Abstract
The corrosion and tribocorrosion of steel in chloride-acetate environment with CO2 and H2S depend on the H2S concentration and are determined by the nature of sulfide films on the surface. The corrosion and tribocorrosion of steel in the solution, saturated with carbon dioxide, is less than in the presence of hydrogen sulfide. Iron carbonate is unstable in solution and does not affect processes. The corrosion of steel in a solution with mixtures of CO2 and H2S slows down during first 200–300 h of exposure due to formation of iron sulfide film. Over time, the corrosion rate increases due to the transformation of sulfides and the defective surface layers formation. Wear resistance of steel in a solution with mixtures of CO2 and H2S decreases with an increase in the H2S concentration and the duration of exposure. Ulcerative corrosion was found on the friction surface. The protective effect of sulfide films was not detected. An increase of wear resistance at the beginning of friction in the chloride-acetate solution, saturated with H2S was found. A film of mackinawite is formed on the friction surface. Due to layered structure, it acts as a solid lubricant in the friction zone, which reduces the wear of steel and the coefficient of friction. Over time, mackinawite transforms into troilite and acts as an additional abrasive. Numerous ulcers were found on the surface as a result of corrosion and hydrogen-initiated cracking.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Corrosion-mechanical destruction of steels and alloys in environments containing hydrogen sulfide and carbon dioxide is one of the most dangerous types of structural material damage in the oil and gas production and processing industries. Carbon dioxide dissolved in mineralized environments accelerates the total corrosion of steels, and dissolved hydrogen sulfide initiates total and pitting corrosion with simultaneous hydrogenation of steels, which causes corrosion and corrosion-mechanical destruction [1,2,3,4,5,6]. In such systems, due to cavitation, erosion or contact of solid bodies, the problem of tribocorrosion can arise [7, 8]. Tribocorrosion of metals is a result of chemical and mechanical processes on the surface under frictional contact in corrosive environments. It includes synergistic interaction of mechanical, chemical and electrochemical processes. Secondary structures on the surface can protect it by acting as a lubricant, or accelerate the destruction of the material by acting as an additional abrasive.
A lot of attention is devoted to study the mechanism of corrosion-mechanical destruction of steels [7, 8], but the effect of hydrogen sulfide and carbon dioxide on the tribocorrosion characteristics of steels has not been sufficiently studied. Such environments are characterized by an increased corrosion rate, hydrogenation, and the formation of sulfides and carboxides, which can be both an additional abrasive and reduce the friction coefficient [9, 10].
Investigation the effect of hydrogen sulfide and carbon dioxide on the tribocorrosion of pipe steels, taking into account their corrosion behavior, the nature of surface films, and hydrogenation allow to understand the mechanisms corrosion-mechanical destruction of metals under frictional contact. The tribocorrosion properties of 17Mn1Si steel were studied under the influence of different concentrations of carbon dioxide and hydrogen sulfide in the chloride-acetate solution.
2 Materials and Methods
Ferrite-pearlite pipeline steel 17Mn1Si (mass. %: 0.17 C; 0.47 Si; 1.4 Mn; < 0.3 Cr; 0.3 Ni; < 0.3 Cu, Fe—the bal.) was studied. Before experiments, the specimens (d = 9 mm, l = 30 mm) were polished up to a roughness Rz = 2.5 μm, cleaned in deionized water, acetone and then dried.
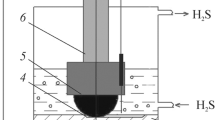
Corrosion and tribocorrosion tests of steel were developed in solution of 5% NaCl+0.5% CH3COOH (pH 2.7), which is the basis of the NACE solution—standard for studying corrosion and corrosion-mechanical destruction in hydrogen sulfide environments [11]. Experiments were performed in chloride-acetate solution, saturated by CO2 or H2S, and with their mixtures (CO2+100 mg/dm3 H2S and CO2+500 mg/dm3 H2S) at a temperature of 25 °C and a total pressure of 0.1 MPa. A gas mixture of hydrogen sulfide and carbon dioxide of appropriate partial pressures was passed through the solution. Experiments were started after 2 h of bubbling.
Hydrogen sulfide was obtained by hydrolysis of aluminum sulfide, which guarantees its purity. The concentration of hydrogen sulfide in the solution was determined by iodometrical method.
After corrosion test, a specimens were mechanically cleaned from corrosion products with a rubber bar and a solution of hydrochloric acid with urotropin. The corrosion rate Km (g m−2 h−1) of steel was determined gravimetrically as the mass loss per unit area within 1 h. A total durability of corrosion test was 720 h.
The concentration of hydrogen in steel was determined using the analyzer LECO DH-603.
Tribocorrosion tests of steel were developed in CO2/H2S environments under ball-on-flat configuration. The load on the counterbody (Al2O3 ball of Ø9 mm) was 10 N, the stroke length was 16 mm, the period of reciprocating movement was 6 s. Tests were performed in freshly prepared solutions and in solutions after exposition of specimens for 10, 20, and 30 days to form corrosion products of different composition and morphology. The duration of the friction test was 1200 s. A coefficient of friction was recorded using strain gauges, which were glued to the rod and connected to a computer via an analog–digital device. The average value of the friction coefficient during reciprocating movement was calculated as (µ + ǀ-µǀ)/2. Wear of material was determined using the electron microscope by the width of the friction track.
Metallographic analysis was performed using a scanning electron microscope EVO-40XVP (Carl Zeiss) with a system of microanalysis on energy-dispersive X-ray spectrometer INCA ENERGY 350. The diffractograms of surface films were obtained using X-ray diffractometer DRON-3.0 M with Cu-Kα and Co-Kα irradiation.
Research results were averaged based on 3–5 experiments.
3 Results and their Discussion
The effect of hydrogen sulfide concentration on the corrosion rate of 17Mn1Si steel in a solution of 5% NaCl + 0.5% CH3COOH + CO2/H2S was studied.
The corrosion rate in the solution, saturated with carbon dioxide, is less than in the presence of hydrogen sulfide, but it increases over time (Fig. 1). Iron carbonate is unstable in a chloride-acetate solution with pH2.7 and carbonate films does not protect the surface from corrosion. Addition from 100 to 2800 mg/dm3 H2S to the solution increases corrosion rate from ~ 3 to ~ 6 times after 72 h exposure. Corrosion slows down during the first 240–300 h of test, due to formation of sulfide films on the surface. The corrosion rate decreases twice during 720 h in a solution with 100 mg/dm3 H2S. An increase of H2S concentration from 500 to 2800 mg/dm3 accelerates the corrosion from 1.5 to 2.6 times after 720 h of exposure (Fig. 1). At the beginning of exposure, a dense film of mackinawite is formed on the steel surface, which inhibits corrosion. As the duration of exposure increases, the rate of corrosion increases due to the transformation of mackinawite to troilite and the formation of defective surface layers [10, 12].
Reactions of sulfide formation are accompanied by the release of hydrogen. Hydrogenation of steel causes specific damage—hydrogen-initiated cracking and blistering, which deteriorates its physical, mechanical and operational properties [15, 16]. Concentration of absorbed hydrogen in steel after exposure in the solution of 5% NaCl + 0.5% CH3COOH, saturated by CO2 is insignificant (~ 0.5 ppm). Hydrogen absorption intensifies in presence of hydrogen sulfide in the environment. Steel absorbs from ~ 6 to ~ 17 ppm of hydrogen with an increase in H2S concentration from 100 mg/dm3 to saturation (Table 1).
Ulcerative corrosion was found on the steel surface after after 720 h exposure in a solution with carbon dioxide and hydrogen sulfide [12]. Ulcers of a rounded shape with a depth of up to 15 μm are formed on the steel surface in a solution saturated with carbon dioxide, the cracks formation was not recorded (Fig. 2a). In a solution with 100 mg/dm3 H2S, corrosion damage slightly increases, but there are no signs of hydrogen embrittlement (Fig. 2b). The depth of ulcers increases in a solution with 500 mg/dm3 H2S, the cracks appear at the bottom as a result of hydrogen absorption (Fig. 2c). Numerous subsurface cracks are formed in the saturated by hydrogen sulfide solution (Fig. 2d). This is a sign of hydrogen-initiated cracking of steel.
The sulfide film formed on the steel surface at the beginning of exposure in a chloride-acetate solution containing CO2/H2S contributes to reducing the corrosion rate (Fig. 1). It can be expected that this film will have a positive effect also under conditions of tribocorrosion. The wear resistance of 17Mn1Si steel in a chloride-acetate solution, saturated with CO2, H2S, and their mixtures, was studied. In a freshly prepared solution saturated with CO2, the width of the friction track after 20 min is 195 μm, and the coefficient of friction is 0.094 (Table 2). The friction surface does not contain ulcerated damage, only traces of abrasion and plastic deformation of the metal (Fig. 3a). In a chloride-acetate solution with pH2.7, iron carbonate is unstable and does not affect the friction. Wear resistance decreases after long term exposure steel in the solution as a result of corrosion. The corrosion rate in the solution, saturated by CO2, is less than in the presence of H2S. Abrasive particles are formed as a result of ulcerative corrosion of steel and can accelerate wear (Fig. 3b). Wear resistance decreases after long term exposure of steel in the solution. The width of the wear track increases almost linearly over time and after 30 days is 446 μm, and the friction coefficient doubles (Table 2).
The addition of 100 mg/dm3 of H2S in the chloride-acetate solution with CO2 leads to an increase in wear by 70%, and the coefficient of friction by ~ 2 times after 20 min of the exposition (Table 2). In solution with 500 mg/dm3 of H2S the friction coefficient is ~ 3 times higher than without H2S. Ulcerative damage was found on the surface after the friction in the solutions with H2S (Fig. 4). When H2S concentration increases, pH of the solution decreases and corrosion and hydrogenation of steel accelerates (Fig. 1; Table 1). Wear intensifies due to ulcerative corrosion, and hydrogen embrittlement of the friction surface (Fig. 2; Table 2). The sulfides do not form a dense protect film due to continuous abrasion of the surface layer. Wear increases linearly when exposure time increases: after 30 days width of the wear track is ~ 3 times higher than at the beginning of exposure (Table 2).
Wear resistance of steel in a solution, saturated by hydrogen sulfide at the beginning of exposure is better than in an environment with mixtures of CO2 and H2S. The friction coefficient is 0.09 (Fig. 5), and width of the wear track is 270 μm (Table 2). Plastically deformed material without cracks and traces of a powdery substance was found on the friction surface (Fig. 6a).
After 10 h exposure of steel in the solution, the friction coefficient increases by ~ 4 times. (Fig. 5). With an increase in the duration of exposure, corrosion and hydrogenation of steel accelerates. The wear resistance of the surface disimproves due to the cracking and ulcers formation. On the other hand, the formation of a juvenile surface during friction intensifies corrosion processes. The width of the wear track and the friction coefficient increase (Table 2) as a result of abrasive wear [14].
Mackinawite-type sulfides Fex+1Sx are formed on the steel surface at the beginning of exposure in hydrogen sulfide environment [10]. In a solution with CO2 and 100–500 mg/dm3 H2S, sulfides do not form a dense protect film on the friction surface due to permanent abrasion of the surface and insufficient concentration of hydrogen sulfide. At the same time, the formation of sulfides is accompanied by the hydrogenation of steel. The strength and ductility of the surface layers of steel decreases [12], ulcerative corrosion is developed on the surface, which facilitates their destruction during friction.
A sufficiently thick mackinawite film is formed on the surface in solution, saturated by hydrogen sulfide (Fig. 7a; Table 3). The photo [13] placed in the lower right corners of the figure illustrates the layer structure of mackinawite. Multilayer flake-shaped crystals of mackinawite can act as a solid lubricant in the friction zone, and improve the wear resistance of steel [17,18,19].
Mackinawite is unstable and transforms into more stable sulfides, primarily troilite. Over time, a sulfide film consists of a mixture of mackinawite and troilite. The density of such a film is less than that of mackinawite [10, 12]. The Mohs hardness of mackinawite is 2.5, and that of troilite is twice as high (4.8). The needle-like crystals of troilite FeS were found on the surface of 17Mn1Si steel after tests in a chloride-acetate solution, saturated with H2S (Fig. 7b; Table 3). Therefore, the crystals of troilite in the friction zone can play the role of an additional abrasive and deteriorate the wear resistance of steel. When the duration of the steel exposure increases, the concentration of mackinawite in the solution decreases, and that of troilite increases. The nature of friction changes from tribochemical to abrasive, and wear of friction surface increases [7, 14].
Consequently, the corrosion and tribocorrosion of 17Mn1Si steel in chloride-acetate environments containing carbon dioxide and hydrogen sulfide depends on the hydrogen sulfide concentration and is determined by the nature of sulfide films formed on the surface.
The corrosion of steel in a solution, saturated by carbon dioxide, is less than in the presence of hydrogen sulfide. The corrosion rate increases with an increase in hydrogen sulfide concentration and exposure time. Dence film of mackinawite slows down corrosion of steel at the beginning of exposure. Over time mackinawite transforms into troilite, the loose films of which do not perform the function of corrosion protection. Formation of iron sulfides is accompanied by the hydrogen release. Steel absorbs from ~ 5 to ~ 16 ppm of hydrogen with an increase in H2S concentration from 100 mg/dm3 to saturation.
Ulcerative corrosion was found on the steel surface after exposure in a solution with carbon dioxide and hydrogen sulfide. Ulcers without a cracks formation are observed in a solution saturated with carbon dioxide. A depth of ulcers increases, and a sign of hydrogen-initiated cracking of steel appears in a solutions with ≥ 500 mg/dm3 of hydrogen sulfide.
Sulfides do not form a dence film, which protects against wear under friction in solution of 5% NaCl+0.5% CH3COOH+CO2 with 100 and 500 mg/dm3 of hydrogen sulfide. Film is continuously erased during friction, and hydrogenation of surface layers reduces wear resistance. Tribocorrosion is the result of tribochemical and abrasive wear.
In a solution saturated with hydrogen sulfide, a denser film of mackinawite can be formed on the friction surface. Due to a layered structure, the film can act as a solid lubricant in the friction zone, which reduces wear of steel and the coefficient of friction. The obtained results are correlated with friction parameters in a chloride-acetate solution containing only hydrogen sulfide [9]. Over time, mackinawite transforms into troilite. Hard needle-like crystals of troilite play the role of an abrasive during friction, the wear resistance of steel is significantly reduced.
4 Conclusions
The corrosion and tribocorrosion of steel in chloride-acetate environment with CO2 and H2S depend on the H2S concentration and are determined by the nature of sulfide films on the surface.
The corrosion and tribocorrosion of steel in the solution, saturated by CO2, is less than in the presence of H2S. The protective effect of surface film was not detected.
The corrosion of steel in a solution with mixtures of CO2 and H2S slows down during first 200–300 h of exposure due to formation of iron sulfide film. Over time, the corrosion rate increases due to the transformation of sulfides and the defective surface layers formation.
Wear resistance of steel in a solution with mixtures of CO2 and H2S decreases with an increase in the H2S concentration and the duration of exposure. Ulcerative corrosion was found on the friction surface. The protective effect of sulfide films was not detected.
An increase of wear resistance at the beginning of friction in the chloride-acetate solution, saturated with H2S was found. A film of mackinawite is formed on the friction surface. Due to layered structure, it acts as a solid lubricant in the friction zone, which reduces the wear of steel and the coefficient of friction. Over time, mackinawite transforms into troilite and acts as an additional abrasive. Numerous ulcers were found on the surface as a result of corrosion and hydrogen-initiated cracking.
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Sui P, Sun J (2018) Effect of temperature and pressure on corrosion behaviour of X65 carbon steel in water-saturated CO2 transport environments mixed with H2S. Int J Greenh Gas Control 73:60–69. https://doi.org/10.1016/j.ijggc.2018.04.003
Ren Ch, Liu D (2005) Corrosion behavior of oil sulfide in simulant solution with hydrogen sulfide and carbon dioxide. Mater Chem Phys 93:305–309. https://doi.org/10.1016/j.matchemphys.2005.03.010
Zhao XH, Feng YR (2018) Electrochemical Corrosion Behavior of 15Cr-6Ni-2Mo Stainless Steel with/without stress under the coexistence of CO2 and H2S. Int J Electrochem Sci 13:6296–6309. https://doi.org/10.20964/2018.07.59
Plennevaux C, Kittel J (2013) Contribution of CO2 on hydrogen evolution and permeation in low alloy steel exposed to H2S environment./ Electrochem. Commun 26:17–20. https://doi.org/10.1016/j.elecom.2012.10.010
Zhang GA, Zeng Y, Guo XP (2012) Electrochemical corrosion behavior of carbon steel under dynamic high pressure H2S/CO2 environment. Corros Sci 65:37–47. https://doi.org/10.1016/j.corsci.2012.08.007
Sun W, Nesic S (2006) Kinetics of iron sulfide and mixed iron sulfide-carbonate scale precipitation in CO2/H2S corrosion. Corros., P.06644\1–06644\26.
Landolt D, Mischler S (2011) Tribocorrosion of Passive Metals and Coatings, ISBN:978-1-84569-966-6. Woodhead Publishing, Cambridge
Landolt D (2006) Electrochemical and materials aspects of tribocorrosion systems. J Phys 39(S01):3121–3127. https://doi.org/10.1088/0022-3727/39/15/S01
Khoma M, Vynar V, Vasyliv Ch (2022) Tribo corrosion of 17Mn1Si Steel in Chloride Acetate Environments at the Different Concentrations of Hydrogen Sulfide. J. Bio- Tribo-Corros 8:53. https://doi.org/10.1007/s40735-022-00655-3
Khoma M, Vasyliv Ch (2021) Influence of the hydrogen sulfide concentration on the corrosion and hydrogenation of pipe steels (A survey). Mat Sci 57:308–318. https://doi.org/10.1007/s11003-021-00546-x
NACE Standard SP0110–2010. Wet Gas Internal Corrosion Direct Assessment Methodology for Pipelines. – Houston, TX: NACE, 2010.
Khoma M, Pokhmurskii V (2023) Corrosion-mechanical properties and susceptibility to hydrogenation of pipe steel in the presence of carbon oxide and hydrogen sulfide in environment. – Phys.-chem. Mech Mat 2:80–87
Wen X, Bai P (2018) Review of recent progress in the study of corrosion products of steels in a hydrogen sulphide environment. Corros Sci 139:124–140. https://doi.org/10.1016/j.corsci.2018.05.002
Du S, Hamdi M (2020) Experimental and FEM analysis of mar behavior on amorphous polymers. Wear. 444:203155. https://doi.org/10.1016/j.wear.2019.203155
Balitskii O, Kolesnikov V (2020) Hydrogen effect on the high-nickel surface steel properties during machining and wear with lubricants. Arch Mat Sci Eng 104(2):49–57. https://doi.org/10.5604/01.3001.0014.4894
Balitskii O, Kolesnikov V (2019) Wear resistance of hydrogenated high nitrogen steel at dry and solid state lubricants assistant friction // Arch. Mat Sci Eng 98(2):57–67. https://doi.org/10.5604/01.3001.0013.4607
Molero G, etc. (2019) Experimental and numerical determination of adhesive strength in semi-rigid multi-layer polymeric systems. Polym Test 75:85–92. https://doi.org/10.1016/j.polymertesting.2019.01.012
Zhang N, Zhuang D-M (2000) Microstructure of iron sulfide layer as solid lubrication coating produced by low-temperature ion sulfurization. Surf Coat Technol 132:1–5. https://doi.org/10.1016/S0257-8972(99)00661-1
Wang H-D, Xu B-X (2005) Mild corrosion behavior on sulphurized steel surface during friction. Appl Surf Sci 252(5):1704–1709. https://doi.org/10.1016/j.apsusc.2005.03.159
Funding
National Academy of Sciences of Ukraine, scientific project № III-11-21.
Author information
Authors and Affiliations
Contributions
MK is the project manager. VV prepared the figures 3-7. MC prepared the tables 1-3. CV wrote the main manuscript text. NR prepared the figures 1-2 All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Consent to Publish
This work has not been published previously. This article is not under consideration for publication elsewhere. If accepted, the article will not be published elsewhere in the same form, in any language, without the written consent of the publisher.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khoma, M., Vynar, V., Chuchman, M. et al. Tribocorrosion of Steel in Chloride-Acetate Environment at the Different Concentrations of Hydrogen Sulfide and Carbon Dioxide. J Bio Tribo Corros 9, 58 (2023). https://doi.org/10.1007/s40735-023-00781-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-023-00781-6