase in the melting point and viscosity of the slag. Therefore, the over-blowing of oxygen should be avoided because there is a risk of impairing the overall stability of the process.
Oxygen Effect on the Interfacial Tension Between Metal and Slag
Over-blowing of oxygen can also influence the liquid metal surface state. After the impurities are removed, continuous oxygen blowing causes copper loss at the slag/metal interface, which is schematically shown in Fig. 7. The shape of the slag/metal interface was observed by several researchers when the gas was strongly blown [6,7,8, 30]. In the current study, due to the limitation of the gas flow rate, it could not be injected strongly enough to push out the slag layer. Instead, injected oxygen just flows inside the slag phase, increasing the oxygen potential of the slag and possibly causing a reaction to remove impurities at a specific region of the slag/metal interface.
Certainly, the slag/metal interface is dynamically affected by the oxygen flow as shown in Fig. 8. Small metal droplets are dispersed at the interface in a form similar with the film type emulsification mentioned in the study of Chung and Cramb [22]. Some droplets appear to be created by tearing off the surface of the copper melt. Due to the oxygen potential of the melt, the overall shape of the interface is changed and becomes similar with that proposed by other researchers [20, 21]. It appears that changes of the surface and/or interfacial state and increasing instability of the interface by gas can trigger ejection of droplets.
Various researchers have investigated the effect of the oxygen potential on the surface tension of liquid copper, as shown in Fig. 9 [11,12,13,14]. The surface tension of copper melt was calculated using the Langmuir adsorption isotherm model as a function of the oxygen content of liquid copper as given in Eq. (3) [10, 21].
In Eq. (3), σ0 (mN m−1) is the surface tension of pure liquid copper which is assumed that some impurities do not influence significantly on surface tension. It can be calculated as a function of temperature [15]. \(K_{{\text{O}}}\) is the adsorption coefficient of oxygen at the metal surface, and \(a_{{\text{O}}}\) is the activity of oxygen in the liquid copper with reference to 1 wt% standard state. The \(K_{{\text{O}}}\) value for liquid copper is approx. 40 at 1400 °C [33]. \(\Gamma_{{{\text{sat}}}}\) is the excess surface quantity (moles m−2), which is the same as the number of oxygen atoms present at the copper surface, which can be derived by Eq. (4) [11]. This value was derived from literature data [12, 13]. Gallois and Lupis [11] also experimentally revealed that this variable is the saturation value of the oxygen monolayer at the liquid copper surface. Since the measured oxygen content is less than the oxygen solubility in pure liquid copper, it was assumed that all oxygen was dissolved into copper melt [16, 17]. The change in the surface tension of liquid copper, which is dominantly influenced by the oxygen contents, is shown in Fig. 10a. As mentioned above, as the oxygen in liquid copper increases, the surface tension rapidly decreases.
Not only the surface tension of the liquid copper, but also the surface tension of the slag is changed by reaction with copper melt and oxygen. The surface tension of the slag is expressed as Eq. (5) which is proposed by previous researchers [34,35,36,37].
where \(\sigma_{{\text{s}}}\) represents surface tension of slag, \(\sigma_{{{\text{p}},i}}\) means surface tension of pure component \(i\) which is temperature dependent and \(X_{i}\) is the mole fraction of each component. These values are summarized in Table 2, which were investigated by Mills and Keene [34], and Tanaka and Hara [35]. Generally, FeO acts to greatly increase the surface tension of slag, while Fe2O3 acts in the opposite tendency [34, 35]. Therefore, over-blown oxygen not only greatly lowers the surface tension of the copper melt, but also lowers the surface tension of the slag. The variation of the surface tension of slag as a function of Fe3+/Fe2+ ratio is shown in Fig. 10b. The change in surface tension of the slag does not appear to be significant compared to the change in the surface tension of the liquid copper, from which it can be suggested that the change in the surface tension of liquid copper will dominate in the slag/metal interfacial tension.
The interfacial tension of the liquid copper-slag system can be calculated by using the change of the surface tension of each phase calculated using Eqs. (3) and (5). The interfacial tension between liquid copper and slag can be a clear indicator of the interfacial state because it can represent physical state of interface. This can be calculated through the Girifalco-Good equation, which is shown in Eq. (6) [36].
where \(\sigma_{{{\text{s}}/{\text{m}}}}\) represents the interfacial tension between slag and metal and \(\varphi\) is interaction parameter as a function of FeO content as given in Eq. (7)[35,36,37]. Here, \(X_{{{\text{FeO}}}}\) is the mole fraction of FeO in the slag. The relationship between FeO content and interfacial tension is shown in Fig. 11a. As mentioned above, FeO is dominant factor that increases surface tension of slag and interaction parameter. Interfacial tension which is determined by surface tension of metal and slag has significant effect on copper loss as shown in Fig. 11b. From the point where most of the impurities are removed, the reaction step moves to the over-blowing regime. In the over-blowing regime, oxygen steadily reduces interfacial tension and thus slag/metal interface becomes more unstable. As the interfacial tension decreases lower than approx. 600 mN/m, copper loss significantly occurs. This result is qualitatively in good agreement with the experimental findings reported by other researchers for an iron/slag system [38]. In the case of ‘no slag’ experiment, the iron silicate (fayalite base) slag formed by oxidation of silicon and iron was solidified during oxygen blowing. Thus, although it has low interfacial tension, copper loss was rarely detected.
Droplet formation, which corresponds to spontaneous emulsification, is closely related to the instability of the slag/metal interface. In particular, the chemical effect of oxygen seems to be the dominant factor affecting the instability of interface [39]. Copper ejection into slag seems to be more activated due to the reduction of interfacial tension between slag and liquid copper. Decreased interfacial tension means slag and liquid copper can be intermixed physically at the interface. As a result, it causes instability of the interface and provides the driving force of copper ejection from the surface of copper melt.
On the contrary for the chemical effect of oxygen blowing, the physical effect of oxygen blowing or bubbling seems to be weak. This can be revealed through the Blowing number (\(N_{{\text{B}}}\)), which was suggested by Subagyo et al. [40], and copper contents in slag depending on Eq. (8) [38,39,40].
where \(\rho_{{{\text{gas}}}}\) (kg m−3) and \(v_{{{\text{gas}}}}\) (m s−1) means density and velocity of injected gas (O2 in the present study), \(\sigma_{{\text{m}}}\) (mN m−1) is surface tension of metal (copper in the present study) which can be obtained by Eq. (3), g is gravitational acceleration (m s−2) and \(\rho_{{\text{m}}}\) (kg m−3) is density of metal (Cu). The effect of gas blowing on droplet formation behavior can be predicted by using this parameter. In the present experimental conditions, the calculated Blowing number is very small, i.e., \(N_{{\text{B}}} \sim 10^{ - 4}\), from which the gas cannot cause ‘splashing’ of metal droplets [29, 38, 39]. It was reported that the \(N_{{\text{B}}}\) should be greater than unity to induce splashing of metal droplets or emulsification by only physical turbulence due to injected gas. In other words, it is difficult for droplet formation to occur by only the physical effect of gas blowing in the present experimental conditions. Therefore, it can be concluded that the dominant factor affecting the copper loss is the chemical behavior of oxygen rather than gas blowing itself.
Concerning the relationship between the surface tension of melt and amount of copper loss, some researchers have reported the opposite tendency [7, 9]..It was proposed that the amount of metal droplets increases in the condition where the surface tension of the melt is high. This seems to be the result of the large physical effect of the oxygen gas jet. A more detailed investigation is needed to determine whether the tendency of droplet formation can change depending on the dominance of the physical and/or chemical effects of oxygen.
Effect of Oxygen Potential on the Morphology of Copper in the Slag
The oxygen potential can affect the phase of the copper droplets through the slag. In the case where oxygen is not sufficiently supplied after the removal of impurities, droplets entrapped inside the slag remain in the form of metallic particles. The typical morphology of the slag for the case mentioned above is shown in Fig. 12a. In contrast, when oxygen is over-blown, the copper inside the slag is mainly found as copper oxide, as shown in Fig. 12b. In the case of bottom blowing, copper in the slag can also exist as oxide. When comparing the case where oxygen is supplied sufficiently to the case where it is not, this difference seems clear and there is evidence to suggest that the oxygen potential determines the copper phase in slag.
Alternatively, some droplets found near the slag/refractory interface were a mixed phase of metallic copper and oxide. This unique morphology is shown in Fig. 13. In most other cases, this oxide and metal mixed (dual) phase is not observed. However, droplets physically mixed with spinel are observed in all cases. The outside of the mixed phase is surrounded by oxide. This is evidence that the following reaction occurred at the slag/droplet interface.
For the above reaction to occur at the slag/metal interface, a sufficient oxygen potential must exist, which is achieved by oxygen over-blowing. In addition, from this, it can be seen that even in small scale systems, differences of the oxygen potential may occur in regions where oxygen is intensively injected and regions that are not. This finding supports the notion that oxygen potential gradients can occur. A similar mixed (metal + oxide) phase was also observed by other researchers for the oxidation of FeO in over-blown BOF slag [31, 32, 41]. The excessive supply of oxygen, enough to generate oxide, is not desirable in terms of metal recovery [1, 26,27,28]. As a result, oxygen blowing can influence not only the slag composition but also the metal droplet phase.
The overall reaction and phenomena due to oxygen blowing are summarized in Fig. 14. When oxygen blowing begins, oxygen is introduced into the molten copper to remove impurities in the melt. In this stage, oxygen is mostly used in the oxidative removal process, so the accumulated oxygen contents in the melt are negligible. After most of the impurity removal by oxidation is complete, oxygen is used to increase the oxygen potential of the slag/metal interface and thus interfacial tension is lowered. In the over-blowing regime, oxygen lowers the surface tension of the melt and slag by increasing the Fe3+/Fe2+ ratio in the slag, causing copper droplets to be generated by increasing instability at the slag/metal interface. The copper recovery can be performed through the subsequent process such as slag cleaning furnace treatment. However, because the slag cleaning process is also an energy intensive and time consuming, the occurrence of a serious droplet emulsification in the slag must be prevented.
Conclusions
The effects of oxygen injection on the surface tension of liquid metal, interfacial tension and the amount of droplet ejection into slag were investigated. The impurities such as iron and silicon in the copper melt were removed in a predictable order by oxidation determined through thermodynamic principle. During the impurity removal regime, injected oxygen does not accumulate in the melt. The oxygen injection method has a significant influence on the composition change of the slag because the oxygen potential of the slag is changed. After most of the impurities have been removed through oxidation, excessive oxygen blowing or bubbling greatly reduces the surface tension of the copper melt by increasing the oxygen potential. Because the interface is disturbed by the decreased interfacial tension between slag and metal, the increased instability of interface can induce ejection of droplets. Oxygen is involved in interfacial phenomenon such as change of interfacial tension and droplet ejection into liquid slag. However, the physical effect of gas blowing is negligible and the chemical effect of oxygen is the dominant factor affecting the copper loss into slag. In other words, the interfacial tension, which is excessively lowered by oxygen accumulation, can be a factor that causes the copper loss to be greater. Moreover, since the over-blowing of oxygen can cause a serious metal loss due to an interfacial disturbance, the oxygen blowing must be carefully controlled not only in the primary copper smelting but also in the processing of secondary resources.
References
Schlesinger M, King M, Sole K, Davenport WG (2002) Extractive metallurgy of copper. Elsevier Science, UK, pp 7–9
Ghodrat M, Samali B, Rhamdhani MA, Brooks G (2019) Thermodynamic-based exergy analysis of precious metal recovery out of waste printed circuit board through black copper smelting process. Energies 12:1–20. https://doi.org/10.3390/en12071313
Schimmel FA (1951) Electrolytic refining of copper from ammoniacal cuprous salt solutions. Ind Eng Chem 43:2943–2948. https://doi.org/10.1021/IE50504A075
Turkdogan ET (1996) Fundamentals of steelmaking. The Institute of Materials, UK, pp 209–220
Poggi D, Minto R, Davenport WG (1969) Mechanisms of metal entrapment in slags. JOM 21:40–45. https://doi.org/10.1007/BF03378796
Turner G, Jahanshahi S (1987) A model investigation on emulsification of metal droplets in the basic oxygen steelmaking processes. ISIJ Int 27:734–739. https://doi.org/10.2355/isijinternational1966.27.734
Han Z, Holappa L (2003) Mechanisms of iron entrapment into slag due to rising gas bubbles. ISIJ Int 43:292–297. https://doi.org/10.2355/isijinternational.43.292
Han Z, Holappa L (2003) Bubble bursting phenomenon in gas/metal/slag systems. Metall Mater Trans B 34B:525–532. https://doi.org/10.1007/s11663-003-0020-2
Hahn I, Neuschutz D (2002) Ejection of steel and slag droplets from gas stirred steel melts. Ironmak Steelmak 29:219–223. https://doi.org/10.1179/030192302225004115
Belton GR (1976) Langmuir adsorption, the Gibbs adsorption isotherm, and interfacial kinetics in liquid metal systems. Metall Mater Trans B 7B:35–42. https://doi.org/10.1007/BF02652817
Gallois B, Lupis CHP (1981) Effect of oxygen on the surface tension of liquid copper. Metall Mater Trans B 12B:549–557. https://doi.org/10.1007/BF02654326
Monma K, Suto H (1961) Effect of dissolved sulphur, oxygen, selenium and tellurium on the surface tension of liquid copper. Trans JIM 2:148–153. https://doi.org/10.2320/matertrans1960.2.148
Sakai T, Ip SW, Toguri JM (1997) Interfacial phenomena in the liquid copper-calcium ferrite slag system. Metall Mater Trans B 28:401–407. https://doi.org/10.1007/s11663-997-0105-4
Abbasi M, Lee J, Shin M, Kim Y, Kang Y (2014) Effect of oxygen adsorption on surface tension of liquid copper: experiments and thermodynamic models. Appl Surf Sci 313:116–122. https://doi.org/10.1016/j.apsusc.2014.05.153
Harrison DA, Yan D, Blairs S (1977) The surface tension of liquid copper. J Chem Thermodyn 9:1111–1119. https://doi.org/10.1016/0021-9614(77)90112-4
Sano K, Sakao H (1955) Physical chemistry research on copper making (4th report) Copper and CO-CO2-SO2 thermodynamic study to open for equilibrium test with mixed gas. J Jpn Inst Met 19:655–659. https://doi.org/10.2320/jinstmet1952.19.11_655
Oberg KE, Friedman LM, Boorstein WM, Rapp RA (1973) The diffusivity and solubility of oxygen in liquid copper and liquid silver from electrochemical measurements. Metall Trans 4:61–67. https://doi.org/10.1007/BF02649605
Good RJ (1992) Contact angle, wetting, and adhesion: a critical review. J Adhesion Sci Technol 6:1269–1302. https://doi.org/10.1163/156856192x00629
Gopal ES (1963) Emulsion science. Academic Press, USA, p 1
Bainbridge GS, Sawistowski H (1964) Surface tension effects in sieve plate distillation columns. Chem Eng Sci 19:992–993. https://doi.org/10.1016/0009-2509(64)85108-3
Cramb AW, Jimbo I (1989) Calculation of the interfacial properties of liquid steel–slag systems. Steel Res 60:157–165. https://doi.org/10.1002/srin.198900893
Chung Y, Cramb AW (2000) Dynamic and equilibrium interfacial phenomena in liquid steel-slag systems. Metall Mater Trans B 31B:957–971. https://doi.org/10.1007/s11663-000-0072-5
Ip SW, Toguri JM (1992) Entrainment behavior of copper and copper matte in copper smelting operations. Metall Mater Trans B 23B:303–311. https://doi.org/10.1007/BF02656285
Sigworth GK, Elliott JF (1974) The thermodynamics of dilute liquid copper alloys. Can Metall Q 13:455–461. https://doi.org/10.1179/CMQ.1974.13.3.455
Turkdogan ET (1980) Physical chemistry of high temperature technology. Academic Press, USA, pp 11–20
Mazumdar D (2015) A first course in iron and steelmaking. Universities Press, India, pp 198–210
Meyer HW, Porter WF, Smith GC, Szekely J (1968) Slag-metal emulsions and their importance in BOF steelmaking. J Met 20:35–42. https://doi.org/10.1007/BF03378731
Sano N, Lu WK, Riboud PV, Maeda M (1997) Advanced physical chemistry for process metallurgy, USA, pp 347–379.
Deo B, Boom R (1993) Fundamentals of steelmaking metallurgy. Prentice Hall International, UK, pp 79, 176–177.
Naito KI, Kaizawa A, Kitagawa I, Sasaki N, Asahara N, Ogawa Y, Inomoto T, Matsuo M (2013) Behavior of top-blowing lance jets in BOF. Nippon Steel Tech Rep 104:33–41
Bellemans I, De Wilde E, Blanpain B, Moelans N, Verbeken K (2017) Investigation of origin of attached Cu-Ag droplets to solid particles during high-temperature slag/copper/spinel interactions. Metall Mater Trans B 48B:3058–3073. https://doi.org/10.1007/s11663-017-1088-4
De Wilde E, Bellemans I, Campforts M, Guo M, Blanpain B, Moelans N, Verbeken K (2016) Investigation of high-temperature slag/copper/spinel interactions. Metall Mater Trans B 47B:3421–3434. https://doi.org/10.1007/s11663-016-0805-8
Morita Z, Kasama A (1980) Effect of a slight amount of dissolved oxygen on the surface tension of liquid copper. Trans Jpn Inst Met 21:522–530. https://doi.org/10.2320/matertrans1960.21.522
Mills KC, Keene BJ (1987) Physical properties of BOS slags. Int Mater Rev 32:1–120. https://doi.org/10.1179/095066087790150296
Tanaka T, Hara S (1999) Application of thermodynamic databases to evaluation of interfacial tension between liquid steels and molten slags. Z Metall 90:348–354
Girifalco LA, Good RJ (1957) A theory for the estimation of surface and interfacial energies. I. derivation and application to interfacial tension. J Phys Chem 61:904–909
Shin M, Cho S, Lee J, Park JH (2010) In-situ observation of dynamic interfacial phenomena between CaO-(SiO2 or Al2O3) slag and Fe-11%Cr alloy near the MgO-6%C refractory at 1823 K. Met Mater Int 16:495–499. https://doi.org/10.1007/s12540-010-0623-5
Dogan N, Brooks GA, Rhamdhani MA (2009) Analysis of droplet generation in oxygen steelmaking. ISIJ Int 49:24–28. https://doi.org/10.2355/isijinternational.49.24
Scheller PR, Lee J, Tanaka T (2014) Treatise on process metallurgy, vol 2. Elsevier, UK, pp 111–118
Subagyo, Brooks GA, ColeyIrons KSGA (2003) Generation of droplets in slag-metal emulsions through top gas blowing. ISIJ Int 43:983–989. https://doi.org/10.2355/isijinternational.43.983
Jalkanen H, Vehvilainen J, Poijarvi J (2003) Copper in solidified copper smelter slags. Scand J Metall 32:65–70. https://doi.org/10.1034/j.1600-0692.2003.00536.x
Acknowledgements
This research was partly supported by the Korea Institute of Energy Technology Evaluation and Planning (KETEP, Grant Number 20217510100080), and also partly supported by the Korea Institute for Advancement of Technology (KIAT, Grant Number P0002019) for the Competency Development Program for Industry Specialists, funded by the Ministry of Trade, Industry & Energy (MOTIE), Korea.
Author information
Authors and Affiliations
Corresponding author
Additional information
The contributing editor for this article was Il Sohn.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Park, J., Lee, J. & Park, J.H. Effect of Oxygen Blowing on Copper Droplet Formation and Emulsification Phenomena in the Converting Process. J. Sustain. Metall. 7, 831–847 (2021). https://doi.org/10.1007/s40831-021-00421-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-021-00421-8