Abstract
Background
The prognosis of patients with epidermal growth factor receptor (EGFR) mutation-positive lung cancer has improved significantly since the advent of EGFR tyrosine kinase inhibitors (EGFR-TKIs). We aimed to investigate the relationship between patient characteristics, EGFR genotype, therapeutic agents, and the prognosis of the patients with EGFR mutation-positive lung cancer.
Methods
This retrospective cohort study analyzed 198 Japanese patients with unresectable EGFR mutation-positive lung cancer who were treated with EGFR-TKIs at Toho University Sakura Medical Center from April 2006 to December 2021. Factors associated with overall survival (OS) were analyzed using Cox proportional hazards analysis.
Results
Patients who received osimertinib had a significantly longer OS than did those not receiving it (median OS, 36.2 versus 20.7 months; p < 0.001).There were significant differences in OS between patients with EGFR mutation who received osimertinib as first-line treatment, T790M-positive patients who received osimertinib as second- or later-line treatment, and those who did not receive it (median OS, 28.2 versus 40.2 versus 20.7 months; p = 0.003). However, in T790M-negative patients, no significant difference in OS was noted between those who did and did not receive osimertinib as post-treatment (median OS, 28.0 versus 40.0 months; p = 0.619). Multivariate Cox proportional hazards analysis showed that osimertinib treatment was associated with longer OS (hazard ratio, 0.480; 95% confidence interval, 0.326–0.707; p < 0.001).
Conclusion
The patients who were T790M-positive in the first-line treatment with first or second-generation EGFR-TKIs and were given osimertinib as the second or later line treatment had a better prognosis than the patients who were T790M-negative in the first-line treatment with first or second-generation EGFR-TKIs and could not receive osimertinib.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This 15-year-long follow-up study found that overall survival was significantly longer in patients who received osimertinib than in those not receiving osimertinib. |
Multivariate Cox proportional hazards analysis revealed that osimertinib treatment was associated with longer overall survival. |
Treatment of Japanese patients with EGFR mutation-positive lung cancer with osimertinib of any line of treatment period may prolong survival. |
1 Introduction
Around 40% of Japanese patients with non-small-cell lung cancer (NSCLC) have epidermal growth factor receptor (EGFR) mutation-positive disease [1]. Moreover, a previous study found that 90% of Japanese EGFR mutation-positive lung cancers have either exon19 deletion mutation or L858R, and that EGFR tyrosine kinase inhibitors (EGFR-TKIs) were able to promote significantly longer progression-free survival (PFS) and overall survival (OS) than chemotherapy among such patients [2]. As such, first-generation EGFR-TKIs gefitinib and erlotinib, second-generation EGFR-TKIs afatinib and dacomitinib, and the third-generation EGFR-TKI osimertinib have been endorsed for insurance coverage in Japan as of May 2024. All such drugs have outperformed platinum-containing chemotherapy in global phase III studies [3,4,5,6,7,8,9,10,11]. Furthermore, the FLAURA trial demonstrated that osimertinib promoted significantly longer PFS and OS than gefitinib and erlotinib [12, 13], making it the most recommended first-line treatment for EGFR mutation-positive lung cancer by various guidelines. However, a subgroup analysis of the FLAURA study found that osimertinib was not superior to gefitinib and erlotinib in terms of OS in the L858R-positive group or the Asian population. Hence, the effect of osimertinib on prolonging OS in the Asian population remains unknown.
Immune checkpoint inhibitors (ICIs) work against tumors by inhibiting programmed cell death-1 (PD-1), programmed cell death-ligand 1 (PD-L1), and cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), which prevent immune evasion of tumor cells. Several international phase III trials have recently shown that ICIs promote longer PFS and OS than platinum-containing combination therapy in lung cancer without genetic mutations [14,15,16,17,18,19,20,21,22,23,24,25,26]. However, the impact of ICIs on the OS of patients with EGFR mutation-positive lung cancer remains unknown.
Few studies have investigated the relationship between patient background, therapeutic agents, and the prognosis of EGFR mutation-positive lung cancer in the real world [27,28,29]. Furthermore, all such studies were conducted before osimertinib had been recommended as a treatment for EGFR mutation-positive lung cancer. Unfortunately, only a few reports on OS have been available since the introduction of osimertinib. Therefore, this single-center, retrospective cohort study was designed to investigate the real-world relationship between patient background, EGFR mutation type, osimertinib treatment, and the prognosis of EGFR mutation-positive lung cancer in the Japanese population.
2 Patients and Methods
2.1 Patients
This retrospective study included patients diagnosed with advanced EGFR mutation-positive lung cancer who were ineligible for curative radiation therapy or surgery and were treated with EGFR-TKIs at Toho University Medical Center Sakura Hospital between April 2006 and December 2021. Patients who satisfied the following criteria were excluded: (1) those who underwent surgery or radiotherapy for the treatment of lung cancer, (2) those whose treatment start date was unknown, (3) those whose follow-up was incomplete, or (4) those who had previously participated in clinical trials. This study was conducted following the principles of the Declaration of Helsinki and was approved by the ethics committee of Toho University Sakura Hospital (approval no.: S22012, approval date: 28 September 2022). This retrospective cohort study used an opt-out method for consent. Consent for the use of personal information was obtained by posting the study and the hospital’s personal information policy on the website.
2.2 Data Collection
Clinical data for each patient were collected using the Toho University Medical Center Sakura Hospital database. The following information was collected from all study participants: age at diagnosis, sex, smoking history, histological diagnosis, clinical stage, presence of brain metastases, Eastern Cooperative Oncology Group (ECOG) performance status (PS), and treatment information (treatment regimen, treatment start date, side effects, last follow-up date, and date of death). Tumor stage was determined using the eighth edition of the American Joint Committee on Cancer. Tumors were diagnosed using specimens obtained through bronchoscopic biopsy, pleural fluid, and biopsy specimens from metastatic lesions. EGFR mutations were detected using the peptide nucleic acid-locked nucleic acid polymerase chain reaction clamp method, Oncomine Dx Target Test Multi-CDx System (Ion Torrent PGM Dx Sequencer; Thermo Fisher Scientific), and the cobas EGFR mutation test version 2 (Roche Molecular Diagnostics, Inc, CA, USA) using tissue samples used for diagnosis or newly obtained samples obtained via liquid biopsy of pleural fluid or plasma. The Oncomine Dx Target Test MultiCDx System and the cobas EGFR Mutation Test version 2 were used to identify T790M mutations.
2.3 Statistical Analysis
The primary outcome was OS, which was defined in this study as the duration from the start of primary treatment to the date of all-cause mortality or last follow-up. The OS was determined using a data cutoff date of 31 March 2022. Data for patients who survived until the cutoff date were censored using the last recorded date of the patient. In this study, OS was used to evaluate the efficacy of EGFR-TKIs, whereas time to treatment failure, defined as the duration from ICI treatment onset to the end of treatment for any factor, was used to evaluate the efficacy of ICIs. Multivariate Cox proportional hazards analysis was used to determine prognostic factors. The Kaplan–Meier method was used to estimate OS, and differences between subgroups were compared using the log-rank test. A p < 0.05 was considered statistically significant. IBM SPSS Statistics version 25.0 (IBM CO., Armonk, NY, USA) was used for statistical analysis. Noncategorical and categorical data were expressed as mean ± standard deviation (SD) and percentages, respectively.
3 Results
3.1 Patient Characteristics and Treatment
From April 2006 to December 2021, 255 patients who visited Toho University Medical Center Sakura Hospital were diagnosed with EGFR mutation-positive lung cancer, and 57 patients were excluded according to the criteria [unknown primary treatment start date (n = 5), not treated at our hospital (n = 5), began treatment before 2006 (n = 1), undergone surgery or radiotherapy for lung cancer treatment (n = 39), incomplete follow-up (n = 6), and started treatment for other cancers after starting lung cancer treatment (n = 1)]. Ultimately, 198 patients were included for analysis (Fig. 1).
The average age of the enrolled patients was 70.3 ± 9.8 years, with 85 (42.9%) and 113 (57.1%) males and females, respectively. Histologically, all patients had adenocarcinoma. There were 152 (76.7%) and 46 (23.3%) patients with a PS of 0–1 and 2 or higher, respectively, whereas 184 (92.9%) patients had stage 4 disease at diagnosis. Exon 19 deletion, L858R, and minor mutations were observed in 89 (44.9%), 93 (47.0%), and 16 (8.1%) patients, respectively. Among all included patients, 103 (52.0%) received cytotoxic chemotherapy of any treatment line, 158 (79.8%) received first or second-generation EGFR-TKIs, and 93 (47.0%) received osimertinib. ICIs were administered to 29 (14.7%) patients. The baseline clinical characteristics of the patients and the anticancer drugs they received throughout their treatment are summarized in Table 1.
3.2 T790M Test
Among the patients who received first- and second-generation EGFR-TKIs and experienced disease progression, 54 (35.5%) underwent T790M molecular testing. Tissue samples, pleural fluid samples, and plasma samples were collected from 22 (40.7%), 12 (22.2%), and 20 (37.0%) patients, respectively. Accordingly, 25 (46.3%) patients tested positive for T790M mutation, among whom 15 (60.0%) had exon19 deletion and 10 (40.0%) had L858R.
3.3 Overall Survival
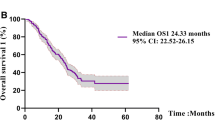
At the end of the follow-up period, 162 (81.8%) of the 198 patients died, with a median follow-up duration of 42.1 months and a median OS of 24.3 months. Patient characteristics included in the Cox regression model were “age,” “sex,” “ECOG-PS,” “stage,” “brain metastases,” and “EGFR mutation type.” Anticancer drugs administered throughout the treatment period were divided into four categories, namely cytotoxic chemotherapy, first- and second-generation EGFR-TKIs, osimertinib, and ICI, all of which were included as a factor in the Cox regression model for multivariate analysis. The first- and second-generation EGFR-TKIs administered were gefitinib, erlotinib, and afatinib. Multivariate Cox proportional hazards analysis of survival showed that exon 19 deletion [hazard ratio (HR), 0.358; 95% confidence interval (CI), 0.193–0.665; p = 0.001], L858R (HR, 0.485; 95% CI, 0.263–0.895; p = 0.021), and a history of osimertinib treatment (HR, 0.480; 95% CI, 0.326–0.707; p < 0.001) were factors for a favorable prognostic. Meanwhile, male sex (HR, 1.394; 95% CI, 1.007–1.929; p = 0.045), ECOG-PS (HR, 1.825; 95% CI, 1.532–2.174; p < 0.001), and brain metastasis (HR, 1.587; 95% CI, 1.119–2.252; p = 0.010) were factors associated with poor prognosis (Table 2).
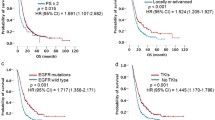
Differences in survival curves between patients with and without a history of osimertinib treatment in all patients and subgroups with exon 19 deletion, L858R, and minor mutation were compared using log-rank test (Fig. 2A–D).
Patients treated with osimertinib had significantly longer OS than those who did not receive the same treatment (median OS, 36.2 versus 20.7 months; p < 0.001; Fig. 2A). Furthermore, the osimertinib-treated group had significantly longer OS than the osimertinib-naive group even in the exon19 deletion (median OS, 39.2 versus 22.9 months; p = 0.028; Fig. 2B) L858R (median OS, 33.9 versus 20.7 months; p = 0.001; Fig. 2C), and minor mutation (median OS, 23.2 versus 9.6 months; p = 0.047; Fig. 2D) subgroups.
3.4 Comparison of OS According to Order of Osimertinib Administration
Patients who had previously received osimertinib were divided into three groups: those who received osimertinib as first-line treatment, those who received it as second- or later-line treatment, and those who did not receive it. Differences in survival were then assessed using the log-rank test.
Among the 48 patients who received osimertinib as second- or later-line treatment, 25 (52.1%) were positive for the T790M, whereas 23 (47.9%) were negative or undetectable. Figure 3 depicts the Kaplan–Meier curves for OS in those who received osimertinib as first-line treatment, those who received osimertinib as second- or later-line treatment, and those who did not receive it.
Kaplan–Meier analysis of OS in patients with EGFR mutation who received osimertinib as first-line treatment, those who received osimertinib as second- or later-line treatment, and those who did not receive it. OS, overall survival; Osi, osimertinib. A: Comparison between patients who received osimertinib as first-line treatment, those who received osimertinib as second- or later-line treatment with or without T790M, and those who did not receive osimertinib; B: comparison between patients who received osimertinib as first-line treatment, T790M mutation-positive patients who received osimertinib as second- or later-line treatment, and those who did not receive it
Significant differences in OS were observed between patients with EGFR mutation who received osimertinib as first-line treatment, those who received osimertinib as second- or later-line treatment with or without T790M, and those who did not receive osimertinib (median OS, 28.2 versus 39.2 versus 20.7 months; p = 0.001; Fig. 3A). Figure 3B presents the Kaplan–Meier curves for OS in those who received osimertinib as first-line treatment, those with T790M who received osimertinib as second-line treatment, and those who did not receive it. The log-rank test revealed significant differences in OS between patients with EGFR mutation who received osimertinib as first-line treatment, T790M -positive patients who received osimertinib as second- or later-line treatment, and those who did not receive it (median OS, 28.2 versus 40.2 versus 20.7 months; p = 0.003; Fig. 3B).
3.5 Comparison of OS in T790M-Negative Cases Who did and did not Receive Osimertinib
A total of 25 patients had negative T790M test results after treatment with first- or second-generation EGFR-TKIs. Moreover, 10 (40.0%) and 11 (44.0%) patients had exon 19 deletion and L858, respectively. Patient characteristics are summarized in Table 3. Notably, seven (28.0%) patients received osimertinib as post-treatment, whereas only one (14.3%) patient was determined to have partial response. Tumor treatment response was determined 3 months after treatment imitation and was based on the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1.
The OS of T790M-negative patients who received osimertinib as posttreatment and those who did not receive osimertinib were compared using the log-rank test (Fig. 4).
The log-rank test and Kaplan–Meier curves showed no significant difference in OS between T790M-negative patients treated with osimertinib and those who did not receive it (median OS, 28.0 versus 40.0 months; p = 0.619; Fig. 4).
3.6 Comparison of Time to Treatment Failure Between Patients who Received ICI and Those Who Received Chemotherapy + ICI
A total of 29 (14.6%) patients received immunotherapy or immunochemotherapy throughout the treatment course. Among them, 22 (75.9%) received only ICI, whereas 7 (24.1%) received immunochemotherapy.
Differences in time to treatment failure between patients who previously received ICI monotherapy and those who received immunochemotherapy are presented in Fig. 5.
Similarly, no significant difference in time to treatment failure was observed between patients who received ICI alone and those who received ICI plus chemotherapy (median time to treatment failure, 2.1 versus 4.5 months; p = 0.085; Fig. 5).
4 Safety
Table 4 shows the incidence of grade 3 or higher adverse events (AEs) that occurred during treatment with each TKI according to the Common Terminology Criteria for Adverse Events, as well as the incidence of AEs that resulted in drug discontinuation and death. Notably, grade 3 or higher AEs occurred in 14/106 (13.2%), 13/81 (16.0%), 6/17 (35.3%), and 8/94 (8.5%) cases who received gefitinib, erlotinib, afatinib, and osimertinib, respectively. Moreover, 16/106 (15.1%), 14/81 (17.3%), 8/17 (47.1%), and 12/94 (12.8%) cases who received gefitinib, erlotinib, afatinib, and osimertinib developed AEs that resulted in drug discontinuation, respectively. One case developed a fatal AE (i.e., interstitial lung disease) during treatment with gefitinib. The most common grade 3 or higher AEs or those resulting in drug discontinuation were hepatotoxicity with gefitinib (5.7%/9.4%); skin rash with erlotinib (8.6%/11.1%); skin rash (16.7%/11.1%), diarrhea (11.1%/22.2%), and anorexia (11.1%/16.7%) with afatinib; and interstitial lung disease (4.3%/6.4%) with osimertinib.
5 Discussion
The current study found that a history of osimertinib treatment was a positive prognostic factor for patients with EGFR mutation-positive lung cancer considering that it extended OS regardless of administration timing or EGFR mutation type. Among patients with EGFR mutation-positive lung cancer, ICIs did not improve OS following EGFR-TKI use.
Our results showed that patients with EGFR mutation-positive lung cancer who had previously used osimertinib had a better prognosis than those who had not used osimertinib. In fact, the global phase III FLAURA trial revealed osimertinib promoted a significantly longer OS than first-generation EGFR-TKIs (gefitinib and erlotinib) among treatment-naive patients with EGFR-positive lung cancer (38.6 versus 31.8 months) [9]. In contrast, a subgroup analysis of the FLAURA trial found that osimertinib promoted a shorter OS than did first-generation EGFR-TKIs among Asians and patients with the L858R. In a network meta-analysis of 13 randomized controlled trials (RCTs), Holleman et al. found that osimertinib as first-line therapy for EGFR mutation-positive lung cancer promoted better PFS and OS than did gefitinib, erlotinib, afatinib, and dacomitinib [30]. In a retrospective cohort study of Japanese patients, Uryu et al. discovered that osimertinib as first-line treatment may promote a better prognosis than would first-generation EGFR-TKIs among patients with EGFR mutation-positive lung cancer [31]. These findings are similar to those presented in our study and support the notion that a history of osimertinib treatment is a favorable prognostic factor for Japanese patients with EGFR mutation-positive lung cancer. Unlike previous studies, our study analyzed the administration history of each drug as a prognostic factor, which differs from patient to patient, thereby providing novel data. In real-world studies, osimertinib has not been consistently used as first-line treatment owing to switching from EGFR-TKIs or late-line re-administration owing to side effects. Until 2016, osimertinib had not been covered by insurance and could not be used in clinical practice. In 2016, Japanese guidelines recommended the use of osimertinib as second- or later-line therapy for patients with T790M-positive lung cancer previously who received EGFR-TKIs, with its use being subsequently extended to first-line therapy by 2018. Whether osimertinib was administered and the order of its administration were determined based on the Japanese guidelines and the discretion of the attending physician. Therefore, we believe that the findings presented herein, particularly regarding the impact of previous use of osimertinib on OS, will be useful. Furthermore, a subgroup analysis of our data revealed that patients with a history of osimertinib use had significantly prolonged OS, regardless of EGFR mutation type. We found a tendency toward better OS in the group that received later-line osimertinib, a finding consistent with those presented in previous studies [32,33,34,35,36]. In the sequential osimertinib group, T790M mutations were found in 71.4% (15/21) and 43.5% (10/23) of the patients with exon 19 deletion and L858R, respectively. Although the T790M test was difficult to perform in some cases, our data are consistent with those presented in previous reports showing that patients with exon 19 deletion mutation were likely to have the T790M mutation [37], increasing the desirability of osimertinib treatment in either treatment line in patients with exon 19 deletion. A phase II study evaluating the efficacy of osimertinib in T790M-negative, EGFR mutation-positive patients who exhibited progression after treatment with first- and second-generation EGFR-TKIs in Japan reported that osimertinib promoted moderate tumor activity [38]. In contrast, our study showed that post-treatment with osimertinib did not prolong OS in T790M-negative cases. Thus, a history of osimertinib treatment may be a positive prognostic factor for Japanese patients with EGFR-positive lung cancer. However, those negative for T790M may not necessarily experience improved prognosis.
Our results showed that ICI administration after EGFR-TKI did not affect the survival of patients with EGFR mutation-positive lung cancer. Subgroup analysis and meta-analysis of multiple international clinical trials revealed that ICI monotherapy did not improve PFS or OS in EGFR mutation-positive lung cancer [39,40,41,42,43]. In a phase II study investigating the efficacy of pembrolizumab monotherapy in patients with EGFR mutation-positive lung cancer showed a response rate of 0% [44]. In the current study, up to 22 of the 29 patients (75.9%) received ICI monotherapy. Our findings showed that ICI treatment did not affect the survival of patients with EGFR mutation-positive lung cancer, echoing the results reported in previous clinical trials [39,40,41,42,43,44]. However, considering that several RCTs comparing ICI plus platinum combination chemotherapy to platinum combination chemotherapy excluded patients with EGFR-positive lung cancer, the efficacy of ICI plus platinum combination chemotherapy in patients with EGFR-positive lung cancer remains unknown [17, 18, 21, 22, 45]. The IMPOWER150 trial, one of the few RCTs that accepted patients with EGFR-positive lung cancer as participants, investigated the synergistic effect of adding atezolizumab to the regimen containing carboplatin/paclitaxel plus bevacizumab, an angiogenesis inhibitor. Notably, a subgroup analysis of the IMPOWER150 trial found that ICI improved OS in patients with EGFR mutation-positive lung cancer (HR, 0.80; 95% CI, 0.65–0.98). A real-world multicenter retrospective study by Hu et al. found that after treatment with EGFR-TKIs, ICI plus platinum combination chemotherapy outperformed ICI monotherapy in terms of PFS and OS [46]. Moreover, a meta-analysis of RCTs by Qian et al. found that ICI-based combination therapy was superior to chemotherapy in PFS in patients with EGFR mutation-positive lung cancer after EGFR-TKI treatment. However, they showed that regardless of whether ICI monotherapy or combination therapy was administered, no significant difference in OS was observed between immunotherapy and chemotherapy [47]. Our findings revealed that a history of ICI administration was not associated with OS in patients with EGFR mutation-positive lung cancer and that no significant difference in median time to treatment failure existed between ICI plus platinum combination chemotherapy and ICI monotherapy, supporting the findings presented in the meta-analysis by Qian et al.
The current study determined the frequency of grade 3 or higher AEs occurring during treatment with each EGFR-TKI, those that required drug discontinuation, and those that resulted in death. Accordingly, grade 3 or higher AEs were reported in 13.2%, 16.0%, 35.3%, and 8.5% of cases who received gefitinib, erlotinib, afatinib, and osimertinib, respectively. AEs resulting in drug discontinuation occurred in 15.1%, 17.3%, 47.1%, and 12.8% of cases who received gefitinib, erlotinib, afatinib, and osimertinib, respectively. Grade 3 or higher AEs and those leading to discontinuation were less common with osimertinib and more common with afatinib. The most common AEs occurring throughout the treatment period with each EGFR-TKI were liver disorder with gefitinib; skin rash with erlotinib; skin rash, diarrhea, and loss of appetite with afatinib; and interstitial lung disease (ILD) with osimertinib. These AEs were generally similar to those reported in the global clinical trials for each EGFR-TKI. However, the overall incidence of AEs in the current study was lower than those presented in the clinical trials [3,4,5,6,7,8,9,10,11,12,13]. Given the retrospective nature of our analysis based on real-world data, our results may differ from those presented in previous prospective studies owing to the presence of some AEs deemed tolerable and did not require discontinuation even when they satisfied the criteria for grade 3 AEs, as well as some AEs determined to be caused by drugs other than EGFR-TKIs or treatment of comorbidities. ILD (6.4%) was more common among AEs that caused discontinuation during osimertinib treatment than among other AEs, including skin rash (1.1%), diarrhea (2.1%), anorexia (2.1%), and liver injury (1.1%). However, a comparison between grade 3 or higher AEs with osimertinib and those occurring owing to other EGFR-TKIs, namely, gefitinib and erlotinib, revealed no significant difference in the incidence of ILDs during treatment. In the Japanese subset of the FLAURA study, the incidence of ILD in the osimertinib group was reported to be 12.8%, which was higher than that in the gefitinib group. Nonetheless, the incidence of grade 3 or higher ILD was similar in both groups [48], which was consistent with our findings. Although osimertinib has been associated with fewer AEs leading to treatment discontinuation than other EGFR-TKIs, it has been known to promote high incidence rates of ILD in the Japanese population and should warrant caution.
The current study has several limitations worth noting. Given the single-center, retrospective design of this study, selection bias could have been present. Among the patients who had a history of osimertinib treatment, around 52% used osimertinib after treatment with first- or second-generation EGFR-TKIs. Thus, an “immortal time bias” was present until T790M positivity. Furthermore, our sample size was quite small, and our results may differ from analyses with larger sample sizes. The follow-up period varied by case because the study determined the data cutoff period.
6 Conclusions
The patients who were T790M-positive in the first-line treatment with first or second-generation EGFR-TKIs and were given osimertinib as the second or later line treatment had a better prognosis than the group of patients who were T790M-negative in the first-line treatment with first or second-generation EGFR-TKIs and could not receive osimertinib.
References
Saito M, Shiraishi K, Kunitoh H, Takenoshita S, Yokota J, Kohno T. Gene aberrations for precision medicine against lung adenocarcinoma. Cancer Sci. 2016;107(6):713–20. https://doi.org/10.1111/cas.12941.
Sekine I, Shintani Y, Shukuya T, et al. A Japanese lung cancer registry study on demographics and treatment modalities in medically treated patients. Cancer Sci. 2020;111(5):1685–91. https://doi.org/10.1111/cas.14368.
Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–57. https://doi.org/10.1056/NEJMoa0810699.
Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–8. https://doi.org/10.1056/NEJMoa0909530.
Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121–8. https://doi.org/10.1016/S1470-2045(09)70364-X.
Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–42. https://doi.org/10.1016/S1470-2045(11)70184-X.
Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–46. https://doi.org/10.1016/S1470-2045(11)70393-X.
Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327–34.
Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15(2):213–22. https://doi.org/10.1016/S1470-2045(13)70604-1.
Wu YL, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18(11):1454–66. https://doi.org/10.1016/S1470-2045(17)30608-3.
Mok TS, Wu Y-L, Ahn M-J, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376(7):629–40. https://doi.org/10.1056/NEJMoa1612674.
Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113–25. https://doi.org/10.1056/NEJMoa1713137.
Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382(1):41–50. https://doi.org/10.1056/NEJMoa1913662.
Garon EB, Hellmann MD, Rizvi NA, et al. Five-year overall survival for patients with advanced non-small-cell lung cancer treated with pembrolizumab: results from the phase I KEYNOTE-001 study. J Clin Oncol. 2019;37(28):2518–27. https://doi.org/10.1200/JCO.19.00934.
Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–33. https://doi.org/10.1056/NEJMoa1606774.
Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–30. https://doi.org/10.1016/S0140-6736(18)32409-7.
Garassino MC, Gadgeel S, Speranza G, et al. Pembrolizumab plus pemetrexed and platinum in nonsquamous non-small-cell lung cancer: 5-year outcomes from the phase 3 KEYNOTE-189 study. J Clin Oncol. 2023;41(11):1992–8. https://doi.org/10.1200/JCO.22.01989.
Novello S, Kowalski DM, Luft A, et al. Pembrolizumab plus chemotherapy in squamous non-small-cell lung cancer: 5-year update of the phase III KEYNOTE-407 study. J Clin Oncol. 2023;41(11):1999–2006. https://doi.org/10.1200/JCO.22.01990.
Herbst RS, Giaccone G, de Marinis F, et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N Engl J Med. 2020;383(14):1328–39. https://doi.org/10.1056/NEJMoa1917346.
Socinski MA, Nishio M, Jotte RM, et al. IMpower150 final overall survival analyses for atezolizumab plus bevacizumab and chemotherapy in first-line metastatic nonsquamous NSCLC. J Thorac Oncol. 2021;16(11):1909–24. https://doi.org/10.1016/j.jtho.2021.07.009.
West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(7):924–37. https://doi.org/10.1016/S1470-2045(19)30167-6.
Horn L, Mansfield AS, Szczęsna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220–9. https://doi.org/10.1056/NEJMoa1809064.
Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019;381(21):2020–31. https://doi.org/10.1056/NEJMoa1910231.
Brahmer JR, Lee JS, Ciuleanu TE, et al. Five-year survival outcomes with nivolumab plus ipilimumab versus chemotherapy as first-line treatment for metastatic non-small-cell lung cancer in CheckMate 227. J Clin Oncol. 2023;41(6):1200–12. https://doi.org/10.1200/JCO.22.01503.
Paz-Ares L, Ciuleanu TE, Cobo M, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial [published correction appears in Lancet Oncol. 2021 Mar;22(3): e92]. Lancet Oncol. 2021;22(2):198–211. https://doi.org/10.1016/S1470-2045(20)30641-0.
Borghaei H, Gettinger S, Vokes EE, et al. Five-year outcomes from the randomized, phase III trials CheckMate 017 and 057: Nivolumab versus docetaxel in previously treated non-small-cell lung cancer [published correction appears in J Clin Oncol. 2021;39(10):1190]. J Clin Oncol. 2021;39(7):723–33. https://doi.org/10.1200/JCO.20.01605.
Zhou X, Cai L, Liu J, et al. Analyzing EGFR mutations and their association with clinicopathological characteristics and prognosis of patients with lung adenocarcinoma. Oncol Lett. 2018;16(1):362–70. https://doi.org/10.3892/ol.2018.8681.
Inoue A, Yoshida K, Morita S, et al. Characteristics and overall survival of EGFR mutation-positive non-small cell lung cancer treated with EGFR tyrosine kinase inhibitors: a retrospective analysis for 1660 Japanese patients. Jpn J Clin Oncol. 2016;46(5):462–7. https://doi.org/10.1093/jjco/hyw014.
Chang HC, Wang CC, Tseng CC, et al. Do patient characteristics affect EGFR tyrosine kinase inhibitor treatment outcomes? A network meta-analysis of real-world survival outcomes of East Asian patients with advanced non-small cell lung cancer treated with first-line EGFR-TKIs. Thorac Cancer. 2023;14(32):3208–16. https://doi.org/10.1111/1759-7714.15111.
Holleman MS, van Tinteren H, Groen HJ, Al MJ, Uyl-de Groot CA. First-line tyrosine kinase inhibitors in EGFR mutation-positive non-small-cell lung cancer: a network meta-analysis. Onco Targets Ther. 2019;12:1413–21. https://doi.org/10.2147/OTT.S189438.
Uryu K, Imamura Y, Shimoyama R, et al. Stepwise prolongation of overall survival from first to third generation EGFR-TKIs for EGFR mutation-positive non-small-cell lung cancer: the Tokushukai REAl-world Data project (TREAD 01). Jpn J Clin Oncol. 2024. https://doi.org/10.1093/jjco/hyad162.
Hochmair MJ, Morabito A, Hao D, et al. Sequential afatinib and osimertinib in patients with EGFR mutation-positive non-small-cell lung cancer: final analysis of the GioTag study. Future Oncol. 2020;16(34):2799–808. https://doi.org/10.2217/fon-2020-0740.
Papadimitrakopoulou VA, Mok TS, Han JY, et al. Osimertinib versus platinum-pemetrexed for patients with EGFR T790M advanced NSCLC and progression on a prior EGFR-tyrosine kinase inhibitor: AURA3 overall survival analysis. Ann Oncol. 2020;31(11):1536–44. https://doi.org/10.1016/j.annonc.2020.08.2100.
Dal Maso A, Lorenzi M, Ferro A, et al. Real-world data on treatment outcomes in EGFR-mutant non-small-cell lung cancer patients receiving osimertinib in second or further lines. Future Oncol. 2021;17(19):2513–27. https://doi.org/10.2217/fon-2021-0356.
Cheema P, Cho BC, Freitas H, et al. A real-world study of second or later-line osimertinib in patients with EGFR T790M-positive NSCLC: the final ASTRIS data. Future Oncol. 2023;19(1):61–75. https://doi.org/10.2217/fon-2022-0919.
Hori T, Yamamoto K, Ito T, Ikushima S, Omura T, Yano I. Upfront Use of First-/Second-Generation EGFR-TKI followed by osimertinib shows better prognosis than upfront osimertinib therapy in Japanese patients with non-small-cell lung cancer with exon 19 deletion: a single-center retrospective study. Biol Pharm Bull. 2023;46(6):788–95. https://doi.org/10.1248/bpb.b22-00794.
Ke EE, Zhou Q, Zhang QY, et al. A higher proportion of the EGFR T790M mutation may contribute to the better survival of patients with exon 19 deletions compared with those with L858R. J Thorac Oncol. 2017;12(9):1368–75. https://doi.org/10.1016/j.jtho.2017.05.018.
Takeda M, Shimokawa M, Nakamura A, et al. A phase II study (WJOG12819L) to assess the efficacy of osimertinib in patients with EGFR mutation-positive NSCLC in whom systemic disease (T790M-negative) progressed after treatment with first- or second-generation EGFR TKIs and platinum-based chemotherapy. Lung Cancer. 2023;177:44–50. https://doi.org/10.1016/j.lungcan.2023.01.011.
Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–39. https://doi.org/10.1056/NEJMoa1507643.
Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–50. https://doi.org/10.1016/S0140-6736(15)01281-7.
Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837–46. https://doi.org/10.1016/S0140-6736(16)00587-0.
Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–65. https://doi.org/10.1016/S0140-6736(16)32517-X.
Lee CK, Man J, Lord S, et al. Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lung cancer-a meta-analysis. J Thorac Oncol. 2017;12(2):403–7. https://doi.org/10.1016/j.jtho.2016.10.007.
Lisberg A, Cummings A, Goldman JW, et al. A phase II study of pembrolizumab in EGFR-mutant, PD-L1+, Tyrosine Kinase Inhibitor Naïve patients with advanced NSCLC. J Thorac Oncol. 2018;13(8):1138–45. https://doi.org/10.1016/j.jtho.2018.03.035.
Paz-Ares L, Ciuleanu TE, Cobo M, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial [published correction appears in Lancet Oncol. 2021 Mar;22(3):e92]. Lancet Oncol. 2021;22(2):198–211. https://doi.org/10.1016/S1470-2045(20)30641-0.
Hu J, Huang D, Wang Y, et al. The efficacy of immune checkpoint inhibitors in advanced EGFR-Mutated non-small cell lung cancer after resistance to EGFR-TKIs: real-world evidence from a multicenter retrospective study. Front Immunol. 2022;13: 975246. https://doi.org/10.3389/fimmu.2022.975246. (Published 2022 Sep 9).
Qian X, Guo X, Li T, et al. Efficacy of immune checkpoint inhibitors in EGFR-Mutant NSCLC patients with EGFR-TKI resistance: a systematic review and meta-analysis. Front Pharmacol. 2022;13: 926890. https://doi.org/10.3389/fphar.2022.926890.
Ohe Y, Imamura F, Nogami N, et al. Osimertinib versus standard-of-care EGFR-TKI as first-line treatment for EGFRm advanced NSCLC: FLAURA Japanese subset. Jpn J Clin Oncol. 2019;49(1):29–36. https://doi.org/10.1093/jjco/hyy179.
Acknowledgments
The authors thank all patients and their families for participating in this study. We thank Chiaki Nishimura of CN Medical Research Inc. (Tokyo Japan).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The authors received no special funding for this study.
Conflict of Interests
The authors declare no competing interests.
Ethics Approval and Consent to Participate
This study and consent procedures were approved by the ethics committee of Toho University Sakura Medical Center (approval number: S22012, approval date: 28 September 2022). Patient consent for the use of personal information was obtained by adopting the opt-out method and by posting the research content and policy on personal information on the hospital's website.
Consent for Publication
Not applicable.
Availability of Data and Materials
The data sets used in this study are available from the corresponding author upon reasonable request.
Code Availability Statement
Not applicable.
Authors’ Contributions
Conceptualization: W.H. and T.K.; data collection, formal analysis, and writing—original draft preparation: T.K.; supervision: S.A. and M.Y.; writing—review and editing: W.H.; approval of final manuscript: all authors. All authors read and approved the final version.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Takashima, K., Wakabayashi, H., Murakami, Y. et al. Prognostic Factors in Japanese EGFR Mutation-Positive Non-Small-Cell Lung Cancer: A Real-World Single-Center Retrospective Cohort Study. Drugs - Real World Outcomes (2024). https://doi.org/10.1007/s40801-024-00449-8
Accepted:
Published:
DOI: https://doi.org/10.1007/s40801-024-00449-8