Abstract
The inhibitive ability of Suphtrim drug on aluminium 6063 alloy was examined in the presence of 0.5 M H2SO4 solution employing three-electrode system. The corrosion rate (Cr) and corrosion current density (jcorr) decrease with increase in the volume concentration of the inhibitive drug. The reduction in the values of Cr and jcorr is an indication that the inhibitive drug molecules adsorbed on the metal surface, forming slight deposits which minimize the attack of hydrogen and sulphide ion on the active sites of the metal. The inhibitive suphtrim drug (ISD), at maximum test volume concentration of 20 ml offered inhibition efficiency of 52.55%. The corrosion rate of the 20 ml suphtrim drug inhibited sample was 0.2021 mm/year, while the as-received or control sample possessed corrosion rate of 0.5933 mm/year. The close values of Ecorr and overlapping nature of the polarization curves indicated that ISD acted as a mixed-type inhibitor. Adsorption of ISD molecules on the aluminium alloy was found to largely follow Langmuir adsorption isotherm (LAI) with correlation regression coefficient (R2) value of 0.9144, and moderately follow Freundlich adsorption isotherm (FAI) with an R2 value of 0.7395. The close values of R2 to unity showed that the inhibitor significantly adsorbed on the metal. The Morphology study via SEM micrograph affirmed the adsorption of ISD molecules on the surface of the aluminium alloy. These results showed that ISD compares favourably with most exiting drug inhibitors.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The deterioration of aluminium and aluminium alloys via corrosion is still a concern to manufacturing industries, especially in marine, aerospace, automotive, food packing and photoelectric material producing industries [1, 2]. Although, aluminium and its alloy offer higher degrees of resistance to corrosion relative to mild steel in the aggressive environment due to the formation of oxide layers on their surfaces over time. However, these oxide layers are eventually destroyed by the environmental contaminants on prolonged exposure [3,4,5]. Constant or even occasional exposure of machinery components and other mechanical devices made of aluminium to sulphide, hydrogen and chloride ion could be detrimental to the life span of the material, due to pitting initiation and subsequent material failure [6,7,8]. The danger with the pitting of materials is that it might not be noticed until failure occurs, particularly if the growth of the pit is inward [9, 10]. Therefore, continuous examination and re-examination of inhibitive devices that can effectively protect commonly used engineering materials like aluminium and its alloys from corrosion and other forms of degradation have been the focus of researchers [11,12,13].
Aluminium and aluminium alloy degradation prevention, protection and maintenance have continued to be a subject of global interest due to their numerous engineering applications and some inherent superior properties they exhibit compared to varieties of industrial materials, which make them one of the indispensable and widely used materials in the world of technology [14, 15]. However, depreciation in the properties of aluminium and its alloys have been broadly linked to contact with saline, acidic and alkaline media by copious studies [5, 16,17,18,19], and as a result, suitable and durable corrosion protection measures should be appropriately utilized. Good numbers of investigation have acknowledged the use inhibitors as an effective corrosion reduction measure in corrosive media [20,21,22,23], although some inhibitors were found to have a toxic impact on the environment [24, 25]. Thus, it has become a necessity to examine the constituent and eco-friendliness of an inhibitor before usage. Interestingly, several chemical inhibitors have shown great inhibitive performance, with moderate toxicity, nevertheless, many of them were found to be more expensive compared to organic or natural inhibitors. The use of organic inhibitors for corrosion protection of metals has grown in leaps and bounds in recent years due to their eco-friendly nature [26,27,28].
More so, inhibitive organic compounds possess the ability to adsorb on the metal substrate surface and thereby forming a barrier film against the attack by corrosive species in the medium [29]. The corrosion inhibitive tendencies of organic inhibitors are also a function of the properties of corrosive media, the surface quality of metal and the constituent of the inhibitor itself [30, 31]. Therefore, those factors demand the cautious choice of inhibitors for metals. A lot of inhibitive antibiotic drugs have offered a significant improvement in the corrosion resistance of metals. A recently used expired Ibuprofen drug on mild steel in 0.5 M H2SO4 exhibited maximum inhibition efficiency of 63.25%. The sample inhibited with 20 ml of Ibuprofen drug had the maximum inhibition efficiency, and it possessed the corrosion rate of 3.8838 mm/year while the as-received steel possessed 10.567 mm/year, which indicated that, drastic enhancement in corrosion resistance, was achieved [32]. The examination of the inhibitive action of expired Ambroxol drug on the corrosion of mild steel in 1 M HCl showed that inhibition efficiency increases with increasing the concentration of the Ambroxol drug inhibitor [33]. Hexamine is an antibiotic that has also been widely used for corrosion inhibition. Hexamine was able to offer the corrosion inhibitive efficiency of 52.9% after 288 h of exposure of cast iron pipes to an aqueous salt solution of 2% NaCl [34]. In a similar manner, a reasonable inhibition efficiency of 47.1% was observed with the use of 1.2 g of hexamine for the inhibition of Aluminium 6063 in 3.65% NaCl solution [35]. In this study, the inhibition performance of Suphtrim drug was examined on Aluminium 6063 alloy in the presence 0.5 M H2SO4 solution using potentiodynamic polarization technique, computational comparisons and SEM micrographs. The choice of inhibitive suphtrim drug (ISD) solution, whose composition is sulfamethoxazole and trimethoprim, is as a result of the fact that it belongs to the sulfonamide antimicrobial class of medicines. Sulfonamides have been found efficient and nontoxic inhibitors for the corrosion of mild steel in acidic medium, and their inhibition performance was attributed to spontaneous adsorption which occurs via physical adsorption mechanism [36].
2 Experimental Procedures
2.1 Sample Preparation
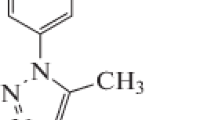
The percentage weight of aluminium 6063 alloy constituents is shown in Table 1. The aluminium alloy was prepared into coupons of dimension (15 × 15 × 2) mm, and cleaned with emery paper of various degrees. The coupons were then rinsed thoroughly in distilled water so as wash away the rusty particles, which sticks on the metal samples due to descaling effect of emery paper. The 0.5 M of H2SO4 solution, which is the simulated corrosive environment, was prepared using doubled distilled water. Every 50 ml of the Suphtrim inhibitive drug solution contains 200 mg of sulphamethoxazole and 40 mg/5 ml of trimethoprim. The structural formula of sulphamethoxazole and trimethoprim is shown in Fig. 1.
2.2 Potentiodynamic Polarization Experiment
A three-electrode cell was used with the aluminium alloy as working electrode, saturated calomel electrode as the reference electrode, and graphite rod as the counter electrode. Copper wire was fused to each of the working electrodes and then implanted in epoxy resin. The epoxy resins were opened underneath to allow the metal and medium contact. The polarization measurements were carried out using Autolab PGSTAT 101 Metrohm potentiostat/galvanostat with NOVA software of version 2.1.2 in connection with the three-electrode cell in 0.5 M H2SO4 solution, at room temperature. The immersion lasted for 10 min, and the steady-state potential/open-circuit potential (OCP) was attained. Polarization curves were recorded from − 1.5 V to + 1.5 V with a scan rate of 0.005 V/s. Each of the experiments was carried out four different times in different 200 ml of 0.5 M H2SO4 solution, varying the volume of suphtrim inhibitive drug. This was done to ensure reproducibility. The corrosion potential (Ecorr), polarization resistance (Pr) and corrosion current density (jcorr) data were estimated from the Tafel plots.
3 Results and Discussion
3.1 Potentiodynamic Polarization Measurement
The Potentiodynamic polarization curves and data obtained via extrapolation of Tafel curves are shown in Table 2 and Fig. 2. It is evident in Table 2 that the corrosion rate (Cr) and corrosion current density (jcorr) of the aluminium alloy decreases with increase in volume concentration of the inhibitor. The as-received or control sample exhibited the highest corrosion rate and corrosion current density of 0.5933 mm/year and 0.529 μA/cm2, respectively. These values suggest that the corrosive medium had more damage on the active sites of the metal with the absence of inhibitor. The progressive corrosion rate and corrosion current density reduction effect observed as a result of increasing inhibitor volume could therefore be ascribed to the ability of the molecules of the inhibitor to adsorb on the metal and thereby blocking anodic and cathodic sites of the metal [39]. The increase in polarization resistance (Pr) with increasing inhibitor volume concentration was also indicated that the inhibitor actively offered some degrees of resistance to the polarization of the metal in the acidic medium. The inhibitive performance of Suphtrim drug could be traceable to the presence of sulphamethoxazole, which has a large number of functional adsorption centres such as –SO2–NH– group, –NH2 group, O and N heteroatoms and aromatic rings [40]. The corrosion reduction effect could also be attributed to the presence of trimethoprim which can form complexes with metals through the nitrogen of pyrimidinyl ring, and these complexes could eventually act as inhibitive substances [41]. It is also worthy of note that the Ecorr values are very close, more so, the cathodic and anodic branches of the uninhibited and inhibited were observed to overlap as shown in Fig. 2, which indicated the inhibitive drug acted as a mixed-type inhibitor in 0.5 M H2SO4 at room temperature [42,43,44].
3.2 Open-Circuit Potential (OCP) Measurement
The open-circuit potential represents the working electrode potential relative to the reference electrode when there is none existence of potential or current in the cell. The change in the OCP results is polarization, which is due to the current flow across the electrode/electrolyte interface [45]. Figure 3 shows the OCP versus exposure time for Suphtrim drug inhibited and uninhibited aluminium alloy in 0.5 M H2SO4. It could be seen that the potential of the uninhibited alloy was constantly at -0.3 V. With the addition of 5 ml of Suphtrim drug inhibitor, the potential dropped to -0.5 V. Although the potential changes for some periods, but became constant between 75 and 120 s. The initial potentials of 10, 15 and 20 ml Suphtrim drug inhibited samples were observed to be -0.15, -0.1 and -0.3 V. Continuous drop in potential was observed for these samples. However, the voltage of 10 ml Suphtrim drug inhibited sample became slightly stable between 155 and 120 s at a voltage of about -0.52 V, 15 ml Suphtrim drug inhibited sample became more stable between 75 and 120 s at a voltage of about -0.55 V, while 20 ml Suphtrim drug inhibited sample became more stable between 60 and 120 s at a voltage of approximately -0.6 V. It could, therefore, be concluded that steady-state potential state was attained within these periods of constant or near-constant voltages [46, 47].
3.3 Mechanism of Inhibition Efficiency and Adsorption Study
Table 3 shows the values of surface coverage (θ) and the inhibition efficiency (IE), which were calculated from Eqs. (1 and 2), respectively [48,49,50]. Increase in the surface coverage and inhibition efficiency as the volume concentration of ISD increases indicated that the inhibitive drug adsorbs on the surface of the metal [51], and this suggests that, at lower volume concentration of ISD, the deteriorating effect of corrosive ions in 0.5 M H2SO4 on the metal will be higher.
where jcorr = inhibited corrosion current densities and jocorr = uninhibited corrosion current density.
To have a comprehensive understanding of adsorption mechanism of the ISD, C/θ, Log θ and Log C were calculated as shown in Table 3. The relationship between C/θ and C (volume concentration) was used to obtain Langmuir adsorption isotherm and a linear correlation, while Log θ and Log C was used to obtain Freundlich adsorption isotherm and a linear correlation. Equations 3 and 4, respectively, indicate the Langmuir and Freundlich isotherm adsorption act [52, 53], which provides a comprehensive understanding of the synergy between inhibitor and metal, and the actions between the metallic complexes in the coverage region. The Langmuir and Freundlich isothermal plots in Figs. 4 and 5, respectively, indicate the linear relationship that take place as result of inhibitor concentration increment. The plots revealed that the inhibitor continuously adsorb on the surface of aluminium alloy. The values of R2 for Langmuir and Freundlich absorption isothermal were 0.9144 and 0.7395, respectively. This R2 values are in close range the work ref.[54, 55]. These R2 values revealed that the corrosion protection of aluminium alloy by ISD had been accomplished their closeness to unity. However, the adsorption was more Langmuir isotherm favourable than Freundlich.
The Langmuir adsorption isotherm law,
The Freundlich adsorption isotherm law,
where C is Concentration of the corrosion inhibitor, θ is degree of surface coverage and k = adsorption equilibrium constant.
3.4 SEM micrograph examination
The SEM micrographs of Suphtrim drug inhibited samples are shown in Fig. 6. It was observed form the SEM micrographs that the inhibitive drug adsorbed more on the sample inhibited with 20 ml of Suphtrim drug than the one with 10 ml. This could possibly be the reason the sample possesses better corrosion resistance performance characteristic than other samples. The micrographs unveiled minimal deterioration of the metal surface, as a result of the chemical interaction between the inhibitor and the ions in the corrosive medium. More pits were observed in Fig. 6a, indicating the possibility of more surface and internal deteriorations. The presence of a few flakes on the micrograph suggests the presence of corrosion products such as oxides and hydroxide of Aluminium [56]. The minimal breakdown of the passive film observed with Fig. 6b revealed that thin layer covering was formed on the metal by the synergetic effect of sulphamethoxazole and trimethoprim molecules in Suphtrim drug, which reduces the ingression of the sulphide ion. More so, the combined effect of the heteroatoms of sulphamethoxazole and trimethoprim enables its adsorption on the interface of aluminium via physical and chemical reaction mechanism [36, 57].
4 Conclusions
-
The corrosion experiment carried out in 0.5 M H2SO4 solution at room temperature showed that the ISD enhanced the corrosion resistance of the aluminium alloy. The efficiency of inhibition was found to increase with an increasing volume concentration of inhibitor.
-
The ISD, at maximum test volume concentration of 20 ml offered inhibition efficiency of 52.55%, and the corrosion rate of the inhibited sample was 0.2021 mm/year while the as- received or control sample possessed corrosion rate of 0.5933 mm/year. The difference in corrosion rate indicated that the sulphamethoxazole and trimethoprim constituent of the inhibitor minimized deterioration of the metal active sites.
-
The close values of Ecorr and overlapping nature of the polarization curves indicated that ISD acted as a mixed-type inhibitor.
-
Adsorption of ISD molecules on the Aluminium alloy was found to largely follow Langmuir adsorption isotherm with correlation regression coefficient R2 value of 0.9144 and moderately follow Freundlich adsorption isotherm with an R2 value of 0.7395. The close values of R2 to unity showed that the inhibitor adsorbed on the metal.
-
Morphology study via SEM micrograph affirmed the adsorption of ISD molecules on the surface of aluminium alloy. This was ascribed to the synergetic effect of the heteroatoms of sulphamethoxazole and trimethoprim, which enables adsorption of ISD molecules on the interface of aluminium via physical and chemical reaction mechanism.
References
Rathod KN, Vashi RT (2016) Inhibition effect of ammonium dichromate on the corrosion of aluminium in phosphoric acid. IJCS 4(1):37–42
Wahid MA, Siddiquee AN, Khan ZA (2019) Aluminum alloys in marine construction: characteristics, application, and problems from a fabrication viewpoint. Mar Syst Ocean Technol. 15:70–80
Wu G, Dash K, Galano ML, O’Reilly KA (2019) Oxidation studies of Al alloys: Part II Al-Mg alloy. Corros Sci 155:97–108
Fayomi OSI, Abdulwahab M, Popoola AP, Asuke F (2015) ‘Corrosion resistance of AA6063-Type Al-Mg-Si alloy by silicon carbide in sodium chloride solution for marine application. J Mar Sci Appl 14(4):459–462
Krishnaveni K, Ravichandran J (2014) Effect of aqueous extract of leaves of Morinda tinctoria on corrosion inhibition of aluminium surface in HCl medium. Trans Nonferrous Met Soc China 24(8):2704–2712
Zhang S, Zhang T, He Y, Feng Y, Du X, Ma B, Zhang T (2019) Effect of coastal atmospheric corrosion on fatigue properties of 2024–T4 aluminum alloy structures. J Alloy Compd 802:511–521
Mrema E, Itoh Y, Kaneko A, Hirohata M (2018) Corrosion of aluminium alloy A6061–T6 members embedded in alkaline materials. Corros Eng, Sci Technol 53(2):102–113
Li S, Church BC (2018) Effects of sulfate and nitrate anions on aluminum corrosion in slightly alkaline solution. Appl Surf Sci 440:861–872
Melchers RE (2020) A review of trends for corrosion loss and pit depth in longer-term exposures. Corros Mater Degrad 1(1):42–58
Soltani Asadi Z, Melchers RE (2018) Long-term external pitting and corrosion of buried cast iron water pipes. Corros Eng, Sci Technol 53(2):93–101
Liu Y, Visser P, Zhou X, Lyon SB, Hashimoto T, Gholinia A, Thompson GE, Smyth G, Gibbon SR, Graham D, Mol JM (2016) An investigation of the corrosion inhibitive layers generated from lithium oxalate-containing organic coating on AA2024-T3 aluminium alloy. Surf Interface Anal 48(8):798–803
Xhanari K, Finsgar M, Hrncic MK, Maver U, Knez Z, Seiti B (2017) Green corrosion inhibitors for aluminium and its alloys: a review. RSC Adv 7(44):27299–27330
Xhanari K, Finsgar M (2016) Organic corrosion inhibitors for aluminium and its alloys in acid solutions: a review. RSC Adv 6(67):62833–62857
El-Dahan HA, Soror TY, El-Sherif RM (2005) Studies on the inhibition of aluminum dissolution by hexamine–halide blends: Part I. Weight loss, open circuit potential and polarization measurements. Mater Chem Phys 89(2–3):260–267
Fayomi OSI, Akande IG, Popoola API (2018) Corrosion protection effect of chitosan on the performance characteristics of A6063 alloy. J BioTribo Corros 4(4):73
El-Deeb MM, Alshammari HM, Abdel-Azeim S (2017) Effect of ortho-substituted aniline on the corrosion protection of aluminum in 2 mol/L H2SO4 solution. Can J Chem 95(5):612–619
Halambek J, Berković K, Vorkapić-Furač J. Laurus nobilis L. ‘Oil as green corrosion inhibitor for aluminium and AA5754 aluminium alloy in 3% NaCl solution’. Materials Chemistry and Physics. 2013 ;137(3):788–795.
Abdallah YM. Electrochemical studies of phenyl sulphonyl ethanone derivatives compounds on corrosion of aluminum in 0.5 M H2SO4 solutions. Journal of Molecular Liquids. 2016; 219:709–719.
Moore KL, Sykes JM, Hogg SC, Grant PS (2008) Pitting corrosion of spray formed Al–Li–Mg alloys. Corros Sci 50(11):3221–3226
Umoren SA, Eduok UM (2016) ‘Application of carbohydrate polymers as corrosion inhibitors for metal substrates in different media’: a review. Carbohyd Polym 140:314–341
Akin M, Nalbantoglu S, Cuhadar O, Uzun D, Saki N (2015) Juglans regia L. Extract as green inhibitor for stainless steel and aluminium in acidic media. Res Chem Intermediates 41(2):899–912
El Moll H, Alenezi KM, Abdel-Latif MK, Halouani H, El-Deeb MM (2020) Water-soluble calix [4] arenes as inhibitors for the corrosion of aluminium in 2 M H2SO4 solution. Int J Electrochem Sci 15:252–264
Wang Y, Chen Y, Zhao Y, Zhao D, Zhong Y, Qi F, Liu X (2017) A ‘Reinforced organic-inorganic layer generated on surface of aluminium alloy by hybrid inhibitors’. J Mol Liq 225:510–516
Raja PB, Ismail M, Ghoreishiamiri S, Mirza J, Ismail MC, Kakooei S, Rahim AA (2016) Reviews on corrosion inhibitors: a short view. Chem Eng Commun 203(9):1145–1156
Winkler DA, Breedon M, White P, Hughes AE, Sapper ED, Cole I (2016) Using high throughput experimental data and in silicon models to discover alternatives to toxic chromate corrosion inhibitors. Corros Sci 106:229–235
Ryl J, Brodowski M, Kowalski M, Lipinska W, Niedzialkowski P, Wysocka J (2019) Corrosion inhibition mechanism and efficiency differentiation of dihydroxybenzene isomers towards aluminum alloy 5754 in alkaline media. Materials 12(19):3067
Al-Amiery AA, Kassim FA, Kadhum AA, Mohamad AB (2016) Synthesis and characterization of a novel eco-friendly corrosion inhibition for mild steel in 1 M hydrochloric acid. Sci Rep 6(1):1–3
Kumar R, Yadav OS, Singh G (2017) Electrochemical and surface characterization of a new eco-friendly corrosion inhibitor for mild steel in acidic media: a cumulative study. J Mol Liq 237:413–427
Umoren SA, Obot IB, Ebenso EE, Okafor PC, Ogbobe O, Oguzie EE (2006) ‘Gum arabic as a potential corrosion inhibitor for aluminium in alkaline medium and its adsorption characteristics. Anti-Corros Methods Mater 53(5):277–282
Fayomi OSI, Akande IG, Oluwole OO, Daramola D (2018) Effect of water-soluble chitosan on the electrochemical corrosion behaviour of mild steel. Chem Data Collect 17–18:321–326
Elgahawi H, Gobara M, Baraka A, Elthalabawy W (2017) ‘Eco-friendly corrosion inhibition of AA2024 in 3.5% NaCl using the extract of Linum usitatissimum seeds. J Bio-Tribo Corros 3(4):55
Fajobi MA, Fayomi OSI, Akande IG, Odunlami OA (2019) Inhibitive performance of ibuprofen drug on mild steel in 05 M of H2SO4 acid. J Bio-Tribo Corros 5(3):79
Geethamani P, Narmatha M, Dhanalakshmi R, Aejitha S, Kasthuri PK (2019) Corrosion inhibition and adsorption properties of mild steel in 1 M hydrochloric acid medium by expired ambroxol drug. J Bio-Tribo Corros 5(1):16
Issa HM, Alshatteri AH (2018) Corrosion Prevention of Cast Iron Industrial Water Pipes: A Preliminary Comparative Study of Hexamine and Aniline Inhibitors. J Garmian Univ. 5(2):67–83
Fayomi OSI, Akande IG (2019) Corrosion mitigation of aluminium in 36.5% NaCl medium using hexamine. J Bio-Tribo Corros 5(1):23
Ebenso EE, Arslan T, Kandemi̇rlı F, Love I, Ogretır C, Saracoglu M, Umoren SA (2010) Theoretical studies of some sulphonamides as corrosion inhibitors for mild steel in acidic medium. Int J Quant Chem. 110(14):2614–2636
Uhlemann T, Seidel S, Muller CW (2018) Site-specific binding of a water molecule to the sulfa drugs sulfamethoxazole and sulfisoxazole: a laser-desorption isomer-specific UV and IR study. Phys Chem Chem Phys 20(10):6891–6904
Bhattacharya B, Das S, Lal G, Soni SR, Ghosh A, Reddy CM, Ghosh S (2020) Screening, crystal structures and solubility studies of a series of multidrug salt hydrates and cocrystals of fenamic acids with trimethoprim and sulfamethazine. J Mol Struct 1199:127028
Fayomi OSI, Atayero AA, Mubiayi MP, Akande IG, Adewuyi PA, Fajobi MA, Ayara WA (2019) Mechanical and opto-electrical response of embedded smart composite coating produced via electrodeposition technique for embedded system in defence application. J Alloy Compd 773:305–313
Ramde T, Rossi S, Bonou L (2016) Corrosion inhibition action of Sulfamethoxazole for brass in acidic media. Int J Electrochem Sci 11:6819–6829
Ibrahim FM, Hammza RA, Fadhil DH (2019) Synthesis and characterization of Trimethoprim metal complexes used as corrosion inhibitors for carbon steel in acid media. Int J Corros Scale Inhib 8(3):733–742
Perumal S, Muthumanickam S, Elangovan A, Karthik R, Mothilal KK (2017) Bauhinia tomentosa leaves extract as green corrosion inhibitor for mild steel in 1M HCl medium. J. BioTribo Corros 3(2):13
Dohare P, Chauhan DS, Hammouti B, Quraishi MA (2017) ‘Experimental and DFT investigation on the corrosion inhibition behavior of expired drug Lumerax on mild steel in hydrochloric acid anal. Bioanal’ Electrochem 9:762
Abd-El-Nabey BA, Goher YM, Fetouh HA, Karam MS (2015) Anticorrosive properties of chitosan for the acid corrosion of aluminium. Port Electrochim Acta 33(4):231–239
Norsworthy R (2014) Understanding corrosion in underground pipelines: basic principles. In Underground Pipeline Corrosion. 3–34
Akande IG, Oluwole OO, Fayomi OSI (2019) Optimizing the defensive characteristics of mild steel via the electrodeposition of Zn-Si3N4 reinforcing particles. Def Technol 15(4):526–532
Gupta RK, Malviya M, Verma C, Quraishi MA (2017) Aminoazobenzene and diaminoazobenzene functionalized graphene oxides as novel class of corrosion inhibitors for mild steel: experimental and DFT studies. Mater Chem Phys 198:360–373
Haque J, Verma C, Srivastava V, Quraishi MA, Ebenso EE (2018) Experimental and quantum chemical studies of functionalized tetrahydropyridines as corrosion inhibitors for mild steel in 1 M hydrochloric acid. Results Phys 9:1481–1493
Anejjar A, El Mouden OI, Batah A, Bouskri A, Rjoub A (2018) Corrosion inhibition potential of ascorbic acid on carbon steel in acid media. Appl J Environ Eng Sci 3(1):36–46
Zakaria K, Negm NA, Khamis EA, Badr EA (2016) Electrochemical and quantum chemical studies on carbon steel corrosion protection in 1 M H2SO4 using new eco-friendly Schiff base metal complexes. J Taiwan Inst Chem Eng 61:316–326
Niouri W, Zerga B, Sfaira M, Taleb M, Touhami ME, Hammouti B, Mcharfi M, Al-Deyab SS, Benzeid H, Essassi EM (2014) Electrochemical and chemical studies of some benzodiazepine molecules as corrosion inhibitors for mild steel in 1 M HCl. Int J Electrochem Sci 9:8283–8298
Kaco H, Talib NA, Zakaria S, Jaafar SN, Othman NK, Chia CH, Gan S (2018) Enhanced corrosion inhibition using purified tannin in HCl medium. Malays J Anal Sci 22(6):931–942
Abeng FE, Idim VD, Obono OE, Magu TO (2017) Adsorption and adsorption isotherm: application to corrosion inhibition studies of mild steel in 2 M HCl. World Sci News 77(2):298–313
Hameed RA, Al-Shafey HI, Abu-Nawwas AH (2014) 2-(2, 6-dichloranilino) phenyl acetic acid Drugs as Eco-Friendly Corrosion Inhibitors for Mild Steel in 1M HCl. Int J Electrochem Sci 9:6006–6019
Verma C, Chauhan DS, Quraishi MA (2017) Drugs as environmentally benign corrosion inhibitors for ferrous and nonferrous materials in acid environment: an overview. J. Mater. Environ. Sci. JMES 8(11):4040–4051
Gao B, Zhang X, Sheng Y (2008) Studies on preparing and corrosion inhibition behaviour of quaternized polyethylenemine for low carbon steel in sulphuric acid. Mater Chem Phys 108(2–3):375–381
Fayomi OSI, Bamgboye OA, Durodola BM, Inam WA, Daniyan AA (2017) Adsorption and corrosion inhibition properties of floxapen compound on the electrochemical characteristics of type-A5-series a luminium in sodium chloride solution. Int J Microstruct Mater Prop 12(5–6):391–401
Acknowledgment
Surface Engineering Research Centre, Covenant University, Ota, Nigeria is deeply acknowledged for the provision of laboratory facilities to carry out this research. University of Ibadan is also profoundly appreciated for providing enabling research environment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This manuscript is an original work of the authors. Thus, there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Akande, I.G., Fayomi, O.S.I. & Oluwole, O.O. Anticorrosion Potential of Inhibitive Suphtrim Drug on Aluminium Alloys in 0.5 M H2SO4. J Bio Tribo Corros 6, 132 (2020). https://doi.org/10.1007/s40735-020-00429-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-020-00429-9