Abstract
Mahua oil was epoxidized to improve its lubrication properties. Furthermore, copper oxide nanoparticles were added to the chemically modified mahua oil in certain proportions, followed by tribological testing using a pin-on-disc tribometer under different conditions. The physicochemical properties of the lubricants were also determined. Improvements in the viscosity, viscosity index, and flash point were observed on addition of copper oxide nanoparticles. Rheological analysis revealed that all the lubricant samples showed Newtonian behavior, presenting a linear relationship between shear rate and shear stress. Addition of copper oxide nanoparticles to the modified mahua oil resulted in better lubricity, but their content was limited to 0.4%. A reduction in the friction coefficient and improved antiwear properties were achieved when adding nanoparticles at concentrations of 0.2% and 0.4%. Scanning electron microscopy (SEM) imaging also revealed a better surface when nanoparticles were added at concentrations up to 0.4% due to effective lubrication of the surface.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Several studies have been conducted to analyze the renewable energy resources available in our country. Increased energy demands from different sectors and the impending depletion of fossil-fuel reserves have forced the Indian government to take relevant measures to consider alternative approaches [1]. This study was conducted to fill some of the remaining gaps regarding the development of such alternative energy sources. Lubrication enables friction to be reduced by applying a lubricant to contacting metal parts. Its functions are (i) to produce a protective lubricant film between the metals during their contact, (ii) to reduce the heat produced, and (iii) to remove metal debris. However, disposal of used mineral oils results in environmental pollution which must be addressed. Due to human negligence, oil spillage onto land and particularly into aquatic environments is of considerable concern [2]. Spillage of oils into the sea can occur during offshore oil transfers and from oil tankers [3].
The fast depletion of petroleum reserves and increased pollution levels of the environment have raised several concerns [4,5,6]. Various steps have been considered in this regard to minimize such harmful environmental effects. Because of their sustainability, biobased lubricants or biolubricants derived from vegetable oils represent one of the available options. Biobased lubricants derived from vegetable oils offer good lubricity, higher viscosity index, higher flash point, and good antiwear properties as compared with conventional lubricants [7, 8]. Despite the several advantages of such nonedible oils, certain challenges need to be addressed before their commercial application, among which their oxidation stability represents a major drawback [8]. The poor oxidation stability of vegetable oils is related to their high content of unsaturated fatty acids. Mahua (Madhuca indica) oil consists of a polar group with a long hydrocarbon chain, thus acting as a surfactant when adsorbed on surfaces due to the development of a protective film. The polarity of vegetable oils is one of the prominent factors contributing to their wear reduction abilities [9]. The epoxidation process can also be applied to raw mahua oil to make it more sustainable by preventing the oxidation process [10].
To improve the properties of such materials, certain additives are available, but they are nonbiodegradable and toxic in nature [11]. This study focuses on biodegradable oil with the introduction of nanoparticles suitable for tribological applications. Very few studies have been conducted on such inclusion of nanoparticles for tribological applications. Xie et al. [12] evaluated the effect of silicon dioxide and molybdenum disulfide nanoparticles on the tribological properties of engine oil. They outlined the effect of the quantity of nanoparticles on the stress-bearing limit and stability of the grease film. In addition, addition of molybdenum disulfide in lubricants has been shown to influence the load-bearing limit and strength of the oil film. Mirjavadi et al. [13] conducted tribological analysis of TiO2 nanoparticles as additives. Addition of TiO2 also results in grain size refinement, in addition to their good dispersion stability [14]. Shi et al. [15] investigated the tribological characteristics of hybrid graphene–copper particles. Addition of these hybrid nanoparticles to a solid lubricant resulted in a reduction of the friction coefficient by 35% and the wear by 50%. Borda et al. [16] determined the friction and wear characteristics of mineral and synthetic oils with copper as additive. A reduction in the friction coefficient was observed for the mineral oil with addition of up to 0.3%.
However, none of these studies reported on the effect of copper oxide nanoparticles on mahua oil as a novel feedstock. Based on previous studies, it can be concluded that nanoparticles are suitable to improve the properties of vegetable oils. The presence of saturated fatty acids in vegetable oils enables their better lubricity [17]. Mahua oil contains large amounts of saturated and monosaturated fatty acids, making it a suitable candidate lubricant.
In the investigation presented herein, raw mahua oil was used as the reference oil, and further chemical medication through the epoxidation process was carried out to improve its oxidation stability. In addition, CuO nanoparticles were added to the modified oil to verify the lubrication characteristics of the resulting lubricants.
2 Materials and Methods
Figure 1 shows the methodology adopted in this study. Details of the process are described in further sections.
2.1 Fatty Acid Profile of Mahua Oil
For evaluation purposes, raw mahua oil was procured from M/s KS Essentials, New Delhi. This oil mainly contains triglyceride esters of fatty acids and glycerol, which were estimated according to European standard method EN14103:2003.
To analyze the fatty acid profile, gas chromatography (HP 6890 series 2) with a flame ionization detector was used. This device consists of a capillary column with length of 30 m, a film thickness of 0.25 µm, and an internal diameter of 0.32 mm. Helium was supplied as carrier gas at a flow rate of 1 ml/min. The 1-μl sample was injected using a 6890 series injector (Agilent).
Table 1 presents the amount of fatty acids present in the mahua oil. The mahua oil contained 39% saturated fatty acids and 61% unsaturated fatty acids. The most dominant constituent of mahua oil was oleic acid, contributing around 58.6%. A large amount of linoleic acid (12.4%) was also present in the mahua oil. The mahua oil also possesses low-temperature properties that promote its consideration for use in lubrication applications. The iodine number of the oil determines its unsaturation. According to literature [18], the iodine number of an oil should be less than 115 if it is to be considered for use as a lubricant.
2.2 Chemical Modification of Mahua Oil
For epoxidation, a 250-ml Soxhlet apparatus consisting of a three-necked glass reactor with a five.-blade stirrer was used. The mixture was stirred using a motor in the setup. The setup was immersed in a hot water bath. Acetic acid was mixed with hydrogen peroxide in equal proportions using sulfuric acid as catalyst to obtain peracetic acid using quantities based on literature. Around 10.72 ml mahua oil was mixed with 37.12 ml peracetic acid. The mixture was heated at 20 °C for about 4.5 h to achieve complete reaction. The resulting solution was poured into water, and the expoxidized oil rose to the surface. After leaving the mixture for about 1 h, water was extracted from the lower surface of the burette. The remaining oil was heated for about 1–2 h to completely remove any remaining moisture. The mixture was thus epoxidized for further analysis.
2.3 Nanolubricant Development
CuO nanoparticles (45 nm diameter, 6321 kg/m3 density) with purity of 99.9% were supplied by M/s Sigma Aldrich, Bangalore. The size of the nanoparticles was 45 nm, as stated by the supplier with proof. The nanoparticles were mixed into the epoxidized oil at 0.2%, 0.4%, and 0.8% on a weight percentage basis. Surface modifier (Triton X-100, M/s Triveni Chemical, Gujarat) was used to stabilize the nanoparticles in the epoxidized oil. The amount of Triton X-100 was 50 wt% versus the nanoparticles. To obtain a steady suspension of nanoparticles, they were mixed using a magnetic stirrer at 800 rpm/min for 2.0 h. The mixture was then agitated for 40 min using an ultrasonic probe sonicator (M/s Samarth Electronics, Thane) at 60 °C.
To verify the size of the nanoparticles, SEM was carried out at magnifications from 50× to 300,000×. The nanopowder was ultrasonicated for around 30 min with acetone before SEM. The mixture was then kept on a polished Si wafer plate (10 mm × 10 mm) and dried using a dryer. Figure 2 shows an image of the CuO nanoparticles, clearly revealing its amorphous structure. Using ImageJ software, the particle size was found to range from 42 to 50 nm.
The dispersion stability of the CuO nanoparticles in the epoxidized oil was analyzed using a Zetasizer (Nano ZS, Malvern) procured from M/s ATA Scientific Instruments. This equipment can measure particle sizes ranging from 0.3 nm to 10 µm.
2.4 Analytical Methods
The viscosity was evaluated using a viscometer (M/s Swastik systems and services, New Delhi) based on the ASTM D-445 standard at atmospheric pressure and temperatures of 40 °C and 100 °C. The test was conducted three times, and the mean value was used to reduce the error and ensure reliability.
To calculate the viscosity index of the samples, Eq. 1 was utilized according to the ASTM D-2270:
where U is the kinematic viscosity at 40 °C, and L and H are the kinematic viscosity at 40 °C with viscosity index of 0 and 100, respectively, having the same kinematic viscosity at 100 °C as the oil whose viscosity index was to be determined.
The flash point and pour point of the oil were measured according to ASTM D-92 (Cleveland open-cup method) and ASTM D-97, respectively, using proper apparatus. The acid value and the density were measured based on standard methods published in literature [19].
2.5 Tribological Testing
2.5.1 Material
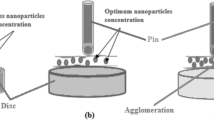
LM 13 alloy was procured from M/s Bharat Aerospace Metals, Mumbai and used as the pin material for the test. Aluminum is a low-density material that can resist corrosion due its surface passivation. Aluminum is also a relatively soft, durable, lightweight, ductile, and malleable metal [20, 21]. This material was thus applied a the piston material facing the maximum friction with hardness of 98 HRB. This material can also resist wear and corrosion. For the disc, EN 31 steel was used as it offers high hardness (62 HRC) and wear resistance. The pin was cylindrically shaped by turning on a lathe. One end of the pin was made spherical to ensure a point contact with the disc. The pin had a diameter of 10 mm and length of 30 mm. The pin was further polished using emery paper with grit sizes of 200, 400 600, and 1200 nm before performing the experiment on the machine.
2.5.2 Test Setup
The pin-on-disc machine was purchased from M/s DUCOM, Bangalore, India and used to investigate the friction and wear characteristics with the application of each lubricant. Figure 3 shows the setup used. The friction force was obtained from the equipment with the help of the load cell attached to the tribometer. To obtain the friction coefficient, the friction force was normalized by the applied load. The lubricant was added dropwise to the interfaces with the help of a pump operated by an electric motor. Table 2 presents the values of the parameters used in this analysis. The conditions applied are based on work reported in literature [22,23,24,25].
3 Results and Discussion
3.1 Analysis of Physicochemical Properties
Table 3 presents the physicochemical properties of the lubricants. An increase in the viscosity of the raw oil was observed after chemical modification. This is due to dissolution of the double bonds associated with raw vegetable oil [26]. Addition of nanoparticles at concentrations of 0.2%, 0.4%, and 0.8% resulted in an increase in the viscosity. The maximum viscosity was attained when adding 0.8%. This increase in the viscosity on nanoparticle addition is in good agreement with previous studies [27,28,29].
The viscosity index (VI) results indicate the temperature range that each lubricant can resist during operation. A high VI value indicates little change of the kinematic viscosity with temperature. Meanwhile, a higher value of VI ensures that the lubricant sample provides good and stable lubricity across a wide operating temperature range. According to Zulkifli et al. [30], the lubrication film becomes extremely thin at high temperatures but very thick at low temperatures. The modification of the raw oil improved its VI value. Also, a better VI was obtained on addition of nanoparticles.
Raw mahua oil was more dense in comparison with the epoxidized oil. The epoxidized oil contained a long straight saturated chain that confirms its lower density. On addition of nanoparticles to the epoxidized oil, the density increased due to the mass concentration.
With increasing concentration of nanoparticles, an increase in the flash point was observed, which is favorable as an increase in the flash point enables an increased upper operating temperature for the lubricant. This increase in the upper operating temperature corresponds to the greater stability and improved thermophysical properties of the CuO nanoparticles. Hence, the enhanced flash point can be considered to be advantageous in terms of enhancing the lubricity characteristics of the epoxidized oil. On addition of nanoparticles, the pour point increased up to the concentration of 0.4% but then reduce at the concentration of 0.8%. Increasing the temperature resisted the flow of the lubricant, and a higher concentration of nanoparticles did not enable a better flow due to agglomeration on the metallic surfaces [28, 31,32,33].
Figure 4 shows the size distribution of the nanoparticles dispersed in the nanolubricant samples obtained using the Zetasizer (Nano ZS, Malvern). It was observed that the average size of the CuO nanoparticles when added at concentrations of 0.2%, 0.4%, and 0.8% was 143, 155, and 208 nm, respectively, being approximately three times higher than the original size. This increase in size of the nanoparticles after dispersion is due to their mild agglomeration. The size of the CuO nanoparticles increased after mixing due to the amorphous morphology of the CuO nanoparticles [34].
3.2 Flow Behavior
Figure 5 shows the variation of the shear stress with the shear rate for all the lubricant samples. At both temperatures, a linear trend emerges, confirming the Newtonian characteristic of the samples considered in this work. The mahua oil showeds the maximum shear rate with respect to the epoxidized oil. A further reduction in the shear rate was obtained on addition of the nanoparticles to the epoxidized oil. On increasing the nanoparticle concentration to 4%, a greater shear rate was measured. This occurs due to the breakdown of the lubricant layers associated with the fluid [35]. The change in viscosity of the samples with respect to the shear rate is depicted in Fig. 6. Negligible changes were observed in the viscosity of the tested samples, confirming their Newtonian behavior. The same trend was also observed in the study by Kerni et al. [36].
3.3 Frictional Behavior
The frictional behavior of the lubricant samples at load of 40 N and sliding speed of 200 rpm is shown in Fig. 7. The raw mahua oil showed the maximum frictional behavior with respect to the other samples due to its failure to form an effective lubricant film on the metallic surfaces. The epoxidized oil presented the minimum coefficient of friction in comparison with the raw mahua oil. The presence of polyol esters in the epoxidized oil results in the minimum friction, as they adsorb easily onto the surfaces and protect the lubricant film from breakdown. This reasoning is in agreement with previous studies [37].
On addition of nanoparticles to the epoxidized oil, an improvement in friction was achieved for the concentrations of 0.2% and 0.4%, but addition of 0.8% nanoparticles resulted in an increment in the friction. Addition of nanoparticles up to the optimum concentration had a positive effect, as they were properly dispersed on the asperities present on the surface and provided better lubrication. According to Chinas and Spikes [38], nanoparticles penetrate the contact area and then deposit on the surface, as they are smaller in size or similar to the thickness of the lubricant film. Addition of amounts of nanoparticles above the optimum concentration resulted in their agglomeration on the surface, causing more friction or wear of the parts during their sliding contact [39].
3.4 Effect of Sliding Speed on Frictional Behavior
The coefficient of friction (COF) was measured at different sliding speeds with a load of 40 N (Fig. 8). The coefficient of friction at the higher sliding speed was minimum, with more friction at reduced sliding speeds. At low speed, the film formed on the surface is thinner, leading to contact between the surfaces and thus greater friction. At higher speed, the boundary lubrication regime increased, leading to an improvement of the thickness of the lubricant film. The same trend with sliding speed was also observed in previous work [40]. The thick film forms a protective layer, thus reducing friction. The minimum coefficient of friction was observed at 600 rpm, with mahua oil and epoxidized oil showing COF values of 0.0178 and 0.0167. Addition of nanoparticles to the epoxidized oil resulted in the minimum coefficient of friction by forming a better protective lubricant film on the surface. With an increase in the sliding speed, the nanoparticles exhibit a rolling mechanism that assists the lubricant film in resisting the pressure exerted on the surface [41].
3.5 Wear
Figure 9 shows the wear of the pin at load of 40 N and sliding speed of 200 rpm. The maximum friction was attained at the speed of 200 rpm, so this result was considered in the wear analysis. The wear of the pin was based on the differences obtained by weighing the pin during the test. The wear of the pin was minimum for the epoxidized oil in comparison with the base oil, as is evident from Fig. 9. The epoxidized oil forms an improved lubricant layer between the surfaces during their contact. The epoxidized oil contains polyesters which promote adsorption on the surface, resulting in minimum wear [42].
Addition of CuO nanoparticles at concentrations up to 0.4% also resulted in minimum wear of the pin when compared with the epoxidized oil. The reduction in the wear was around 3.8%. The effect of the nanoparticles on the antiwear properties depends on their proper dispersion in the lubricant. CuO nanoparticles have the ability to disperse properly in solution, which promotes the antiwear characteristics [43, 44].
3.6 Worn Surface Analysis
Figure 10 shows SEM images of the samples after testing at sliding speed of 200 rpm and load of 40 N. Figure 10a shows SEM images after testing with mahua oil. Delamination of the surface is observed, and the occurrence of the plowing effect results in scuffing of the surface. For the epoxidized mahua oil (Fig. 10b), no surface delamination was observed, with the formation of a smooth surface. This occurred due to the formation of –O– cross-linking on the surface, which protects it by assisting formation of the lubricant film [37]. Addition of nanoparticles at concentrations up to 0.4% results in less wear, as they provide a protective film between the surfaces during their motion. Figure 10c shows an image when adding a concentration of 0.4%. Minimum damage to the surface was observed due to the better protective film formed on the surface. However, further increase in the nanoparticle concentration resulted in greater wear. Figure 10d shows the effect of adhesion and abrasion on the surface when the amount of copper oxide nanoparticles was further increased, revealing that pits formed on the surface due to the plowing effect [28].
4 Conclusions
Friction and wear analyses of mahua oil were carried out to investigate the effect of addition of CuO nanoparticles at different concentrations. Based on the results of this work, the following conclusions can be drawn:
-
1.
An improvement in the physicochemical properties including the viscosity, viscosity index, flash point, and density was achieved with addition of nanoparticles to the chemically modified mahua oil.
-
2.
All the lubricants exhibited Newtonian behavior, showing a linear relationship between shear rate and shear stress. Negligible changes in viscosity at different shear rates were observed.
-
3.
The epoxidized oil showed improved lubricity in comparison with raw mahua oil by reducing friction due to the presence of polyesters in the chemical structure of the oil, resulting in the successful formation of a protective film on the surface.
-
4.
Addition of copper oxide nanoparticles to the chemically modified oil improved the lubricity of the samples. With addition at concentrations of up to 0.4%, better results were obtained in terms of reducing the friction. Their addition to the lubricant assisted the formation of a better protective film between the surfaces, preventing direct contact between the metals during their motion.
-
5.
The use of raw mahua oil resulted in the maximum damage to the surface in comparison with the other lubricants. The use of epoxidized mahua oil resulted in less damage to the surface, due to both adhesion and abrasion effects and the presence of esters in its molecular structure. Addition of nanoparticles at concentrations of up to 0.4% to the modified oil resulted in less wear and damage to the surface. However, more grooves were formed at the 0.8% nanoparticle concentration.
5 Future Work
The present work could be extended by considering the effect of the nanoparticle size and shape on the lubrication mechanism of the chemically modified oil. A comparative study between different types of nanoparticles would also be an interesting option for future work.
References
Deshpande S, Anekar N, Vagge S, Joshi A (2019) Wear behavior of spheroidal graphite cast iron in biodiesel blends. J Bio Tribo Corros 6:4
Chukwuka KS, Alimba CG, Ataguba GA, Jimoh WA (2018) Chapter 9: the impacts of petroleum production on terrestrial fauna and flora in the oil-producing region of Nigeria A2—Ndimele, Prince E. In: The political ecology of oil and gas activities in the Nigerian aquatic ecosystem. Academic Press, pp. 125–142
Ssempebwa JC, Carpenter DO (2009) The generation, use and disposal of waste crankcase oil in developing countries: a case for Kampala district, Uganda. J Hazard Mater 161:835–841
Wachtmeister H, Henke P, Höök M (2018) Oil projections in retrospect: Revisions, accuracy and current uncertainty. Appl Energy 220:138–153
Aleklett K, Höök M, Jakobsson K, Lardelli M, Snowden S, Söderbergh B (2010) The peak of the oil age—analyzing the world oil production reference scenario in World Energy Outlook 2008. Energy Policy 38:1398–1414
Hajjari M, Tabatabaei M, Aghbashlo M, Ghanavati H (2019) A review on the prospects of sustainable biodiesel production: a global scenario with an emphasis on waste-oil biodiesel utilization. Renew Sustain Energy Rev 72:445–464
Zulkifli N, Masjuki H, Kalam M, Yunus R, Azman S (2014) Lubricity of bio-based lubricant derived from chemically modified jatropha methyl ester. J Tribol 1:18–39
Panchal TM, Patel A, Chauhan DD, Thomas M, Patel JV (2017) A methodological review on bio-lubricants from vegetable oil based resources. Renew Sustain Energy Rev 70:65–70
Zareh-Desari B, Davoodi B (2016) Assessing the lubrication performance of vegetable oil-based nano-lubricants for environmentally conscious metal forming processes. J Clean Prod 135:1198–1209
Reeves CJ, Siddaiah A, Menezes PL (2017) A review on the science and technology of natural and synthetic biolubricants. J Bio Tribo Corros 3:11
Singh Y, Chaudhary V, Pal V (2020) Friction and wear characteristics of the castor oil with TiO2 as an additives. Mater Today Proc. https://doi.org/10.1016/j.matpr.2020.02.612
Xie H, Jiang B, Liu B, Wang Q, Xu J, Pan F (2016) An investigation on the tribological performances of the SiO2/MoS2 hybrid nanofluids for magnesium alloy-steel contacts. Nanoscale Res Lett 11:329
Mirjavadi SS, Alipour M, Emamian S, Kord S, Hamouda AMS, Koppad PG et al (2017) Influence of TiO2 nanoparticles incorporation to friction stir welded 5083 aluminum alloy on the microstructure, mechanical properties and wear resistance. J Alloys Compd 712:795–803
Sharifi Asl N, Mirsalehi SE, Dehghani K (2019) Effect of TiO2 nanoparticles addition on microstructure and mechanical properties of dissimilar friction stir welded AA6063-T4 aluminum alloy and AZ31B-O magnesium alloy. J Manuf Process 38:338–354
Shi S-C, Jiang S-Z (2020) Influence of graphene/copper hybrid nanoparticle additives on tribological properties of solid cellulose lubricants. Surf Coat Technol 389:125655
Guzman Borda FL, Ribeiro de Oliveira SJ, Seabra Monteiro Lazaro LM, KalabLeiróz AJ (2018) Experimental investigation of the tribological behavior of lubricants with additive containing copper nanoparticles. Tribol Int 117:52–58
Singh Y, Garg R, Kumar A (2016) Tribological behavior of pongamia oil as a lubricant additive. Energy Sour Part A Recov Util Environ Effects 38:2406–2412
Acharya N, Nanda P, Panda S, Acharya S (2017) A comparative study of stability characteristics of mahua and jatropha biodiesel and their blends. J King Saud Univ Eng Sci 20(2):511–517
Akbar E, Yaakob Z, Kamarudin SK, Ismail M, Salimon J (2009) Characteristic and composition of Jatropha curcas oil seed from Malaysia and its potential as biodiesel feedstock feedstock. Eur J Sci Res 29:396–403
Tulashie SK, Kotoka F (2020) The potential of castor, palm kernel, and coconut oils as biolubricant base oil via chemical modification and formulation. Therm Sci Eng Prog 16:100480
Hwang Y, Lee C, Choi Y, Cheong S, Kim D, Lee K et al (2011) Effect of the size and morphology of particles dispersed in nano-oil on friction performance between rotating discs. J Mech Sci Technol 25:2853–2857
Kapsiz M, Durat M, Ficici F (2011) Friction and wear studies between cylinder liner and piston ring pair using Taguchi design method. Adv Eng Softw 42:595–603
Imran A, Masjuki HH, Kalam MA, Varman M, Hasmelidin M, Mahmud KAHA et al (2013) study of friction and wear characteristic of jatropha oil blended lube oil. Procedia Eng 68:178–185
Ruiz-Andres M, Conde A, de Damborenea J, Garcia I (2015) Friction and wear behaviour of dual phase steels in discontinuous sliding contact conditions as a function of sliding speed and contact frequency. Tribol Int 90:32–42
Raina A, Anand A (2017) Tribological investigation of diamond nanoparticles for steel/steel contacts in boundary lubrication regime. Appl Nanosci 7:371–388
Saboya RMA, Cecilia JA, García-Sancho C, Sales AV, de Luna FMT, Rodríguez-Castellón E et al (2017) Synthesis of biolubricants by the esterification of free fatty acids from castor oil with branched alcohols using cationic exchange resins as catalysts. Ind Crops Prod 104:52–61
Yu H-L, Xu Y, Shi P-J, Xu B-S, Wang X-L, Liu Q (2008) Tribological properties and lubricating mechanisms of Cu nanoparticles in lubricant. Trans Nonferr Met Soc China 18:636–641
Awang NW, Ramasamy D, Kadirgama K, Najafi G, Che Sidik NA (2019) Study on friction and wear of cellulose nanocrystal (CNC) nanoparticle as lubricating additive in engine oil. Int J Heat Mass Transf 131:1196–1204
Farfan-Cabrera LI, Gallardo-Hernández EA, Gómez-Guarneros M, Pérez-González J, Godínez-Salcedo JG (2020) Alteration of lubricity of Jatropha oil used as bio-lubricant for engines due to thermal ageing. Renew Energy 149:1197–1204
Zulkifli N, Kalam M, Masjuki H, Shahabuddin M, Yunus R (2013) Wear prevention characteristics of a palm oil-based TMP (trimethylolpropane) ester as an engine lubricant. Energy 54:167–173
Ali MKA, Xianjun H, Mai L, Bicheng C, Turkson RF, Qingping C (2016) Reducing frictional power losses and improving the scuffing resistance in automotive engines using hybrid nanomaterials as nano-lubricant additives. Wear 364–365:270–281
Aravind A, Joy ML, Nair KP (2015) Lubricant properties of biodegradable rubber tree seed (Hevea brasiliensis Muell. Arg) oil. Ind Crops Prod 74:14–19
Asnida M, Hisham S, Awang NW, Amirruddin AK, Noor MM, Kadirgama K et al (2018) Copper (II) oxide nanoparticles as additive in engine oil to increase the durability of piston-liner contact. Fuel 212:656–667
Gu K, Chen B, Chen Y (2013) Preparation and tribological properties of lanthanum-doped TiO2 nanoparticles in rapeseed oil. J Rare Earths 31:589–594
Hernández Battez A, González R, Viesca JL, Fernández JE, Díaz Fernández JM, Machado A et al (2008) CuO, ZrO2 and ZnO nanoparticles as antiwear additive in oil lubricants. Wear 265:422–428
Kerni L, Raina A, Haq MIU (2019) Friction and wear performance of olive oil containing nanoparticles in boundary and mixed lubrication regimes. Wear 426–427:819–827
McNutt J, He Q (2016) Development of biolubricants from vegetable oils via chemical modification. J Ind Eng Chem 36:1–12
Chinas-Castillo F, Spikes HA (2000) The behavior of colloidal solid particles in elastohydrodynamic contacts. Tribol Trans 43:387–394
Khajuria A, Akhtar M, Pandey Manish K, Singh Mayur P, Raina A, Bedi R et al (2019) Influence of ceramic Al2O3 particulates on performance measures and surface characteristics during sinker EDM of stir cast AMMCs. World J Eng 16:526–538
Singh Y, Garg R, Kumar S (2019) Effect of sliding speed and temperature on the tribological behavior of pongamia oil-based blended lubricant. Energy Sources Part A Recov Util Environ Effects 41:468–480
Delgado MA, Quinchia LA, Spikes HA, Gallegos C (2017) Suitability of ethyl cellulose as multifunctional additive for blends of vegetable oil-based lubricants. J Clean Prod 151:1–9
do Valle CP, Rodrigues JS, Fechine LMUD, Cunha AP, QueirozMalveira J, Luna FMT et al (2018) Chemical modification of Tilapia oil for biolubricant applications. J Clean Prod 191:158–166
Salimon J, Salih N, Yousif E (2011) Chemically modified biolubricant basestocks from epoxidized oleic acid: Improved low temperature properties and oxidative stability. J Saudi Chem Soc 15:195–201
Borugadda VB, Goud VV (2014) Epoxidation of castor oil fatty acid methyl esters (COFAME) as a lubricant base stock using heterogeneous ion-exchange resin (IR-120) as a catalyst. Energy Procedia 54:75–84
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chaurasia, S.K., Sehgal, A.K. & Singh, N.K. Improved Lubrication Mechanism of Chemically Modified Mahua (Madhuca indica) Oil with Addition of Copper Oxide Nanoparticles. J Bio Tribo Corros 6, 94 (2020). https://doi.org/10.1007/s40735-020-00387-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-020-00387-2