Abstract
For many decades, scientists in the field of boundary tribology have been working to find an excellent and environment friendly anti-wear additives to minimize wearing of the boundary equipment by improving the anti-wearing ability of the boundary lubricants. Four (4) anti-wear lubricant additives with better properties were carefully designed with the aids of QSPR and MD simulation methodologies. Out of the four newly designed additives, 2, 3, 5-trimethylheptyl acetate with the anti-wear property of 2.0802 mm was found to have better anti-wear lubricant property than its co-additives as well as the standard AW additive, Zinc dipropyl dithiophosphate (ZDDP). All the additives were found to have better boundary dynamic binding energy as well as high dynamic binding temperatures on steel-simulated coated surface than on DLC-coated surface. The boundary dynamic binding energy of all these anti-wear additives was found to be better than the ZDDP. And they could improve the anti-wear property of lubricant at elevated temperature without being decomposed since they have a high dynamic binding tribological temperature. Moreover, all these new anti-wear lubricant additives structures do not contain zinc (catalytic converters deactivator), sulfur (acidic oxide), and phosphorus (exhaust pipe ashes producer) and could be used to replace the widely used additive, ZDDP, which contains zinc and phosphorus and has less active (3.284 mm) additive property. Due to their better structures and properties correlation ability, these two methods could be used to provide a theoretical framework for engineers and other researchers to design a better anti-wear base oil additive before laboratory synthesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Scientists in the field of boundary tribology have been working for many decades to find excellent and environment friendly anti-wear additives to minimize wearing of the boundary equipment, pollution, and consumption of energy by improving the anti-wearing ability of the boundary lubricants. It was reported by some scientists that 50 to 95% of the global energy is usually consumed by the machine's sliding components wearing of different types [1,2,3]. To improve the mechanical sliding components performance and curbing environmental pollution as well as to improve the global economy, wearing caused by the lubricating oil must be modified. Addition of anti-wear additives to the lubricating oil has been recommended by many researchers to modified working equipment lubricating oil wear since heavy duty sliding equipment has been reported to be damaged due to wearing of such equipment [4, 5]. Anti-wear lubricant additives are chemical compounds added to the base stock to improve the lubricating oil properties [1,2,3,4,5,6,7,8].
Since many decades ago, scientists in the field of boundary tribology have been working to find excellent and environment friendly anti-wear additive to minimize wearing of the lubricant boundary problems [9]. Since the last five decades, many different classes of anti-wear polar additive compounds like Organo-sulfur and organo-phosphorus, organo-molybdenum, and nanoparticles have been used in that order extensively. The usual disadvantage with these classes of boundary oil additives was due to their expensiveness and high level of corrosion properties [10,11,12,13,14,15,16]. Another most used anti-wear additive is the multipurpose base oil additive, Zinc dipropyl dithiophosphate (ZDDP). This additive is the most commonly used multifunctional additive in the oil industry since the Second World War. It protects the metal surfaces from wearing by forming sulfides and phosphates film. This multipurpose additive was reported elsewhere that its constituent phosphorus (P) restricts the function of catalytic converters, while zinc (Zn) often produces ashes in the exhaust emissions pipe [15, 16].
Therefore, in silico quantitative structure–property relationships (QSPR) is a computational method that could be used to provide a theoretical framework for engineers and other researchers to design a better anti-wear base oil additive that is devoid of phosphorus, zinc, and sulfur before laboratory synthesis. This QSPR method could be used to save resources by avoiding expensive laboratory trial and error. In silico QSPR could relate the additive's properties with their structures to produce a mathematical linear model that could be used to design novel base oil additives. In view of this, QSPR was used to design some excellent novel anti-wear additives, while molecular dynamic (MD) simulation method was also used to obtain the severe working boundary dynamic binding energies of some novel designed additives and boundary-coated equipment [17,18,19].
2 Experimental Section Details
2.1 Data Set Collections
The set of anti-wear lubricant additives data used for correlating the additive’s anti-wear properties (mm) and the structures together was 25 compounds [20]. Unlike ZDDP, the experimental chemical structures of all the additives do not contain sulfur, phosphorus, and zinc which may produce harmful gases into the environment. These chemical structures of the additives were retrieved and drawn with the aids of Chemdrawn software (Fig. 1). All the two-dimensional drawn additive’s chemical structures contained an excellent anti-wear property (Table 1).
2.2 Molecular Descriptors Generation and QSPR Model Building
The 2D additives chemical structures in Table 1 were saved in cdx file format in ChemDraw software and uploaded unto the Spartan 14 multipurpose software. Using B3LYP (level of theory) and 6–311 (basis set) of density functional theory, Spartan 14 software was used to perform geometrical optimization of all the 25 anti-wear lubricant additives. The optimized additives were saved in sdf and uploaded unto the dragon and padel toolkit software where 4832 molecular descriptors were generated [17, 21]. The 4832 generated molecular descriptors from 25 anti-wear additives were grouped into test (30) and training (70) sets. Using Molegro Data Modeler 3.0, 70% of the training data set was used to generate many mathematical linear QSPR models (Eq. 1), while the test data sets were used to identify the quality of the generated models.
Y predicted anti-wear lubricant additive property (pAW), a0 equation’s intercept, xi developed molecular descriptors ,and ai descriptors coefficients. To test the quality of the constructed/developed QSPR model, some QSPR parameters must be determined by subjecting them into statistical evaluation [22]. The Coefficients of internal additive set, R2 (Eq. 2), adjusted R2adj (Eq. 3), PRESS (Eq. 4) cross-validated q2/R2cv (Eq. 5), and external statistical validation R2ext (Eq. 6), must be less than one, but greater than 0.5 [22].
Moreover, the selected descriptors contribution to the constructed model was derived/identified in terms of mean effect (MF) value in Eq. 7 [23].
where Xpred is the predicted additive variable, Xobs is the observed additive variable, Yobs is the experimental training data set anti-friction additive properties, Ycalc is the calculated training data set on AW properties, Yobs(test) is the experimental AW additive properties for test set, Ypred(test) is the predicted data test set additive properties, N is the number of molecules in the data, R2 is the determination coefficient, and p is descriptors number in the QSPR model, while N−1−p is degree of freedom, j QSPR model’s descriptor, βj descriptor’s coefficient, dij training set data matrix’s descriptor value, m sum of model’s descriptors, and n all training set’s molecules.
2.3 Additive’s Molecular Dynamics (MD) Simulation Methodology
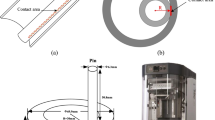
The comprehension of the interactions of various greasing up oil added substances with this material isn't yet completely developed, but it was reported in the literature that high dynamic binding energy and sp3 hybridization between Diamond-like carbon (DLC) and lubricant additives does not accelerate base oil wearing [24,25,26,27]. DLC is an astounding covering material, and it has been accounted to be exceptionally compelling in dealing with the motors fuel energy utilization by forestalling mechanical seizure [28]. To understand, the structural and dynamic binding energy between the DLC/Steel-simulated boundary-coated surfaces and potentially designed lubricant anti-wear additives were accessed, and molecular dynamics (MD) simulation investigation was studied [17, 29, 30]. COMPASS II which is a strong and well-created power field was determined depending on the fitting capacity against a wide scope of inorganic and synthetic organic compounds [31] selected from the studio of the materials 8.0 toolbar. MD simulation binding energy (B.E) calculations were done subsequently after presenting the minimized lubricating base oil additive compound unto the simulation vacuum slab of optimized DLC/Steel crystal (24.82 Å × 24.82 Å × 45.27 Å) surface at a high temperature of 350.15 K and over a range of inter-surface separations. The interaction dynamic binding energy was determined by using Eqs. 8 and 9 [31].
3 Results and Discussion
A molecular modeling study was carried out to examine the property and structure relationship of 25 organic compounds as anti-wear base oil additives. Three QSPR linear models were developed with the aids of the material studio and molegro data modeler 3.0 software to help in designing some novel anti-wear lubricating base oil additives. After performing a diligent examination on all the three developed QSPR linear models, the second model was found to have better coefficients such as internal R2internal (0.90843), external/predicted R2ext (0.7209), adjusted R2adj (0.8507), and cross-validated R2cv (0.90843) [32]. Therefore, the second model was reliable and was used to design organic compounds with better anti-wear lubricant properties since there were no obvious variations between the experimental and predicted properties of the lubricating oil anti-wear p(AW) additives (Table 1).
3.1 1st QSPR Model
p(AW) = 0.95207 * RNCS − 0.85298 * "Weta3.mass" − 0.853297 * "WV.mass" + 0.85239* "Weta2.eneg" + 2.82097. R2ext (0.5692), R2internal (0.7432186), R2adj (0.5972), R2cv (0.64296).
3.2 2nd QSPR Model
p(AW) = 0.00993976 * RNCS + 0.162303 * "Weta3.mass" + 0.00431547 * "WV.mass" + 0.629144 * "Weta2.eneg" + 1.8537. R2ext (0.7209), R2internal (0.90843), R2adj (0.8507), R2cv (0.90843).
3.3 3rd QSPR Model
p(AW) = 0.09934965 * RNCS + 0.74298 * "Weta3.mass" + 0.00431547 * "WV.mass" + 0.629144 * "Weta2.eneg"—3.9753. R2ext (0.684), R2internal (0.6983), R2adj (0.7564), R2cv (0.8285).
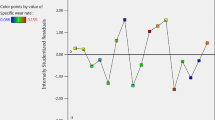
The predicted internal (R2internal = 0.90843) value derived from molegro data modeler 3.0 software was consistentwith the predicted R2 of 0.9084 obtained from the plotted graph (Fig. 2). Also, the external predicted (R2external = 0.7209) value obtained by using Eq. 6 was the same with R2 of 0.7209 value obtained from Fig. 2 plot. Figure 3 also revealed the reliability of the developed QSPR model since the dispersion of coefficient of residual (Experimentally Predicted AW Lubricant Additives) anti-friction lubricant additives on the two sides of the plotted graph was observed [17]. Moreover, QSPR molecular descriptors’ correlation analysis in Table 2 revealed that the coefficient correlation between the generated molecular descriptors was very low, and this was due to the non-significant inter-correlation among those molecular descriptors used in the development of AW QSPR model.
3.4 Evaluation of Designed Anti-wear Additives
The first two steps that were taken for the designing of some anti-wear lubricant additives were the identification of the most contributed molecular descriptor toward the developed QSPR model and selection of additive template from the pool of experimental lubricant additives in Table 1 [33].The excel worksheet of Molegro data modeler 6.3 was used to identify the most relevant descriptor out of the four molecular descriptors generated from the most reliable QSPR model (2nd QSPR model). Figure 4 represents a plot of relevancy values against molecular descriptors. From Table 2 and Fig. 4, it shows WV.Mass molecular descriptor contributed more to the development of the 2nd QSPR model due to high relevancy value than other co-descriptor [17].
Moreover, the second stage was the operation and statistical analysis of applicability domain to identify the set of molecular additives found within the domain and the outliers or influential additive compounds outside the domain, h* (Eq. 10) [34] where m number of descriptors that appear in a QSPR linear model (4) and n is the number of training set molecule only (16).
In Fig. 5, those AW lubricant additives that were found outside the leverage boundary, h*, were called influential compounds because they were not similar to the majority of the lubricant anti-wear additives used for the QSPR model development.
From the applicability domain figure, anti-wear lubricant additives with a serial number 24 (standard residual of − 0.54229) were termed influential compounds [34]. The AW lubricant additive with serial number 10 was found to have a standard residual value of 0.132095 (Table 1 and Fig. 5) and was selected as design template (Fig. 5) to which other structural substituents were attached to this template on the information extracted from WV.mass (3D weighted by atomic masses) molecular descriptor (Table 2). WV.mass is a 3D hydrophobic or hydrogen bonding capacity descriptor [35]. The addition of substituents like –CH3 or –C2H5 on the additive template was found to increase the AW lubricant properties of some four newly designed additives (Table 3). Out of these four, newly designed AW lubricant additives such as 2,3,5-trimethylheptyl acetate (2.0802 mm), 3,4-dimethylhexyl acetate (2.1135 mm), 3-methylnonan-2-yl propionate (2.1112 mm), and 5-methylheptan-2-yl ac`etate (2.1026 mm) were found to possess excellent anti-wear properties than the one reported by other researchers [35]. From Table 3, the lubricant additive with the highest anti-wear value was found to be 2,3,5-trimethylheptyl acetate (2.0802 mm).
3.5 Assessment of Molecular Dynamic Binding Energy
Material studio software was used to optimize all the four designed additives as well as simulated coated sliding surfaces of DLC and steel (Fig. 6) before dynamic energies were calculated one after the other. Equations 8 and 9 were used to calculate the dynamic binding energies between the designed anti-wear additives and DLC as well as steel-coated interfaces. The dynamic binding energies for the newly designed anti-wear lubricant additives on DLC (− 40.812, − 39.299, − 29.299, − 47.066 kcal/mol) and steel (− 12,562, − 448.1, − 1333, − 9162.3 kcal/mol) simulated coated sliding surfaces (Table 3).
All the additives were found to have better boundary dynamic binding energy on steel-simulated coated surface than on DLC-coated surface. Moreover, Fig. 7 shows temperatures of various tribological dynamic binding energies . The boundary dynamic binding energy temperatures range from 148.691 to 274.41 K for all the four anti-wear lubricant additives **on both the DLC and steel surfaces. The 3-methylnonan-2-yl propionate with anti-wear lubricant property of 2.1112 mm and boundary dynamic binding energy of − 1333 kcal/mol were found to have better dynamic binding working temperature of 274.41 K than other co-additives (Fig. 7). The 3-methylnonan-2-yl propionate additive could improve the anti-wear property of lubricant at elevated temperature since it has a high dynamic binding temperature of 274.41 K.
4 Conclusion
Four (4) anti-wear lubricant additives with better properties were carefully designed with the aid of QSPR and MD simulation methods. Out of the four newly designed additives, 2,3,5-trimethylheptyl acetate with the anti-wear property of 2.0802 mm was found to have an excellent anti-wear lubricant property than its co-additives as well as standard additive, ZDDP (3.284 mm), and the one reported by other authors [20]. These four additives were found to have better boundary dynamic binding energy as well as high dynamic temperatures on steel-simulated coated surface than on DLC-coated surface. The boundary dynamic binding energy of all these anti-wear additives was found to be better than the standard anti-wear additive ZDDP and other researchers [36]. Also, the 3-methylnonan-2-yl propionate additive, in particular, could improve the AW property of lubricant at elevated temperature since it has a high dynamic binding temperature of 274.41 K. All the four new anti-wear lubricant additives' structures do not contain zinc (catalytic converters deactivator), sulfur (acidic oxide), and phosphorus (exhaust pipe ashes producer). Any of these newly designed additives could be used to replace the widely used additive ZDDP which contains zinc and phosphorus and has less active (3.284 mm) additive property. Due to their better structures and properties correlation ability, these two methods could be used to provide a theoretical framework for engineers and other researchers to design a better anti-wear base oil additive before laboratory synthesis.
References
Holmberg K, Andersson P, Erdemir A (2012) Global energy consumption due to friction in passenger cars. Tribol Int 47:221–234
Holmberg K, Andersson P, Nylund NO, Makela K, Erdemir A (2014) Global energy consumption due to friction in trucks and buses. Tribol Int 78:94–114
Wen SZ, Huang P (2012) Principles of tribology, 4th edn. Tsinghua University Press, Beijing
Le-Cao D, Khac BC, Le CT, Kim YS, Chung KH (2018) Friction characteristics of mechanically exfoliated and CVD-grown single-layer MoS2. Friction 6(4):395–406
Onodera T, Morita Y, Suzuki A, Koyama M, Tsuboi H, Hatakeyama N, Endou A, Takaba H, Kubo M, Dassenoy F et al (2009) A computational chemistry study on friction of h-MoS2. Part I. Mechanism of single sheet lubrication. J Phys Chem B 113(52):16526–16536
Ansari R, Ajori S, Motevalli B (2012) Mechanical properties of defective single-layered graphene sheets via molecular dynamics simulation. Superlattices Microstruct 51(2):274–289
Smolyanitsky A, Killgore JP (2012) Anomalous friction in suspended graphene. Phys Rev B 86(12):125432
Yoon HM, Jung Y, Jun SC, Kondaraju S, Lee JS (2015) Molecular dynamics simulations of nanoscale and subnanoscale friction behavior between graphene and a silicon tip: analysis of tip apex motion. Nanoscale 7(14):6295–6303
Tang Z, Li S (2014) A review of recent developments of friction modifiers for liquid lubricants (2007-present). Curr Opin Solid State Mater Sci 18:119–139
Chou CC, Lee SH (2008) Rheological behavior and tribological performance of a nanodiamond-dispersed lubricant. J Mater Process Technol 201:542
Hernández-Battez A, González R, Viesca JL, Fernández JE, Díaz Fernández JM, Machado A, Chou R, Riba J (2008) CuO, ZrO2 and ZnO nanoparticles as antiwear additive in oil lubricants. Wear 265:422
Jiao D, Zheng S, Wang Y, Guan R, Cao B (2011) The tribology properties of alumina/silica composite nanoparticles as lubricant additives. Appl Surf Sci 257:5720
Rudnick LR (2010) Lubricant additives. Chemistry and applications, 2nd edn. CRS Press, Boca Raton, p 796
Zhang B, Xu B, Xu Y, Gao F, Shi F, Wu Y (2011) Cu nanoparticles effect on the tribological properties of hydrosilicate powders as lubricantadditive for steel–steel contacts. Tribol Int 44:878
Hong H, Riga AT, Cahoon JM, Vinci JN (1993) Evaluation of overbased sulfonates as extreme-pressure additives in metalworking fluids. Lubr Eng 49:19–24
McDonald RA (2009) Zinc Dithiophosphates. In: Rudnick LR (ed) Lubricant additives, 2nd edn. Marcel Dekker Inc., New York, pp 51–62
Abdulfatai U, Uzairu A, Uba S, Shallangwa GA (2019a) Molecular modelling and design of lubricant additives and their molecular dynamic simulations studies of Diamond-Like-Carbon (DLC) and steel surface coating. Egypt J Pet. https://doi.org/10.1016/j.ejpe.2018.12.004
Ivanciuc O, Ivanciuc T, Cabrol-Bass T (2000) 3D quantitative structure-activity relationships with CoRSA. Comparative receptor surface analysis. Application to calcium channel agonists. Analysis 28:637–642
Wong KY, Mercader AG, Saavedra LM, Honarparvar B, Romanelli GP, Duchowicz PR (2014) QSAR Analysis on tacrine-related acetylcholinesterase inhibitors. J Biomed Sci 21:84
Wang Z, Wang T, Yang G, Gao X, Dai K (2017) Estimating anti-wear properties of esters as potential lubricant based oils using QSTR models with CoMFA and CoMSIA. Friction. https://doi.org/10.1007/s40544-017-0175-5
Todeschini R. Consonni V, Mauri A, Pavan M (2012) DRAGON for widows (Software for the Calculation of Molecular Descriptors), version 6.0, TALETE srl, Milan, Italy
Rogers D, Hopfinger AJ (1994) Application of genetic function approximation to quantitative structure-activity relationships and quantitative structure property relationships. J Chem Inf Comp Sci 34:854–866
Minovski N, Zuperl S, Drgan V, Novi M (2013) Assessment of applicability domain for multivariate counterpropagation artificial neural network predictive models by minimum Euclidean distance space analysis: a case study. Anal Chim Acta 759:28–42. https://doi.org/10.1016/j.aca.2012.11.002
Moriguchi H, Ohara H, Tsujioka M (2016) History and applications of diamond-like carbon manufacturing processes. Sei Tech Rev 82:52–58
Kalin M, Kosovšek J, Remškar M (2013) Nano particle sasnovel lubricating additives in agreen, physically based lubrication technology for DLC coatings. Wear 303:480–485
Kano M, Yasuda Y, Okamoto Y, Mabuchi Y, Hamada T, Ueno T et al (2005) Ultralow friction of DLC vinpresence of glycerolmono-oleate(GMO). Tribol Lett 18:245–251
Neville A, Morina A, Haque T, Voong M (2007) Compatibility between tribological surfaces andlubricantadditives—how friction and wear reduction can be controlled by surface/lube synergies. Tribol Int 40:1680–1695
Oluwaseye A, Uzairu A, Shallangwa G, Abechi S (2017) A novel QSAR model for designing, evaluating, and predicting the anti-MES activity of new 1H-pyrazole-5-carboxylic acid derivatives. JOTCSA 4(3):739–774
Cholakov GS (2011) Towards computer aided design of fuels and lubricants (review). J Univ Chem Technol Metall 46(3):217–236
Michael PA (2004) Introduction to molecular dynamics simulation, vol 23. John von Neumann Institute for Computing, Jülich, pp 1–28
Wymyslowski A, Iwamoto N, Yuen M, Fan H (2008) Molecular modeling and multiscaling issues for electronic material applications. Springer, New York
Tropsha A (2010) Best practices for QSAR model development, validation, and exploitation. Mol Inform 29:476–488
Abdulfatai U, Uzairu A, Uba S (2019b) Molecular docking and quantitative structure-activity relationship study of anticonvulsant activity of aminobenzothiazole derivatives. Beni-Suef Univ J Basic Appl Sci 7:204–214
Netzeva TI, Worth AP, Aldenberg T, Benigni R, Cronin MTD, Gramatica P, Jaworska JS, Kahn S, Klopman G, Marchant CA, Myatt G, Nikolova-Jeliazkova N, Patlewicz GY, Perkins R, Roberts DW, Schultz TW, Stanton DT, Van De Sandt JJM, Tong W, Veith G, Yang C (2005) Current status of methods for defining the applicability domain of (quantitative) structure–activity relationships. Altern Lab Anim 33:155–173
Todeschini R, Gramatica P (1998) New 3D molecular descriptors: the WHIM theory and QAR applications. Persepect Drug Discov Design. https://doi.org/10.1023/A:1027284627085
Abdulfatai U, Uzairu A, Uba S, Shallangwa GA (2019c) Quantitative structure-properties relationship, molecular dynamic simulations and designs of some novel lubricant additives. Egypt J Pet. https://doi.org/10.1016/j.ejpe.2019.05.001
Funding
No direct funding was received for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research Involving Human and Animal Participants
This research does not contain any human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdulfatai, U., Uzairu, A., Shallangwa, G.A. et al. In Silico Modeling, Prediction, and Designing of Some Anti-wear Lubricant Additives. J Bio Tribo Corros 6, 100 (2020). https://doi.org/10.1007/s40735-020-00399-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-020-00399-y