Abstract
Purpose of Review
This paper serves as a review of the anatomic lesions in congenital heart disease that would benefit from pre-surgical evaluation using three-dimensional echocardiography (3DE) and future research needed in 3DE.
Recent Findings
Three-dimensional echocardiography provides a noninvasive evaluation of congenital heart disease in three dimensions to allow for better understanding of anatomic relationships. Specific views of 3DE views are discussed to best access these complex lesions prior to surgery.
Summary
Mechanisms of atrioventricular valve regurgitation are discussed using 3DE. Location of straddling valve, location of ventricular septal defect in double outlet right ventricles, complex anatomy of left ventricular outflow tract obstruction, and complex muscular ventricular septal defect locations are described in detail using 3DE and the best imaging modality from live 3D, 3D zoom, full volume acquisitions, and multiplane reconstruction to aid surgical planning.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Three-dimensional echocardiography (3DE) is rapidly recognized as an important modality in pre-surgical planning in congenital heart disease (CHD) [1, 2•, 3]. The display of the 3DE images is often from the surgical view to facilitate communication between echocardiographers and surgeons. Two-dimensional echocardiographic (2DE) imaging requires the reconstruction of 3D images which can result in miscommunication, as the display of the 2DE images is usually from the ventricle perspectives that is completely different from the enface surgical views of the anatomy. The benefits of 3DE datasets allow the user to manipulate the images in 3D space and allow for better understanding of the specific congenital heart lesions. The purpose of this review is to highlight lesions where 3DE has the most impact on pre-surgical planning.
Normal atrioventricular valve
An understanding of what constitutes the normal atrioventricular (AV) valve is imperative to understanding the abnormalities of AV valves associated with CHD. The annulus, leaflets, commissures, chordal support, and papillary muscles work as a functional unit of the AV valve. Disruption in any of these functional units will result in AV valve incompetence. The goals of transthoracic 3DE AV valve evaluation prior to surgery focus on the 4 major components of the valve: (1) the valve annulus size and shape, (2) leaflet morphology, (3) chordal apparatus, and (4) papillary muscles [4•, 5, 6]. The tricuspid and mitral valves are saddle-shaped with two high points and two low points [7]. This morphology reduces leaflet stress. The mitral annulus is not as elliptical as the tricuspid annulus [7]. When the elliptical annulus of the tricuspid valve is disrupted, there is inability of the tricuspid valve leaflets to coapt resulting in regurgitation [8]. Both the mitral and tricuspid valve leaflets are supported by the attachments of the chords, and when this relationship is disrupted, regurgitation occurs [9•]. The papillary muscles of the mitral and tricuspid valves maintain a relatively constant angle to the leaflets throughout the cardiac cycle. When these angles are changed, there is tethering leading to poor leaflet coaptation that results in valve regurgitation [9•]. Table 1 lists the acquisition of 3DE in different positions for specific congenital heart lesions.
Tricuspid valve abnormalities
The tricuspid valve is the largest of the four valves in the heart and has three leaflets [10,11,12]. The three leaflets cannot be displayed simultaneously using 2DE. With 3DE, the enface view of the tricuspid valve from the right atrium allows accurate identification of the anterior, septal, and posterior leaflets [13]. This can be achieved using 3D zoom or cropping from 3D full volume datasets. The anterior leaflet is the largest of the three leaflets and is supported by a large papillary muscle that attaches to the moderator band and a small papillary muscle of the conus. There are instances where there can be two anterior leaflets or cleft within the anterior leaflet. The septal leaflet of the tricuspid valve has chordae that insert directly into the septum with no papillary muscles. The posterior leaflet is probably the smallest leaflet and is supported by the smaller, more posteriorly situated papillary muscle.
Congenital anomalies of the tricuspid valve include tricuspid valve dysplasia, Ebstein anomalies, tricuspid valve prolapse, and tricuspid stenosis from congenital tricuspid stenosis. 3DE allows for several enface views of the tricuspid valve from the transthoracic approach including parasternal short axis, apical four chamber, and subcostal views. Axial plane has the greatest spatial resolution; thus, when evaluating the tricuspid valve leaflets, the apical four chamber view will provide the most resolution of the valve leaflets. 3DE can readily define the morphologic features of the valve and the associated mechanism(s) for regurgitation, which are key pieces of information the surgeon needs in planning the valve repair.
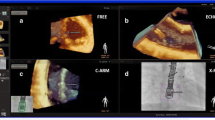
Tricuspid valve dysplasia occurs when the leaflets are thickened and deficient, and have short chordae that tether the valve resulting in inability of the anterior and posterior leaflets to come together with the septal leaflet (Fig. 1; Video 1). A hypoplastic papillary muscle may be seen in conjunction with this lesion [14]. Tricuspid valve dysplasia can occur in isolation or can be associated with other congenital heart diseases, such as pulmonary atresia with intact ventricular septum. Tricuspid valve dysplasia may be repaired with leaflet extension. 3DE will define the thickened valve with short chords.
Atria down view from the surgeon’s perspective of tricuspid valve dysplasia. There are two anterior (A) leaflets or a large anterior tricuspid valve leaflet with a cleft. The septal (S) and posterior (P) leaflets are tethered and not coapting with the anterior leaflets resulting in a large regurgitation site in this tricuspid valve dysplasia. LEAF, leaflet; POST, posterior; SEP, septal.
Ebstein anomaly of the tricuspid valve involves the apical (downward) displacement of insertion of the septal leaflet of the tricuspid valve (≥ 8 mm/m2), redundant elongated anterior tricuspid valve leaflet, failure of delamination in the septal and posterior leaflets, dilation of the AV junction, and right ventricular thinning, enlargement, and dysfunction [14,15,16,17]. The anterior leaflet may have distal linear attachments to the anterior wall of the right ventricle and in some cases can result in right ventricular outflow tract obstruction. There can be significant tricuspid valve regurgitation with progressive right heart failure that can affect these patients early in life and many need surgery to correct this anomaly. With 3DE, the anterior leaflet of the Ebstein valve may be seen attaching to the anterior wall of the right ventricle and right ventricular outflow tract. The relation of the leaflets to the chordal support and papillary muscles can be defined using multiplane reconstruction (MPR).
Congenital tricuspid valve prolapse is seen in patients with elastin abnormalities or corrected transposition of the great arteries, whereas acquired tricuspid valve prolapse occurs in the setting of anterior chest wall trauma, or secondary to closure of ventricular septal defect (VSD), subendocardial ischemia, or papillary muscle dysfunction. Rarely, it presents as cleft of the anterior leaflet of the tricuspid valve with prolapse, resulting in severe tricuspid regurgitation. Isolated congenital cleft of the anterior tricuspid valve leaflet is very rare with a reported incidence of 0.018% [18].
The valve annulus of tricuspid valve stenosis appears relatively large with leaflets that are thickened with commissural fusion and shortened chordae [19, 20]. This lesion is usually associated with other anomalies such as right ventricular outflow tract obstruction or atresia secondary to hypoplasia of the right ventricle. Isolated congenital tricuspid valve stenosis is extremely rare. Patients who have had porcine bioprosthetic valves in the tricuspid position for treatment of Ebstein anomaly may develop tricuspid stenosis over time [21].
Quantification of AV valve regurgitation
Pre-surgical evaluation of AV valve anatomy using 3DE provides insights into the mechanism of valve regurgitation. This includes detailed evaluation of valve annulus, leaflets, sub-valve apparatus, and papillary muscle position that can contribute to valve incompetence for which surgical techniques may correct the dysfunctional component [22–24]. Although the features of the valve components can be evaluated with the heart deflated on cardiopulmonary bypass, 3DE demonstrates the heart in real-time motion to evaluate the valve and delineate regions of leaflet prolapse, tethering, regurgitation jet locations, and commissural abnormalities [4•, 6]. The understanding of the valve prior to surgery provides the surgical team time to reflect on surgical options in fixing the valve.
The use of 3DE to evaluate AV valve in atrioventricular septal defect (AVSD) and the tricuspid valve in hypoplastic heart syndrome (HLHS) has been extensively studied. Valve failure after surgical repair has been reported and carries significant morbidity and mortality [22, 23, 25–27]. In AVSD repair, residual left atrioventricular valve regurgitation (LAVVR) remains up to 30% necessitating re-repair in this population [23, 28]. The mechanism and pathophysiology of post repair LAVVR are not clearly understood with some AV valve failing early despite usual anatomy and an uncomplicated repair. Quantitative 3DE research provides insight on features of LAVVR. Commissural abnormalities, annular dilation, and leaflet prolapse are independent risk factors for LAVVR [4•, 29]. In addition, 3DE shows an association between congenital abnormal sub-valve apparatus and post repair LAVVR [4•, 5]. Features of abnormal insertion of the anterolateral papillary muscle, abnormal chordal insertion to the superior bridging leaflet, imbalance of superior bridging leaflet and inferior bridging leaflet size, and the presence of commissural abnormalities have challenged the complete closure of the zone of apposition in the LAVV [9•, 29]. Guidelines on the use of 3DE valve quantitative assessment in clinical settings and normal values are lacking in congenital heart disease.
Quantitative research using 3DE for the assessment of the tricuspid valve in HLHS has identified the mechanism of valve failure [8, 26, 30, 31•, 32, 33•]. Significant tricuspid valve regurgitation in HLHS results from loss of normal saddle-shaped annulus with flattened annulus, becoming more circular in shape rather than an elliptical shape, and has more annular dilation with lateral displacement of anterior papillary muscle [26, 30, 31•]. There is leaflet tethering in younger patients and more leaflet prolapse in older patients [8, 26, 30]. A recent study identified that septal leaflet tethering prior to tricuspid valve repair will lead to significant postoperative regurgitation [31•]. 3DE accurately defines commissure abnormalities using the ventricle up view and MPR. The 3DE color Doppler readily identifies the regurgitation jet and is more precise when compared to 2DE and surgical saline testing [5, 32, 34]. The current use of 3DE in the preoperative evaluation of tricuspid regurgitation in HLHS remains qualitative. Even though current technology allows accurate evaluation of tricuspid regurgitation jet location and valve failure mechanism, the surgical options for addressing the tricuspid valve remain limited [35]. This highlights the importance of more 3DE research beyond the valve components but into novel approaches for tricuspid valve failure in HLHS. Data from quantitative 3DE research in HLHS tricuspid valve supports the hypothesis that tricuspid valve components appear to adapt to valve stressors from rapid and chronic changes in preload and afterload conditions during staged palliations [26]. Recent software to evaluate tricuspid valve from 3DE from machine learning provides insight into more refined details of the valve [36]. Further understanding of valve development, adaptation, and mechanisms of valve failure in HLHS may facilitate innovation in medical therapy and surgical strategies.
Chordal abnormalities in congenital heart disease
3DE provides accurate imaging of straddling chordae in complex CHD with accurate visualization and understanding of chordal insertion [37]. An understanding of the embryologic development of AV valve straddling [38] coupled with an accurate assessment is critical for surgical determination of a single ventricle versus a biventricular repair. Although 2DE with cross-sectional imaging will determine the presence of tricuspid valve or mitral valve straddle [39], 3DE can provide additional accuracy to the degree of straddle, location of chordae relative to the VSD, and its insertion in the contralateral ventricle, in addition to assessment of the ipsilateral ventricular size [37, 40]. This information is useful in surgical planning. Current surgical approaches to the straddling chord are somewhat based on degree of chordal straddle, classified as type A (the insertion is just on the wrong side of the crest of the septum), type B (insertion on the wrong side of the septum further than 0.5 to 1 cm of the crest of the septum), and type C (where the insertion is into the papillary muscle of the other AV valve or across the contralateral ventricle free wall) [41]. Current surgical approaches include extension of the VSD patch to include the minor straddling chord (type A) or by omitting sutures of the VSD patch at the straddling chord (type B). Surgical translocation of type C AV straddling chordae to the ipsilateral ventricle has been reported [42, 43]. Careful assessment of high-quality 3DE datasets is required. The imaging window is best achieved in the subcostal view as the chordal structure is mostly in the axial imaging plane (Table 1). An ECG gated full volume acquisition from subcostal view allows a wider pyramid volume while maintaining a high temporal resolution and provides anatomic details surrounding the straddling valve such as location and size of the VSD and the outflow tracts. Delineation of the chordal structure and papillary attachments with accuracy can be achieved with MPR from the full volume datasets.
Double outlet right ventricle—location of ventricular septal defect
The understanding of the VSD location in relation to the great arteries in double outlet right ventricle is crucial for surgical repair. 3DE has the advantage of incorporating the VSD in relation to the great arteries in one volume dataset allowing for surgical planning by enhancing the understanding of the anatomic structures. 3DE has the additional advantage of retaining dynamic information on the changing VSD size (especially muscular defects) and the tricuspid valve leaflets and chordal apparatus. The apical 4-chamber view and subcostal 3DE full volume acquisitions have the advantages of incorporating all the relevant structures needed for surgical planning [44]. A rendered cropped view from the ventricular apex will show the relationship of the atrioventricular valves, the VSD, and the relationship of the great arteries. This will identify whether or not the baffle from the left ventricle to the aorta will obstruct tricuspid valve inflow to the right ventricle or from the right ventricle to the pulmonary artery [44]. The cropped anatomic right ventricular view will show the inflow of the tricuspid valve with the relation of the VSD to the aorta and the pulmonary artery. Lastly, MPR will determine the size of the VSD in systole to assess the need for VSD enlargement.
Complex left ventricular outflow obstruction
Complex left ventricular outflow tract obstruction (LVOTO) includes multiple levels of obstruction from sub-aortic ridge, membrane, AV valve chordae, aneurysm of the membranous septum, accessory mitral valve tissue, abnormal insertion of the LAVV papillary muscle, or LVOT tumor. This LVOTO can be obtained with accuracy using 3DE and can provide safe surgical resection. If there are chordal tissues in the mix, critical supporting apparatus of the left AV valve must be preserved and not be injured during surgery [45,46,47,48,49,50]. This process is often made more difficult by having to inspect the LVOT from the aorta through a frequently associated hypoplastic aortic valve, especially in neonates and small children. The 3DE assessment of complex LVOT is feasible with a high inter-reader agreement and is highly accurate [48]. 3DE provides details on the obstructive anatomy and mechanism that is difficult to achieve on 2DE. Full volume rendered 3DE from the left ventricle view facilitates the surgeon’s understanding of the patient’s specific complex lesion with obstructive tissue and other structures such as AV valve supporting apparatus and their insertion relative to the obstructive tissue [47, 48]. The surgeon can then virtually inspect the LVOT from the aortic valve (surgical view) to accurately locate the obstructive and supporting structures [51]. Such detailed preoperative knowledge of the anatomy allows surgeons to plan their approach to reduce intraoperative inspection time and erroneous injury to AV valve supporting structures.
Optimal imaging of LVOTO is achieved by imaging in the parasternal long axis, even though the obstructing tissue (i.e., membrane, LVOT chordae, mass, or muscle bar) may not necessarily be in the axial plane. The apical 5-chamber view would place most obstructive lesions in the axial plane, but any resolution gained is often negated by the lesion being in the far field. While adequate temporal resolution can be achieved for the LVOT obstructing structure using live 3DE mode, an ECG gated full volume multi-beat dataset with its wider field of view is often required for accurate determination of LVOT chordae, their role in the support of the LAVV, and the surrounding structures. The subcostal 3DE full volume acquisition in small infants may complement the parasternal full volume acquisitions. In our experience, MPR will help delineate the complex LVOTO by locating the chordal structures that may or may not be attached to the AV valve.
Complex multiple muscular ventricular septal defects
Surgical closure of complex multiple muscular VSDs from the right ventricle is challenging due to the heavy ventricular trabeculations and multiple exit points. Thus, accurate determination of size and locations of the defects that is communicated to the surgeon is key to successful closure of complex multiple VSDs [52]. While most VSDs can be adequately assessed by 2DE and color Doppler flow mapping, the understanding of complex muscular VSD size, shape, and its location on a non-geometric septal surface is difficult. This requires the interrogation of the defects from multiple views, followed by mental integration of 2DE images to create a mental 3D image of the VSD that may or may not be communicated clearly to the surgeon. 3DE can overcome these difficulties by adding depth perception and enabling presentation of unique enface views of the surface of both the right ventricle and left ventricle [53,54,55,56]. Imaging with 3DE is feasible for VSDs and inter-reader variability is low [55, 57, 58]. It is superior to 2DE in assessing the VSD maximum diameter and the change in VSD area from diastole to systole, as well as accurate determination of the shape of the defect that is important for both catheter intervention and surgical planning [55, 57, 59]. This detailed information becomes even more critical when the complex multiple muscular VSDs require defect closure. The presentation of the accurate locations of multiple muscular defects on the rendered RV septal surface allows appropriate planning of catheter device intervention or the surgical approach for direct suture, patch closure, apical exclusion via the tricuspid valve, or left ventricular patch closure via a left ventricular ventriculotomy. In some instances, the hybrid approach with both catheter and surgery may be necessary.
Imaging of the VSD can be achieved by live 3DE and/or full volume 3DE acquisition. A live 3DE with a narrowed sector (60 degree × 20 degree) beam can be steered to incorporate the entire RV septal wall from the subcostal window [56]. This approach achieves reasonable temporal resolution for both 3DE gray-scale imaging and 3DE color Doppler flow mapping for analysis. Although this approach will sacrifice some details of the surrounding structures, it is useful in unsedated patients where breath-holding is not possible for multi-beat acquisition. If the patient is cooperative, a modified apical or subcostal approach is used to perform an ECG multi-beat 3DE dataset for offline analysis [57]. Acquired datasets will require careful cross-sectional interrogation with the MPR mode to locate the defects and measure their shape and size before tissue rendered septal surface is performed to communicate findings to interventionists and surgeons.
Conclusion
In conclusion, 3DE is a useful and critical modality for pre-surgical planning in many of the CHD lesions. It overcomes the limitations of 2DE and provides understanding of anatomy in 3D space. Future research will need to focus on quantification of valve regurgitation from 3D datasets and 3D printing from 3DE datasets so that valve morphology can be visualized, and further miniaturization of pediatric 3D transthoracic probes to allow for scanning smaller infants with better resolution.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance
Acar P, Abadir S, Paranon S, Latcu G, Grosjean J, Dulac Y. Live 3D echocardiography with the pediatric matrix probe. Echocardiography. 2007;24(7):750–5.
• Simpson J, Lopez L, Acar P, Friedberg MK, Khoo NS, Ko HH, et al. Three-dimensional echocardiography in congenital heart disease: an expert consensus document from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2017;30(1):1–27. This is an important review of using three-dimensional echocardiography congenital heart disease.
Sugeng L, Shernan SK, Salgo IS, Weinert L, Shook D, Raman J, et al. Live 3-dimensional transesophageal echocardiography initial experience using the fully-sampled matrix array probe. J Am Coll Cardiol. 2008;52(6):446–9.
• Takahashi K, Mackie AS, Thompson R, Al-Naami G, Inage A, Rebeyka IM, et al. Quantitative real-time three-dimensional echocardiography provides new insight into the mechanisms of mitral valve regurgitation post-repair of atrioventricular septal defect. J Am Soc Echocardiogr. 2012;25(11):1231–44. This is an important study delineating the mechanisms of valve regurgitation.
Takahashi K, Mackie AS, Rebeyka IM, Ross DB, Robertson M, Dyck JD, et al. Two-dimensional versus transthoracic real-time three-dimensional echocardiography in the evaluation of the mechanisms and sites of atrioventricular valve regurgitation in a congenital heart disease population. J Am Soc Echocardiogr. 2010;23(7):726–34.
Takahashi K, Guerra V, Roman KS, Nii M, Redington A, Smallhorn JF. Three-dimensional echocardiography improves the understanding of the mechanisms and site of left atrioventricular valve regurgitation in atrioventricular septal defect. J Am Soc Echocardiogr. 2006;19(12):1502–10.
Nii M, Roman KS, Macgowan CK, Smallhorn JF. Insight into normal mitral and tricuspid annular dynamics in pediatrics: a real-time three-dimensional echocardiographic study. J Am Soc Echocardiogr. 2005;18(8):805–14.
Nii M, Guerra V, Roman KS, Macgowan CK, Smallhorn JF. Three-dimensional tricuspid annular function provides insight into the mechanisms of tricuspid valve regurgitation in classic hypoplastic left heart syndrome. J Am Soc Echocardiogr. 2006;19(4):391–402.
• Colen T, Smallhorn JF. Three-dimensional echocardiography for the assessment of atrioventricular valves in congenital heart disease: past, present and future. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2015;18(1):62–71. This is an important review of valves, chords, and papillary muscles in atrioventricular septal defect.
Silver MD, Lam JH, Ranganathan N, Wigle ED. Morphology of the human tricuspid valve. Circulation. 1971;43(3):333–48.
Lamers WH, Viragh S, Wessels A, Moorman AF, Anderson RH. Formation of the tricuspid valve in the human heart. Circulation. 1995;91(1):111–21.
Addetia K, Yamat M, Mediratta A, Medvedofsky D, Patel M, Ferrara P, et al. Comprehensive two-dimensional interrogation of the tricuspid valve using knowledge derived from three-dimensional echocardiography. J Am Soc Echocardiogr. 2016;29(1):74–82.
Stankovic I, Daraban AM, Jasaityte R, Neskovic AN, Claus P, Voigt JU. Incremental value of the en face view of the tricuspid valve by two-dimensional and three-dimensional echocardiography for accurate identification of tricuspid valve leaflets. J Am Soc Echocardiogr. 2014;27(4):376–84.
Ammash NM, Warnes CA, Connolly HM, Danielson GK, Seward JB. Mimics of Ebstein’s anomaly. Am Heart J. 1997;134(3):508–13.
Lev M, Liberthson RR, Joseph RH, Seten CE, Eckner FA, Kunske RD, et al. The pathologic anatomy of Ebstein’s disease. Arch Pathol. 1970;90(4):334–43.
Ho SY, Goltz D, McCarthy K, Cook AC, Connell MG, Smith A, et al. The atrioventricular junctions in Ebstein malformation. Heart. 2000;83(4):444–9.
Anderson KR, Zuberbuhler JR, Anderson RH, Becker AE, Lie JT. Morphologic spectrum of Ebstein’s anomaly of the heart: a review. Mayo Clin Proc. 1979;54(3):174–80.
Eichhorn P, Ritter M, Suetsch G, von Segesser LK, Turina M, Jenni R. Congenital cleft of the anterior tricuspid leaflet with severe tricuspid regurgitation in adults. J Am Coll Cardiol. 1992;20(5):1175–9.
Svane S. Congenital tricuspid stenosis. A report on six autopsied cases. Scand J Thor Cardiovasc Surg. 1971;5(3):232–8.
Lewis T. Congenital tricuspid stenosis. Clin Sci. 1945;5(3–4):261–73.
Williams DB, Danielson GK, McGoon DC, Puga FJ, Mair DD, Edwards WD. Porcine heterograft valve replacement in children. J Thorac Cardiovasc Surg. 1982;84(3):446–50.
Hoohenkerk GJ, Bruggemans EF, Rijlaarsdam M, Schoof PH, Koolbergen DR, Hazekamp MG. More than 30 years’ experience with surgical correction of atrioventricular septal defects. Ann Thorac Surg. 2010;90(5):1554–61.
Stulak JM, Burkhart HM, Dearani JA, Cetta F, Barnes RD, Connolly HM, et al. Reoperations after repair of partial atrioventricular septal defect: a 45-year single-center experience. Ann Thorac Surg. 2010;89(5):1352–9.
Stulak JM, Burkhart HM, Dearani JA. Reoperations after repair of partial and complete atrioventricular septal defect. World J Pediatr Congenit Heart Surg. 2010;1(1):97–104.
Barber G, Helton JG, Aglira BA, Chin AJ, Murphy JD, Pigott JD, et al. The significance of tricuspid regurgitation in hypoplastic left-heart syndrome. Am Heart J. 1988;116(6 Pt 1):1563–7.
Kutty S, Colen T, Thompson RB, Tham E, Li L, Vijarnsorn C, et al. Tricuspid regurgitation in hypoplastic left heart syndrome: mechanistic insights from 3-dimensional echocardiography and relationship with outcomes. Circ Cardiovasc Imaging. 2014;7(5):765–72.
Ghanayem NS, Allen KR, Tabbutt S, Atz AM, Clabby ML, Cooper DS, et al. Interstage mortality after the Norwood procedure: results of the multicenter Single Ventricle Reconstruction trial. J Thorac Cardiovasc Surg. 2012;144(4):896–906.
Kaza AK, Colan SD, Jaggers J, Lu M, Atz AM, Sleeper LA, et al. Surgical interventions for atrioventricular septal defect subtypes: the pediatric heart network experience. Ann Thorac Surg. 2011;92(4):1468–75 (discussion 75).
Colen TM, Khoo NS, Ross DB, Smallhorn JF. Partial zone of apposition closure in atrioventricular septal defect: are papillary muscles the clue. Ann Thorac Surg. 2013;96(2):637–43.
Takahashi K, Inage A, Rebeyka IM, Ross DB, Thompson RB, Mackie AS, et al. Real-time 3-dimensional echocardiography provides new insight into mechanisms of tricuspid valve regurgitation in patients with hypoplastic left heart syndrome. Circulation. 2009;120(12):1091–8.
• Shigemitsu S, Mah K, Thompson RB, Grenier J, Lin LQ, Silmi A, et al. Tricuspid valve tethering is associated with residual regurgitation after valve repair in hypoplastic left heart syndrome: a three-dimensional echocardiographic study. J Am Soc Echocardiogr. 2021;34(11):1199–210. This is a study that describes why valve repair fails in hypoplastic left heart syndrome.
Li L, Colen TM, Jani V, Barnes BT, Craft M, Tham E, et al. Dynamic systolic changes in tricuspid regurgitation vena contracta size and proximal isovelocity surface area in hypoplastic left heart syndrome: a three-dimensional color Doppler echocardiographic study. J Am Soc Echocardiogr. 2021;34(8):877–86.
• Mah K, Khoo NS, Martin BJ, Maruyama M, Alvarez S, Rebeyka IM, et al. Insights from 3D echocardiography in hypoplastic left heart syndrome patients undergoing TV repair. Pediatr Cardiol. 2022;43(4):735–43. This study contributes to the difficulty of valve repair in hypoplastic left heart syndrome.
Mah K, Khoo NS, Tham E, Yaskina M, Maruyama M, Martin BJ, et al. Tricuspid regurgitation in hypoplastic left heart syndrome: three-dimensional echocardiography provides additional information in describing jet location. J Am Soc Echocardiogr. 2021;34(5):529–36.
Tsang VT, Raja SG. Tricuspid valve repair in single ventricle: timing and techniques. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2012;15(1):61–8.
Herz C, Pace DF, Nam HH, Lasso A, Dinh P, Flynn M, et al. Segmentation of tricuspid valve leaflets from transthoracic 3D echocardiograms of children with hypoplastic left heart syndrome using deep learning. Front Cardiovasc Med. 2021;8: 735587.
Vogel M, Ho SY, Lincoln C, Anderson RH. Transthoracic three-dimensional echocardiography for the assessment of straddling tricuspid or mitral valves. Cardiol Young. 2000;10(6):603–9.
Wenink AC, Gittenberger-de Groot AC. Straddling mitral and tricuspid valves: morphologic differences and developmental backgrounds. Am J Cardiol. 1982;49(8):1959–71.
Rice MJ, Seward JB, Edwards WD, Hagler DJ, Danielson GK, Puga FJ, et al. Straddling atrioventricular valve: two-dimensional echocardiographic diagnosis, classification and surgical implications. Am J Cardiol. 1985;55(5):505–13.
Khoo NS, Young A, Occleshaw C, Cowan B, Zeng IS, Gentles TL. Assessments of right ventricular volume and function using three-dimensional echocardiography in older children and adults with congenital heart disease: comparison with cardiac magnetic resonance imaging. J Am Soc Echocardiogr. 2009;22(11):1279–88.
Tabry IF, McGoon DC, Danielson GK, Wallace RB, Tajik AJ, Seward JB. Surgical management of straddling atrioventricular valve. J Thorac Cardiovasc Surg. 1979;77(2):191–201.
Reddy VM, McElhinney DB, Brook MM, Silverman NH, Stanger P, Hanley FL. Repair of congenital tricuspid valve abnormalities with artificial chordae tendineae. Ann Thorac Surg. 1998;66(1):172–6.
van Son JA, Hambsch J, Mohr FW. Suspension of straddling tricuspid valve chordae into the appropriate ventricle. Ann Thorac Surg. 1998;65(3):850–2.
Pushparajah K, Barlow A, Tran VH, Miller OI, Zidere V, Vaidyanathan B, et al. A systematic three-dimensional echocardiographic approach to assist surgical planning in double outlet right ventricle. Echocardiography. 2013;30(2):234–8.
Tamin SS, Dillon J, Aizan K, Kadiman S, Latiff HA. An accessory mitral valve leaflet causing left ventricular outflow tract obstruction and associated with severe aortic incompetence. Echocardiography. 2012;29(2):E34–8.
Razzouk L, Applebaum RM, Okamura C, Saric M. The windsock syndrome: subpulmonic obstruction by membranous ventricular septal aneurysm in congenitally corrected transposition of great arteries. Echocardiography. 2013;30(8):E243–8.
Barrea C, Levasseur S, Roman K, Nii M, Coles JG, Williams WG, et al. Three-dimensional echocardiography improves the understanding of left atrioventricular valve morphology and function in atrioventricular septal defects undergoing patch augmentation. J Thorac Cardiovasc Surg. 2005;129(4):746–53.
Dall’Agata A, Cromme-Dijkhuis AH, Meijboom FJ, McGhie JS, Bol-Raap G, Nosir YF, et al. Three-dimensional echocardiography enhances the assessment of ventricular septal defect. Am J Cardiol. 1999;83(11):1576–9 (A8).
Butz T, Meissner A, Plehn G, Raute-Kreinsen U, Gummert J, Trappe HJ. Papillary fibroelastoma prolapsing into the left ventricular outflow tract: diagnosis using three-dimensional TEE. Herz. 2010;35(7):503–5.
Yang HS, Lee KS, Chaliki HP, Tazelaar HD, Lusk JL, Chandrasekaran K, et al. Anomalous insertion of the papillary muscle causing left ventricular outflow obstruction: visualization by real-time three-dimensional echocardiography. Eur J Echocardiogr. 2008;9(6):855–60.
Pisacane C, Pacileo G, Palladino MT, Iacono C, Santoro G, Sarubbi B, et al. Left ventricular outflow tract obstruction in the transposition of great arteries defined by transthoracic three-dimensional echocardiography. Echocardiography. 2001;18(8):695–700.
Kirklin JK, Castaneda AR, Keane JF, Fellows KE, Norwood WI. Surgical management of multiple ventricular septal defects. J Thorac Cardiovasc Surg. 1980;80(4):485–93.
Acar P, Abdel-Massih T, Douste-Blazy MY, Dulac Y, Bonhoeffer P, Sidi D. Assessment of muscular ventricular septal defect closure by transcatheter or surgical approach: a three-dimensional echocardiographic study. Eur J Echocardiogr. 2002;3(3):185–91.
Charakida M, Qureshi S, Simpson JM. 3D echocardiography for planning and guidance of interventional closure of VSD. JACC Cardiovasc Imaging. 2013;6(1):120–3.
Hadeed K, Hascoet S, Amadieu R, Karsenty C, Cuttone F, Leobon B, et al. Assessment of ventricular septal defect size and morphology by three-dimensional transthoracic echocardiography. J Am Soc Echocardiogr. 2016;29(8):777–85.
Sivakumar K, Singhi A, Pavithran S. Enface reconstruction of VSD on RV septal surface using real-time 3D echocardiography. JACC Cardiovasc Imaging. 2012;5(11):1176–80.
van den Bosch AE, Ten Harkel DJ, McGhie JS, Roos-Hesselink JW, Simoons ML, Bogers AJ, et al. Characterization of atrial septal defect assessed by real-time 3-dimensional echocardiography. J Am Soc Echocardiogr. 2006;19(6):815–21.
Cossor W, Cui VW, Roberson DA. Three-dimensional echocardiographic en face views of ventricular septal defects: feasibility, accuracy, imaging protocols and reference image collection. J Am Soc Echocardiogr. 2015;28(9):1020–9.
Mercer-Rosa L, Seliem MA, Fedec A, Rome J, Rychik J, Gaynor JW. Illustration of the additional value of real-time 3-dimensional echocardiography to conventional transthoracic and transesophageal 2-dimensional echocardiography in imaging muscular ventricular septal defects: does this have any impact on individual patient treatment? J Am Soc Echocardiogr. 2006;19(12):1511–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Pei-Ni Jone declares that she has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cardiology/CT Surgery
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 Video 1 Atria down view from the surgeon’s perspective of tricuspid valve dysplasia. There are two anterior (A) leaflets or a large anterior tricuspid valve leaflet with a cleft. The septal (S) and posterior (P) leaflets are tethered and not coapting with the anterior leaflets resulting in a noncoaptation site in this tricuspid valve dysplasia. LEAF, leaflet; POST, posterior; SEP, septal (AVI 3648 KB)
Rights and permissions
About this article
Cite this article
Jone, PN. Using 3D Echocardiography for Surgical Planning in Congenital Heart Disease. Curr Treat Options Peds 8, 129–140 (2022). https://doi.org/10.1007/s40746-022-00245-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40746-022-00245-y