Abstract
Sexual dimorphism (i.e., masculinity in males and femininity in females) is known to affect social perceptions that are important for both mate choice and intrasexual competition, such as attractiveness and dominance. Little is known, however, about the neurophysiological underpinnings mediating sexual dimorphism’s effects on face processing. Here we investigate the neurological correlates of processing sexually dimorphic faces using event-related potentials (ERPs). We employed image transformation techniques to enhance and reduce the sexually dimorphic shape features of male and female faces viewed by women performing a sex categorization task. Sexual dimorphism modulated superior-central N250 magnitude and the peak latency of the N170 and P200. The sex of the face further modulated the amplitude of the P200. These findings extend prior work linking the superior-central N250 to social categorization processes triggered by face shape, and strengthen its functional interpretation in terms of coarse- versus fine-grained categorical judgements. We conclude that ERPs can illuminate the cognitive mechanisms (i.e., mental processes) underlying behavioral responses to sexual dimorphism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Perceptions of physical attractiveness affect many aspects of our lives, such as who we reproduce with (Gangestad, 1993; Gangestad & Buss, 1993), employ (Chiu & Babcock, 2002), and vote for (Klein & Ohr, 2000; Little et al., 2007a), as well as other fundamental aspects of human care (e.g., Bordieri, Solodky, & Mikos, 1985; Dion, 1972; Kurdahi Badr & Abdallah, 2001; Stephan & Langlois, 1984). Facial attractiveness, specifically, may be particularly important in person perception (see, e.g., Little, Jones, & DeBruine, 2011; Thornhill & Gangestad, 1999), although not the only important element involved in prosocial biases toward attractive people (see Maestripieri, Henry, & Nickels, 2017). Evidence suggests that the face is processed differently than the body, beginning at a very early age. For example, the configural processing of faces, which emerges very early in infancy (Le Grand, Mondloch, Maurer, & Brent, 2003; Walton & Bower, 1993), develops earlier than the configural processing of bodies (Slaughter, Heron, & Sim, 2002). Brain imaging studies (e.g., Allison, Puce, Spencer, & McCarthy, 1999; Eimer, 2000; Halit, de Haan, & Johnson, 2000; Kanwisher, McDermott, & Chun, 1997; McCarthy, Puce, Belger, & Allison, 1999; Puce, Allison, & McCarthy, 1999) and single-cell recording studies (Perrett, Hietanen, Oram, & Benson, 1992) show greater activity in certain brain regions (e.g., the fusiform gyrus, Kanwisher et al., 1997) when processing faces compared to other types of stimuli, further suggesting that face processing may play a particularly important role in overall person perception.
Certain facial traits, including bilateral symmetry, facial averageness, color cues to health, and sexual dimorphism (i.e., masculinity in males and femininity in females), are related to attributions of attractiveness with relative consistency across cultures (reviewed in, e.g., Little et al., 2011; Mogilski & Welling, 2017). However, compared to the consistently high preferences for female facial femininity among men (e.g., Perrett et al., 1998; Welling et al., 2008a), preference for male masculinity among women is considerably more variable, with some studies reporting a high preference for relatively masculine men (e.g., Johnston, Hagel, Franklin, Fink, & Grammer, 2001) and others reporting a high preference for relatively feminine men (e.g., Welling et al., 2007). Given that masculinity is associated with measures of long-term health (reviewed in Thornhill & Gangestad, 2006) and negative personality traits (e.g., dishonesty; Perrett et al., 1998), it is likely that women’s preferences for masculinity reflect how women resolve the tradeoff between the benefits (e.g., genetic immunocompetence that may be passed on to offspring) versus costs (e.g., less investment) of choosing a masculine partner. Certainly, preferences for male masculinity appear to be relatively context-specific, whereby preferences for masculinity are higher in conditions where genetic fitness should be prioritized. For example, women prefer masculine men more when environmental conditions are harsher (DeBruine, Jones, Crawford, Welling, & Little, 2010; DeBruine, Jones, Little, Crawford, & Welling, 2011; Little et al., 2007b), when considering men for short-term (i.e., purely sexual) relationships (Little, Jones, Penton-Voak, Burt, & Perrett, 2002), and when conception is more likely (e.g., Gildersleeve, Haselton, & Fales, 2014; Penton-Voak et al., 1999; Welling et al., 2007). Thus, various adaptive functions may be served when social behavior is influenced by others’ sexually dimorphic features (Rhodes, Chan, Zebrowitz, & Simmons, 2003; Thornhill & Gangestad, 1999), but little is known about the brain’s response to these features.
Relatively few studies have examined how event-related potentials (ERPs) are influenced by face shape during face processing, although some work has been done on the brain’s response to another socially important facial cue – that of race. Recently, Balas and Nelson (2010) investigated the effect on White participants of manipulating own-race (White) and other-race (Black) face shape and skin pigmentation within images viewed in a simple orientation discrimination task. They found that the N170, an early-processing component in face recognition (e.g., Bentin & Deouell, 2000; Herzmann, Schweinberger, Sommer, & Jentzsch, 2004; Itier & Taylor, 2004a, 2004b), and a superior-central N250 component were modulated by skin color, independent of face shape, such that the N170 amplitude was larger (i.e., more negative) and the latency shorter to Black faces than to White faces, and that the N250 amplitude was larger for White faces than to Black faces (with no N250 differences in latency in response to skin color). However, only the superior-central N250 exhibited sensitivity to face shape, whereby White face shape made N250 magnitudes less negative compared to Black face shape. As concluded by Balas and Nelson (2010), the superior-central N250 may reflect greater experience with face shapes of own- compared to other-race individuals, although it is possible that this component may relate to the ease of processing face shape more generally. With respect to facial masculinity, although the neurophysiological correlates of perceived sex of the face have been investigated (Mouchetant-Rostaing & Giard, 2003; Schyns, Bonnar, & Gosselin, 2002; Xu, Liu, & Kanwisher, 2005), only one study has investigated perception of sexually dimorphic face shape using ERPs. Cellerino et al. (2007) presented participants with profile views of gender-ambiguous faces and investigated perceptual masculinity based on a sex categorization task. They found that the right parieto-temporal N170 latency positively correlated with perceived masculinity, suggesting that the right parieto-temporal region plays a role in processing sexually dimorphic cues. However, given that the images were intentionally gender-ambiguous, the manipulation used by Cellerino et al. (2007) may not have been sufficiently salient to properly capture the encoding of sexually dimorphic face shape. Similarly, profile views of faces may not provide enough structural information related to sexually dimorphic shape because important dimorphic features are obscured or not fully visible (e.g., jaw width; Penton-Voak et al., 2001).
ERPs allow brain activity to be tracked with high temporal resolution and provide researchers with a way to study the brain’s response to facial manipulations in real-time (i.e., as they happen), thereby offering important insight into the electrophysiology underlying perception. Here, given the importance of sexually dimorphic traits in person perception, we investigate how these cues are processed in the brain using ERPs. We demonstrate that neurophysiological correlates of face processing in women are sensitive to sexually dimorphic cues in male and female faces, providing novel insight into cognitive mechanisms (i.e., mental processes) mediating adaptive responses to facial sexual dimorphism. Given that most behavioral studies on facial sexual dimorphism have focused on women’s responses and given that preferences for sexually dimorphic traits are less consistent across women as compared to men, ERPs were recorded from a sample of young adult women as they categorized faces manipulated in sexually dimorphic shape as male or female. This work builds upon previous research (Cellerino et al., 2007) by using stronger manipulations of sexually dimorphic face shape. We predicted sexual dimorphism effects upon the right parieto-temporal N170, and further predicted effects upon the superior-central N250, on the assumption that processes reflected by this component are not exclusively tuned to race-specific structure (i.e., that this component may reflect expertise with faces more generally). We additionally examined whether sexual dimorphism manipulations trigger different neurophysiological responses (as measured by differences in ERP amplitude and latency) as a function of sex, indicating functionally distinct processing of sexually dimorphic cues in male versus female faces.
Methods
Stimuli
We manipulated images using prototype-based image transformation techniques. First, prototype male and female face images were made by averaging the shape, color, and texture of a group of male and a group of female faces (for more information, see Rowland & Perrett, 1995; Tiddeman, Burt, & Perrett, 2001). Next, 50% of the linear differences in 2D shape between these prototype faces were added to or subtracted from 40 unfamiliar male and 40 unfamiliar female face identities (all with neutral expressions). This generated 40 pairs of male faces and 40 pairs of female faces, with each pair consisting of a version enhanced and a version reduced in sexual dimorphism (see, e.g., Buckingham et al., 2006; Little et al., 2007b; Welling, DeBruine, Little, & Jones, 2009; see also Figure 1). Previous studies have verified that manipulating sexual dimorphism in this way influences perceptions in the predicted manner (DeBruine et al., 2006; Welling et al., 2007). Importantly, the masculine and feminine versions of each image differed equally in 2D shape from the original image, and pairs of images were matched in other regards (e.g. , texture, color, and identity). Also, the image manipulation applied to male and female faces was identical.
Examples of female (top row) and male (bottom row) manipulated faces. Feminized versions are on the left and masculinized versions are on the right. Masculinized and feminized versions differ only in 2D shape and are identical in other regards (e.g., color, texture, and identity). Differences from average face shape are also constrained to be mathematically equivalent for each particular masculinized and feminized individual image. The manipulation therefore produces equivalently atypical, (i.e., distinctive) versions of each base identity
The experiment was implemented using Presentation 9.13, with 4 randomized blocks of 40 trials, each containing 20 of the 80 face pairs (half female). Each image (20 male and 20 female) was presented once within each block in a random order.
Procedure
Heterosexual female participants (N = 17 adult White women, M age = 21 years, SD = 2.89 years) were recruited through the Psychology Subject Pool at the University of Aberdeen (Scotland, United Kingdom) and participated in exchange for course credit. All participants had normal or corrected to normal vision and reported being right-handed. These women classified faces as male or female using a response box while electroencephalogram (EEG) was recorded in an electrically and acoustically shielded chamber. Participants responded right button = female and left button = male during blocks 1 and 3 and the opposite during blocks 2 and 4, as quickly as possible. Each face was presented for 200 ms, followed by a 600 ms blank screen, and then a 500 ms fixation cross followed by a 700-1700 msec blank screen. Trial durations were randomly set at 2000-3000 ms, in 200 ms jumps. This task allows us to measure categorical discrimination based on biological sex, as well as the influence of relevant fine-grained details (i.e., sexually dimorphic traits) in sex categorization, and how these details potentially correlate with ERP components involved in face processing.
ERP Derivation
Sixty-four-channel EEG data files were converted via the Polyrex conversion programme to a 16-bit resolution (gain .05) for processing in Neuroscan Edit 4.3. Following ocular artefact correction, data were bandpass filtered (.1 to 30 Hz), re-referenced to the common average, then epoched (100 ms pre- to 800 ms post-stimulus) and baseline corrected. Trials containing drift or saturation artefacts were rejected and data were smoothed using a 5-point binomial filter. ERPs were created from correct-response trials for the enhanced-male (M trials = 35.7), reduced-male (M trials = 34.8), enhanced-female (M trials = 34.9), and reduced-female (M trials = 34.4) conditions (i.e., masculinized male, feminized male, feminized female, and masculinized female, respectively).
Results
Behavioral Data
One-sample t-tests revealed that participants categorized faces in all conditions more accurately than chance (all t > 40.66, all p < .001, see Figure 2). Repeated-measures ANOVA [within-subjects factors: sex of face (male, female); sexual dimorphism (enhanced, reduced)] revealed only a main effect of sexual dimorphism upon sex discrimination (F 1,16 = 5.88, p = .03, η p 2 = .269), whereby enhanced sexual dimorphism faces were categorized with more accuracy than reduced sexual dimorphism faces (all other p > .39). Reaction time (RT) analyses (from trials where RT > 150 ms, see Kaufmann, Schweinberger, & Burton, 2009) revealed a main effect of sexual dimorphism (F 1,16 = 9.61, p = .007, η p 2 = .375), whereby enhanced sexual dimorphism faces were categorized faster. A marginal main effect of sex of face (F 1,16 = 4.12, p = .06, η p 2 = .205) emerged, reflecting faster identification of male versus female faces, and a sex of face by sexual dimorphism interaction (F 1,16 = 10.41, p = .005, η p 2 = .394). RTs were slower for masculinized female faces than for feminized female faces (t 16 = 5.14, p < .001), with no equivalent effect for male faces (p = .83).
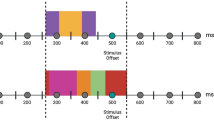
Mean percent accuracy (out of 40, grey bars) and mean RTs (milliseconds, yellow bars) for all four conditions. Faces enhanced in sexual dimorphism (i.e., masculine male faces and feminine female faces) were categorized more accurately (M = 97.1%, SD = .04) and faster (M = 550 msec, SD = 82) than reduced sexual dimorphism faces (M = 95.1%, SD = .04; M = 565 msec, SD = 94). Male faces were also identified faster (M = 546 msec, SD = 95) than female faces (M = 564 msec, SD = 83). RTs were slower for female faces reduced in sexual dimorphism (M = 580 msec, SD = 81) than for female faces enhanced in sexual dimorphism (M = 548 msec, SD = 84), with no equivalent effect for male faces
ERP Data
Figure 3 shows ERPs at left and right lateral inferior sites P7/P8 and the superior-central Pz site where the primary components of interest (N170 and superior-central N250) are located. Repeated-measures ANOVA of N170 mean amplitude (150-180 msec) [within-subject factors: sex of face (male, female); sexual dimorphism (enhanced, reduced); site (P7, P8)] produced no significant main effects or interactions (all p > .08). However, repeating this analysis using N250 mean amplitude data (230-270 msec) at Pz produced a main effect of sexual dimorphism (F 1,16 = 5.67, p = .03, η p 2 = .262) because superior-central N250 amplitude was larger for reduced versus enhanced sexual dimorphism images (see Figure 4 a), with no other effects (all p > .65). Inspection of Fig. 3 shows further differentiation between positive ERPs in each condition at the lateral inferior sites during the 230-270 msec interval. We analyzed this effect using repeated-measures ANOVA with the above factors, which gave only a main effect of sex of face (F 1,16 = 10.25, p < .008, η p 2 = .390; see Figure 4 b) due to increased positivity of ERPs evoked by female versus male images (all other effects p > .6).
Grand average ERPs contrasting all four image conditions at the superior-central Pz site and the lateral inferior P7 and P8 sites. Scalp locations are indicated on the inset. Clear lateral inferior N170 and superior-central N250 are evident and, in addition, a lateral inferior positivity enhanced for female versus male images
(a) Topographic maps depicting left and right hemi-scalp as viewed from the side, illustrating the effect of sexual dimorphism upon the superior-central N250 during the 230-270 msec interval separately for male (top) and female (bottom) faces. Each map was created by subtracting ERPs in the enhanced conditions (i.e., masculinized male and feminized female) from ERPs in the respective reduced conditions (i.e., feminized male and masculinized female). (b) Topographic map of the lateral inferior face gender positivity observed during the 230-270 msec interval, created by subtracting ERPs evoked by male faces (average of the reduced and enhanced male face ERPs) from ERPs evoked by female faces (average of the reduced and enhanced female face ERPs). Electrode sites are shown as black squares, and the voltage range for each map is given in the color scales
Analyses of lateral inferior peak latency data revealed sexual dimorphism by site interactions both for the N170 (F 1,16 = 6.39, p = .022, η p 2 = .285) and (marginally) the subsequent 230-270 msec positivity (F 1,16 = 4.23, p = .056, η p 2 = .209). ERPs peaked faster over the left hemiscalp at P7 for faces enhanced in sexual dimorphism (N170: 163 msec versus 166 msec, t 17 = 2.18, p < .05; 230-270 msec positivity: 251 msec versus 258 msec, t 17 = 2.16, p < .05). Note that prominent superior-central N250 peaks could not be identified reliably in all participants, as was the case in Balas and Nelson’s (2010) study, and so we too cannot provide superior-central N250 latency analyses.
Discussion
Reduced sexual dimorphism resulted in decreased overall accuracy and, for female faces, increased reaction times, suggesting that this manipulation affected within-category (i.e., within-sex) discrimination of facial features that signal biological sex. In other words, sexually dimorphic faces were easier to correctly categorize as male or female, especially for female faces. These behavioral data add to our ERP data; our key finding was that the superior-central N250 was largest for reduced sexual dimorphism faces regardless of their sex, extending Balas and Nelson’s (2010) work by showing that superior-central N250 sensitivity is not limited to racial characteristics of face shape. In line with Balas and Nelson’s (2010) expertise account of the superior-central N250 (see also Scott, Tanaka, Sheinberg, & Curran, 2006, 2008; Tanaka, Curran, Porterfield, & Collins, 2006), our findings suggest that correct categorization of faces with reduced sexual dimorphism requires sensitivity to fine-grained facial features that signal sex. Thus, faces that are less sex-typical (i.e., less sexually dimorphic) are more difficult to categorize with respect to biological sex, as suggested by our behavioral data, and this task difficulty is apparently reflected in enhanced N250 negativity in response to faces with reduced sexual dimorphism. Moreover, previous fMRI work investigating effects of sexual dimorphism on face perception (Rupp et al., 2009) identified five brain regions (i.e., superior temporal gyrus, precentral gyrus, anterior and posterior cingulate, and inferior parietal neocortex) previously related to either face or risk processing that showed increased activation to masculinized versus feminized male faces in women. Our findings complement this work by revealing neurophysiological correlates of face perception processes sensitive to sexual dimorphism, and by showing that common responses can be triggered in response to sexually dimorphic manipulations within male and female faces. These findings yield novel information regarding how features relevant to mate preferences and sex categorization are encoded in the brain.
Participants in our sample were quicker at categorizing male faces than female faces. It is possible that this categorical difference is reflected in the inferior lateral positivity observed during the 230-270 msec interval, which showed reduced positivity to male versus female faces. This positivity could be a P200 effect related to social categorization that is enhanced for same-sex versus opposite-sex images. Similar research has found enhanced P200 effects for “in-group” versus “out-group” individuals in terms of ethnicity (Ito & Urland, 2003, 2005; see also Kubota & Ito, 2007; Stahl, Wiese, & Schweinberger, 2010) and age (Wiese, Schweinberger, & Hansen, 2008). In support of this conclusion, Latinus and Taylor (2006) previously demonstrated faster P200 latency for upright faces when compared to inverted faces, Mooney faces, and non-face stimuli, and this was coupled with faster N170 peak latency. Correspondingly, we find that the peak latencies of the N170 and the subsequent putative P200 over the left hemi-scalp are similarly affected by sexual dimorphism. Although Cellerino et al. (2007) found longer N170 latency to faces perceived as masculine, they used gender-ambiguous faces, which may have been more complex to process. Here we used sex-typical faces that varied in the degree of sexual dimorphism and found that enhanced (i.e., more sex-typical) faces were related to earlier N170 peak latencies, perhaps suggesting, as our behavioral data suggests, that they are easier to process than faces with reduced sexual dimorphism. Alternatively, differences in the observed inferior lateral positivity may reflect greater ease of perceptual processing for mating-relevant (i.e., male) faces, but this interpretation is highly speculative and additional work would be needed to investigate the mate choice specificity of this component. Regardless, because the superior-central and lateral inferior ERP modulations were differentially sensitive to sexual dimorphism and sex, we suggest that these two effects could reflect the concurrent activation of functionally distinct processes (i.e., shape processing versus sex categorization).
There are several limitations of the current work. First, our sample consisted of only 17 women. However, we note that small sample sizes are commonplace in the ERP literature and similar research has made use of comparable participant numbers (i.e., N = 14, 12 female, Balas & Nelson, 2010; N = 20, 10 female, Cellerino et al., 2007). Still, future work could use a larger sample with more varied demographic characteristics (e.g., different ethnicities or sexual orientations). Similarly, future work could test males so that inferences can be made about whether differences between male and female stimuli reflect properties of male versus female faces or same- versus different-sex expertise in face processing. Researchers could also have participants rate or categorize faces for attractiveness within short-term and long-term mating contexts, which has been shown to influence preferences for sexually dimorphic face shape (e.g., Little et al., 2002). Male and female participants could rate the attractiveness of both sexes to assess whether more attractive faces are processed more quickly. Finally, although our results aligned well with the race-related face shape findings reported by Balas and Nelson (2010), suggesting that the N250 component may reflect expertise in correctly categorizing faces based on subtle aspects of face shape, we did not test other aspects of expertise in faces or other stimuli. Future research could include additional conditions related to face shape (e.g., ethnicity) and/or a non-face condition to see whether results are relatively specific to dimorphic face shape or if they generalize to expertise in other domains.
Sexually dimorphic facial cues are important both for women’s mate preferences and for assessing same-sex competitor mate-quality (e.g., Perrett et al., 1998). Therefore, sexual dimorphism manipulations may trigger an evaluative response in regard to mate quality that is common to male and female faces, although perception of mate quality was not directly tested in the current study. Our data are consistent with this conclusion, revealing identical ERP effects (increases in superior-central N250 magnitude and alterations in left lateral inferior ERP peak latencies) triggered by reduced versus enhanced sexual dimorphism in male and female faces. A necessary caveat is that neural generators independently sensitive to male versus female sexual dimorphism may co-exist within the same regions, producing the similar male and female ERP effects related to sexual dimorphism observed here. Importantly, future ERP work can now explore the various individual difference factors (e.g., Jones et al., 2005; Vukovic et al., 2009; Welling et al. 2007, 2008b, 2008c) that influence female sexual dimorphism preferences, which may allow sexual dimorphism effects triggered by male and female faces to be neurally dissociated. This, in turn, should lead to a better functional characterization of the cognitive mechanisms underlying adaptive behavioral responses to sexual dimorphism. These same techniques can later be applied to investigate how other cues to mate quality and attractiveness (e.g., averageness, symmetry, color cues to health; see, e.g., Little et al., 2011; Mogilski & Welling, 2017) are processed in the brain. Furthermore, eventual research could investigate how ERP components associated with sexual dimorphism and other cues to mate quality are influenced by different contexts where cues to genetic fitness should be prioritized, such as under harsher environmental conditions (DeBruine et al., 2010, 2011; Little et al., 2007b), thereby bridging the evolutionary psychology and perceptual neurology literatures.
References
Allison, T., Puce, A., Spencer, D. D., & McCarthy, G. (1999). Electrophysiological studies of human face perception. I: Potentials generated in occipitotemporal cortex by face and non-face stimuli. Cerebral Cortex, 9(5), 415–430. doi:10.1093/cercor/9.5.415.
Balas, B., & Nelson, C. A. (2010). The role of face shape and pigmentation in other-race face perception: An electrophysiological study. Neuropsychologia, 48(2), 498–506.
Bentin, S., & Deouell, L. Y. (2000). Structural encoding and identification in face processing: ERP evidence for separate mechanisms. Cognitive Neuropsychology, 17(1), 35–55. doi:10.1080/026432900380472.
Bordieri, J. E., Solodky, M. L., & Mikos, K. A. (1985). Physical attractiveness and nurses' perceptions of pediatric patients. Nursing Research, 34(1), 24–26.
Buckingham, G., DeBruine, L. M., Little, A. C., Welling, L. L. M., Conway, C. A., Tiddeman, B. P., & Jones, B. C. (2006). Visual adaptation to masculine and feminine faces influences generalized preferences and perceptions of trustworthiness. Evolution and Human Behavior, 27(5), 381–389.
Cellerino, A., Borghetti, D., Valenzano, D. R., Tartarelli, G., Mennucci, A., Murri, L., & Sartucci, F. (2007). Neurophysiological correlates for the perception of facial sexual dimorphism. Brain Research Bulletin, 71(5), 515–522. doi:10.1016/j.brainresbull.2006.11.007.
Chiu, R. K., & Babcock, R. D. (2002). The relative importance of facial attractiveness and gender in Hong Kong selection decisions. International Journal of Human Resource Management, 13(1), 141–155.
DeBruine, L. M., Jones, B. C., Little, A. C., Boothroyd, L. G., Perrett, D. I., Penton-Voak, I. S., et al. (2006). Correlated preferences for facial masculinity and ideal or actual partner’s masculinity. Proceedings of the Royal Society of London B, 273, 1355–1360.
DeBruine, L. M., Jones, B. C., Crawford, J. R., Welling, L. L. M., & Little, A. C. (2010). The health of a nation predicts their mate preferences: Cross-cultural variation in women's preferences for masculinized male faces. Proceedings of the Royal Society B Biological Sciences, 277(1692), 2405–2410.
DeBruine, L. M., Jones, B. C., Little, A. C., Crawford, J. R., & Welling, L. L. M. (2011). Further evidence for regional variation in women's masculinity preferences. Proceedings of the Royal Society B: Biological Sciences, 278(1707), 813–814.
Dion, K. K. (1972). Physical attractiveness and evaluation of children's transgressions. Journal of Personality and Social Psychology, 24(2), 207–213.
Eimer, M. (2000). Event-related brain potentials distinguish processing stages involved in face perception and recognition. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 111(4), 694–705.
Gangestad, S. W. (1993). Sexual selection and physical attractiveness: implications for mating dynamics. Human Nature, 4(3), 205–235.
Gangestad, S. W., & Buss, D. M. (1993). Pathogen prevalence and human mate preferences. Ethology and Sociobiology, 14, 89–96.
Gildersleeve, K. A., Haselton, M. G., & Fales, M. R. (2014). Do women’s mate preferences change across the ovulatory cycle? A meta-analytic review. Psychological Bulletin, 140(5), 1205–1259. doi:10.1037/a0035438.
Halit, H., de Haan, M., & Johnson, M. H. (2000). Modulation of event-related potentials by prototypical and atypical faces. Neuroreport, 11(9), 1871–1875.
Herzmann, G., Schweinberger, S. R., Sommer, W., & Jentzsch, I. (2004). What's special about personally familiar faces? A multimodal approach. Psychophysiology, 41(5), 688–701.
Itier, R. J., & Taylor, M. J. (2004a). Effects of repetition learning on upright, inverted and contrast-reversed face processing using ERPs. NeuroImage, 21(4), 1518–1532.
Itier, R. J., & Taylor, M. J. (2004b). N170 or N1? Spatiotemporal differences between object and face processing using ERPs. Cerebral Cortex, 14(2), 132–142.
Ito, T. A., & Urland, G. R. (2003). Race and gender on the brain: Electrocortical measures of attention to the race and gender of multiply categorizable individuals. Journal of Personality and Social Psychology, 85(4), 616–626.
Ito, T. A., & Urland, G. R. (2005). The influence of processing objectives on the perception of faces: An ERP study of race and gender perception. Cognitive, Affective, & Behavioral Neuroscience, 5(1), 21–36.
Johnston, V. S., Hagel, R., Franklin, M., Fink, B., & Grammer, K. (2001). Male facial attractiveness: Evidence for hormone-mediated adaptive design. Evolution and Human Behavior, 21, 251–267. doi:10.1016/S1090-5138(01)00066-6.
Jones, B. C., Little, A. C., Boothroyd, L., DeBruine, L. M., Feinberg, D. R., Law Smith, M. J., et al. (2005). Commitment to relationships and preferences for femininity and apparent health in faces are strongest on days of the menstrual cycle when progesterone level is high. Hormones and Behavior, 48(3), 283–290.
Kanwisher, N., McDermott, J., & Chun, M. M. (1997). The fusiform face area: A module in human extrastriate cortex specialized for face perception. The Journal of Neuroscience, 17(11), 4302–4311.
Kaufmann, J. M., Schweinberger, S. R., & Burton, A. M. (2009). N250 ERP correlates of the acquisition of face representations across different images. Journal of Cognitive Neuroscience, 21(4), 625–641. doi:10.1162/jocn.2009.21080.
Klein, M., & Ohr, D. (2000). Gerhard or Helmut? The effect of candidates’ ‘nonpolitical’ qualities on the voting decision - The German National Election 1998. Politische Vierteljahresschrift, 41, 199.
Kubota, J. T., & Ito, T. A. (2007). Multiple cues in social perception: The time course of processing race and facial expression. Journal of Experimental Social Psychology, 43(5), 738–752.
Kurdahi Badr, L., & Abdallah, B. (2001). Physical attractiveness of premature infants affects outcome at discharge from the NICU. Infant Behavior & Development, 24(1), 129–133.
Latinus, M., & Taylor, M. J. (2006). Face processing stages: Impact of difficulty and the separation of effects. Brain Research, 1123(1), 179–187.
Le Grand, R., Mondloch, C. J., Maurer, D., & Brent, H. P. (2003). Expert face processing requires visual input to the right hemisphere during infancy. Nature Neuroscience, 6, 1108–1112.
Little, A. C., Burriss, R. P., Jones, B. C., & Roberts, S. C. (2007a). Facial appearance affects voting decisions. Evolution and Human Behavior, 28(1), 18–27. doi:10.1016/j.evolhumbehav.2006.09.002.
Little, A. C., Cohen, D. L., Jones, B. C., & Belsky, J. (2007b). Human preferences for facial masculinity change with relationship type and environmental harshness. Behavioral Ecology and Sociobiology, 61, 967–973. doi:10.1007/s00265-006-0325-7.
Little, A. C., Jones, B. C., & DeBruine, L. M. (2011). Facial attractiveness: Evolutionary based research. Philosophical Transactions of the Royal Society B, Biological Sciences, 366(1571), 1638–1659. doi:10.1098/rstb.2010.0404.
Little, A. C., Jones, B. C., Penton-Voak, I. S., Burt, D. M., & Perrett, D. I. (2002). Partnership status and the temporal context of relationships influence human female preferences for sexual dimorphism in male face shape. Proceedings of the Royal Society B: Biological Sciences, 269(1496), 1095–1100. doi:10.1098/rspb.2002.1984.
Maestripieri, D., Henry, A., & Nickels, N. (2017). Explaining financial and prosocial biases in favor of attractive people: Interdisciplinary perspectives from economics, social psychology, and evolutionary psychology. Behavioral and Brain Sciences, 40, 1–16.
McCarthy, G., Puce, A., Belger, A., & Allison, T. (1999). Electrophysiological studies of human face perception. II: Response properties of face-specific potentials generated in occipitotemporal cortex. Cerebral Cortex, 9(5), 431–444. doi:10.1093/cercor/9.5.431.
Mogilski, J. K., & Welling, L. L. M. (2017). The relative importance of sexual dimorphism, fluctuating asymmetry, and color cues to health during evaluation of potential partners' facial photographs: A conjoint analysis study. Human Nature, 28(1), 53–75. doi:10.1007/s12110-016-9277-4.
Mouchetant-Rostaing, Y., & Giard, M. H. (2003). Electrophysiological correlates of age and gender perception on human faces. Journal of Cognitive Neuroscience, 15(6), 900–910.
Penton-Voak, I. S., Jones, B. C., Little, A. C., Baker, S., Tiddeman, B. P., Burt, D. M., & Perrett, D. I. (2001). Symmetry, sexual dimorphism in facial proportions, and male facial attractiveness. Proceedings of the Royal Society B: Biological Sciences, 268(1476), 1617–1623. doi:10.1098/rspb.2001.1703.
Penton-Voak, I. S., Perrett, D. I., Castles, D. L., Kobayashi, T., Burt, D. M., Murray, L. K., & Minamisawa, R. (1999). Menstrual cycle alters face preference. Nature, 399, 741–742. doi:10.1038/21557.
Perrett, D. I., Hietanen, J. K., Oram, M. W., & Benson, P. J. (1992). Organization and functions of cells responsive to faces in the temporal cortex. Philosophical Transactions: Biological Sciences, 335(1273), 23–30.
Perrett, D. I., Lee, K. J., Penton-Voak, I. S., Rowland, D. R., Yoshikawa, S., Burt, D. M., et al. (1998). Effects of sexual dimorphism on facial attractiveness. Nature, 394, 884–887. doi:10.1038/29772.
Puce, A., Allison, T., & McCarthy, G. (1999). Electrophysiological studies of human face perception. III: Effects of top-down processing on face-specific potentials. Cerebral Cortex, 9(5), 445–458. doi:10.1093/cercor/9.5.445.
Rhodes, G., Chan, J., Zebrowitz, L. A., & Simmons, L. W. (2003). Does sexual dimorphism in human faces signal health? Proceedings of the Royal Society B: Biological Sciences, 270, S93–S95. doi:10.1098/rsbl.2003.0023.
Rowland, D. A., & Perrett, D. I. (1995). Manipulating facial appearance through shape and color. IEEE Computer Graphics and Applications, 15(5), 70–76. doi:10.1109/38.403830.
Rupp, H. A., James, T. W., Ketterson, E. D., Sengelaub, D. R., Janssen, E., & Heiman, J. R. (2009). Neural activation in women in response to masculinized male faces: mediation by hormones and psychosexual factors. Evolution and Human Behavior, 30(1), 1–10. doi:10.1016/j.evolhumbehav.2008.08.006.
Schyns, P. G., Bonnar, L., & Gosselin, F. (2002). Show me the features! Understanding recognition from the use of visual information. Psychological Science, 13(5), 402–409.
Scott, L. S., Tanaka, J. W., Sheinberg, D. L., & Curran, T. (2006). A reevaluation of the electrophysiological correlates of expert object processing. Journal of Cognitive Neuroscience, 18(9), 1453–1465. doi:10.1162/jocn.2006.18.9.1453.
Scott, L. S., Tanaka, J. W., Sheinberg, D. L., & Curran, T. (2008). The role of category learning in the acquisition and retention of perceptual expertise: A behavioral and neurophysiological study. Brain Research, 1210, 204–215.
Slaughter, V., Heron, M., & Sim, S. (2002). Development of preferences for the human body shape in infancy. Cognition, 85(3), B71–B81.
Stahl, J., Wiese, H., & Schweinberger, S. R. (2010). Learning task affects ERP-correlates of the own-race bias, but not recognition memory performance. Neuropsychologia, 48(7), 2027–2040.
Stephan, C. W., & Langlois, J. H. (1984). Baby beautiful: adult attributions of infant competence as a function of infant attractiveness. Child Development, 55(2), 576–585.
Tanaka, J. W., Curran, T., Porterfield, A. L., & Collins, D. (2006). Activation of Preexisting and Acquired Face Representations: The N250 Event-related Potential as an Index of Face Familiarity. Journal of Cognitive Neuroscience, 18(9), 1488–1497. doi:10.1162/jocn.2006.18.9.1488.
Thornhill, R., & Gangestad, S. W. (1999). Facial attractiveness. Trends in Cognitive Science, 3(12), 452–460. doi:10.1016/S1364-6613(99)01403-5.
Thornhill, R., & Gangestad, S. W. (2006). Facial sexual dimorphism, developmental stability, and susceptibility to disease in men and women. Evolution and Human Behavior, 27, 131–144. doi:10.1016/j.evolhumbehav.2005.06.001.
Tiddeman, B. P., Burt, D. M., & Perrett, D. I. (2001). Prototyping and transforming facial texture for perception research. IEEE Computer Graphics and Applications, 21, 42–50. doi:10.1109/38.946630.
Vukovic, J., Jones, B. C., DeBruine, L. M., Little, A. C., Feinberg, D. R., & Welling, L. L. M. (2009). Circum-menopausal effects on women's judgements of facial attractiveness. Biology Letters, 5(1), 62–64. doi:10.1098/rsbl.2008.0478.
Walton, G. E., & Bower, T. G. R. (1993). Newborns form "prototypes" in less than 1 minute. Psychological Science, 4, 203–205.
Welling, L. L. M., DeBruine, L. M., Little, A. C., & Jones, B. C. (2009). Extraversion predicts individual differences in women's face preferences. Personality and Individual Differences, 47(8), 996–998.
Welling, L. L. M., Jones, B. C., & DeBruine, L. M. (2008c). Sex drive is positively associated with women's preferences for sexual dimorphism in men's and women's faces. Personality and Individual Differences, 44(1), 161–170.
Welling, L. L. M., Jones, B. C., DeBruine, L. M., Conway, C. A., Law Smith, M. J., Little, A. C., et al. (2007). Raised salivary testosterone in women is associated with increased attraction to masculine faces. Hormones and Behavior, 52(2), 156–161.
Welling, L. L. M., Jones, B. C., DeBruine, L. M., Little, A. C., & Smith, F. G. (2008b). Exposure to sexually attractive men decreases women’s preferences for feminine faces. Journal of Evolutionary Psychology, 6(3), 219–230.
Welling, L. L. M., Jones, B. C., DeBruine, L. M., Smith, F. G., Feinberg, D. R., Little, A. C., & Al-Dujaili, E. A. S. (2008a). Men report stronger attraction to femininity in women's faces when their testosterone levels are high. Hormones and Behavior, 54(5), 703–708.
Wiese, H., Schweinberger, S. R., & Hansen, K. (2008). The age of the beholder: ERP evidence of an own-age bias in face memory. Neuropsychologia, 46(12), 2973–2985.
Xu, Y., Liu, J., & Kanwisher, N. (2005). The M170 is selective for faces, not for expertise. Neuropsychologia, 43(4), 588–597.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Welling, L.L.M., Bestelmeyer, P.E.G., Jones, B.C. et al. Effects of Sexually Dimorphic Shape Cues on Neurophysiological Correlates of Women’s Face Processing. Adaptive Human Behavior and Physiology 3, 337–350 (2017). https://doi.org/10.1007/s40750-017-0072-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40750-017-0072-1