Abstract
ASTM A194 steel is one of the standard steels used in the manufacture of steel nuts used in high temperature and pressure conditions in the oil and gas industry. In this study, the effect of polyurea coating on improving the corrosion resistance of this alloy and the effect of Ag nanoparticles on the corrosion properties of polyurea coating in 3.5% sodium chloride solution was investigated. Corrosion resistance of the samples was evaluated by potentiodynamic polarization test. Scanning electron microscopy (SEM) and X-ray diffraction spectroscopy (EDS) analyses were used to qualitatively evaluate the surface of the samples coated with polyurea and Ag nanoparticles. The obtained results showed that applying polyurea coating on ASTM A194 steel improved its corrosion resistance significantly. It was also found that addition of Ag nanoparticles had a positive effect on the protective properties of polyurea coating. The results of potentiodynamic polarization tests showed that adding silver nanoparticles to the polyurea coating improved the protective behavior of the coating in the short and long term. This improvement in corrosion behavior can be attributed to the barrier properties and chemical properties of silver nanoparticles in contact with destructive species present in corrosive electrolytes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Corrosion is defined as the destruction and loss of material due to reactions with its surroundings. Corrosion damages can be divided into two categories of direct damages, including the cost incurred due to the damage to the equipment, and indirect damages such as the cost of repairing or replacing the damaged part. The necessity of corrosion control is often summarized in safety and economic considerations. Machines, equipment and components of factories can be broken due to corrosion and can lead to severe health and financial losses.
Generally, the main methods of corrosion protection can be divided into four categories: cathodic protection, anodic protection, use of inhibitors, and the use of coatings [1, 2]. There are several ways to prevent corrosion of steels, among which the use of organic coatings is the most widely used method of corrosion protection of steel equipment exposed to corrosive environments [3,4,5,6,7]. This type of coating creates a relatively thin layer between the piece and its surroundings. Using coatings undoubtedly has the highest corrosion protection among such methods [8,9,10,11].
Thin coatings can provide an effective barrier between metal and its surroundings. Among the wide range of industrial coatings used so far, some studies have been done on the uses of polyurea coating as a high-performance corrosion protection and mechanical strength enhancer [12,13,14].
In the study by Feng and Iroh [13], a polyurea aromatic coating was used to protect 2024 aluminum corrosion in a solution of 3.5% sodium chloride. The results of their electrochemical impedance spectroscopy showed that, due to its permeability and corrosion resistance, the polyurea coating could provide effective protection for aluminum. They showed that corrosion resistance increased with polyurea concentration in coating up to 50%. Also, they reported that the coating lifetime was about 8 years [13].
In another study, Orlov [14] used a polyurea coating to protect the corrosion of drinking water pipelines underground. He observed that by applying the appropriate thickness of the polyurea coating, tubes could be protected up to 50 years. The researchers investigated the effect of polyurea coating on composite cylinders used under the sea [15]. They evaluated the uncoated cylinder with two cylinders with different coating thicknesses. Laboratory and computational results showed that the use of polyurea coating reduced the degradation of cylinders and increased their service life. It was also observed that with increasing the thickness of the coatings used on the cylinders, the rate of degradation of the cylinders decreased.
Additives are among the chemicals, commonly used in small values to obtain special effects [16]. Additives affect the technical characteristics of the coating and the characteristics of the protective layer created. These effects are adhesion, creating foam, dispersion of pigments, stability, flexibility, smoothness and polish, UV resistance, permeability, fire resistance, resistance to microbes, and the like [17, 18].
The production of graphene/polyurea and graphite oxide/polyurea nanocomposites were investigated to improve protective coating properties [19]. The authors observed that by adding 0.2 wt% graphene in the nanocomposite, tensile strength reached the maximum enhancement of 80%. While the tensile strength of nanocomposite decreased by increasing graphite oxide concentration [19]. Moreover, the effect of microcomposite coating has been investigated by adding glass fiber to polyurea to enhance mechanical properties [20]. The results revealed that the microcomposite became stiffer by increasing milled glass volume fraction from 10 to 20% [20]. By using cold spray coating technology, Zhoa et al. [21] made a composite coating on aluminum matrix by combining graphene sheets decorated by Ag nanoparticles. The graphene plates and Ag nanoparticles were uniformly distributed in the coating. Their results indicated that this type of coating had a very high antibacterial activity. They proved the presence of Ag nanoparticles improved the coating properties and prevented the bacterial (Escherichia coli) activity. Torrico et al. [22] prepared anticorrosive coating on metallic surfaces by mixing siloxane and silica at different molar proportions with epoxy. The produced coating provided high thermal stability (> 300 °C) and excellent barrier protection for steel substrate [22]. Effects of SiO2 nanoparticles on corrosion behavior of coating over St 37 steel as substrate were investigated, showing that corrosion resistance was raised by adding SiO2 nanoparticles up to 2 g/L [23]. Manjumeena et al. [24] used the green silver nanoparticles as an environmentally friendly additive to reinforce epoxy resins. This additive increased the both corrosion resistance and antimicrobial activity of the coating. In this research study, three different amounts of Ag nanoparticles, i.e., 1, 3, and 5 wt% Ag nanoparticles, were added to the coating. The corrosion resistance of the coatings was investigated by corrosion tests which the results showed that the best corrosion behavior was in the case of adding 1 wt% Ag nanoparticles to the coating. Furthermore, studies have also shown that this type of coating could protect the surface from the bacteria by preventing the growth of bacteria generated layers.
Nanotechnology is the recognition and control of nanoscale materials producing superior physical, chemical, and biological properties, enabling new and unique uses [25,26,27,28,29,30,31,32,33,34,35]. An increase in the surface-to-volume ratio, which gradually occurs with a reduction in the size of the particle, ends in overcoming the behavior of the atoms at the surface to the behavior of the internal atoms. In recent decades, various studies have been conducted on the methods for the synthesis and application of metal nanoparticles, especially silver nanoparticles [36]. Silver nanoparticles are used in a variety of areas, such as clothing, medical and military textiles, producing different types of filter, packaging industries, water purification, and paint and varnish. Using silver nanoparticles in industrial coatings is done to improve corrosion behavior, to reach antibacterial properties, and to improve mechanical and thermal properties [24].
In marine environments, severe damage is caused by corrosion attacks on the nuts and bolts, causing their mechanical properties decrease and also may cause the failure of them. Failure of such equipment due to its important usage in flange connections of oil and gas pipelines causes many problems including fluid leakage at the flange joint which resulting in safety and life-threatening contamination and stopping production ultimately. In order to control the corrosion and extend the service life of these components in industrial offshore conditions, it is recommended to use polyurea E300 coating because of its excellent mechanical and corrosion properties. The petroleum tapes are commonly used for controlling the corrosion of nuts and bolts in industrial applications. There is no explicit instruction for paint or coating systems for controlling the corrosion of them. To provide the ability to open and tighten the nuts and the bolts, three-layer common coating systems cannot be used. This study tries to fill a gap in the literature by using the improved polyurea coating with Ag nanoparticles (thin layer), offer appropriate protection system for the equipment.
ASTM A194 is one of the steel types used in manufacturing steel nuts which are used frequently in condition of high temperature or high pressure, or both high temperature and pressure, in oil, gas, and petrochemical industries. This group of steels includes all types of carbon steel, alloy steel, martensitic stainless steel, and austenitic stainless steel [2].
This study has examined the effect of polyurea coating on the improvement of ASTM A194 steel corrosion resistance and the effect of silver nanoparticles on the corrosion of polyurea coatings in a simulated seawater. The study, using the potentiodynamic polarization technique, was an effort to examine the protective behavior of polyurea coatings as well as the effect of adding silver nanoparticles in various mass fraction, i.e., 0.5%, 1%, and 2%, into coating on its corrosion resistance in the simulated seawater electrolyte.
2 Material and Method
Silver nanoparticles supplied by US Research Nanomaterials (USA) were used in this study as additive. To prevent the silver nanoparticle oxidation and reduce re-weighting error which lead to wasting nanoparticles, one-gram packages of nanoparticles were purchased. The properties of the mentioned nanoparticle are listed in Table 1.
Also, polyurea (300 cold) with 70% solids was supplied by Eurotaff Co. (Spain) and used as coating on ASTM A194 steel.
Potentiodynamic polarization test was used to evaluate the protective behavior of E300 grade polyurea coating on ASTM A194 steel and the effect of adding silver nanoparticles to polyurea coatings in a 3.5% NaCl solution. In doing so, the corrosion behavior of the samples was evaluated before and after coating by polyurea and before and after the addition of silver nanoparticles to polyurea coatings at mass concentrations of 0.5, 1, and 2 wt%. The main evaluation factors are corrosion current density (icorr) and corrosion potential (Ecorr).
To evaluate the quality of the coating, the surface of the samples was evaluated using scanning electron microscope (SEM) at various magnifications, and the energy-dispersive X-ray spectroscopy analysis (EDS) of the samples was done.
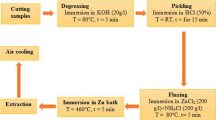
Since the curing agent has less viscosity than the resin (polyurea), a certain amount of it was injected into the silver nanoparticles sachet via a syringe. After complete mixing and ensuring that the nanoparticles had incorporated into the hardener phase well, the nanohardener suspension was stirred at 500 rpm for 15 min at room to achieve a homogenous suspension. ASTM A194 steel bolt of 1 × 1 cm2 was used to provide the bare steel samples as working electrodes. In order to prepare standard electrochemical corrosion samples, the copper wire were soldered to the steel samples for electrical connection and then mounted by resin. The working electrodes were polished using abrasive papers of silicon carbide up to 2500 grit. The samples were degreased by acetone, washed with distilled water and dried by compressed air according to the ASTM G1 standard instructions [37]. The surface cleanliness of the bare steel samples before coating was achieved NACE No.2/SSPC-SP10 requirements by blast cleaning with the use of abrasives. After blasting, the surface profile of the cleaned steel was measured 50 ± 10 micrometer in accordance with ASTM D4417.
After preparing the steel substrate, the polyurea coating was prepared at various concentrations of silver nanoparticles and was applied on the steel surface by immersing. After 24 h, the coated steel samples were cut in 1 × 1 cm2 size with a cutter, and standard samples of corrosion test were prepared. The average dry film thickness (DFT) was determined 150 ± 10 micrometer based on SSPC-PA2 standard.
To conduct the corrosion tests, the Autolab Galvanostat PGSTAT 302 device was used coordinated with the Nova 1.8. To conduct the electrochemical tests on each of the prepared working electrodes, three electrode cells were used, which contained a working electrode (test samples), a platinum auxiliary electrode, and a calomel reference electrode. Potentiodynamic polarization test was conducted at a potentiometric range of − 300 to + 300 mV in relation to the open circuit potential at a scanning speed of 0.5 millivolt per second, and then the potentiodynamic polarization curve of the samples was plotted.
3 Results and Discussion
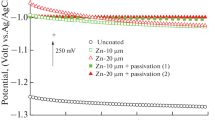
Figure 1 shows potentiodynamic polarization graphs of ASTM A194 uncoated steel, steel with polyurea coating without silver nanoparticles, and three samples with different concentrations of silver nanoparticles (0.5, 1, and 2 wt% silver nanoparticles) in the solution of 3.5% of sodium chloride.
Figure 1 shows that by applying the polyurea coating on the ASTM A194 steel sample, potentiodynamic polarization diagram is significantly shifting toward lower currents and higher potentials. Moreover, by comparing potentiodynamic polarization curve of the steel sample with polyurea coating and polyurea coating with various percentages of silver nanoparticles, one can conclude that by adding silver nanoparticles to the polyurea coating, the graphs of these samples shift toward lower currents and higher potentials.
Table 2 shows the extracted data from polarization curves related to the five samples reported in Fig. 1. In this table, Ecorr is corrosion potential, icorr is corrosion current density, and bc and ba are, anodic and cathodic tafel slopes, respectively.
The results in Table 2 show that by applying the polyurea coating on ASTM A194 steel, the corrosion density decreased by about 109 times relative to the uncoated samples. Additionally, by comparing the sample with polyurea coating and the sample with polyurea coating containing silver nanoparticles additive, it can be concluded that the corrosion density of the coated samples containing additive was improved 1.7, 2.5, and 5.9 times. Thus, the addition of silver nanoparticles improves the corrosion resistance of ASTM A194 steel with polyurea coating. Moreover, the results of Table 2 show that by increasing the concentration of silver nanoparticles from 0.5 to 1%, and from 1 to 2% the corrosion density of the samples decreased and the corrosion behavior of the coating improved. The best corrosion behavior of the three samples listed with silver nanoparticles is related to the sample with 2 wt% silver nanoparticles. This sample has the lowest corrosion density of all tested samples in this study.
The additive quantity is usually depending on the base resin cost. In another word, the cost of additives should not be higher than that of the resin (otherwise, the base resin can be improved to another one). At the time of this study, the polyurea resin was about $ 46/1 kg and silver nanoparticles $23/1 g, so the maximum limit for adding silver nanoparticles was only 2 %wt. Therefore, by increasing the concentration of silver nanoparticles further (> 2%), the nanocomposite coating is not economical.
To investigate corrosion behavior of the sample containing 2% silver nanoparticles in the long term, the experiments were repeated at 3-, 7-, 12-, 18-, and 30-day intervals. Figure 2 illustrates the potentiodynamic polarization diagrams of the polyurea-coated steel containing 2% silver nanoparticles (PU + 2.0 wt% Ag) in 3.5% sodium chloride solution on different days. Table 3 provides the data for these five samples.
According to Table 3, the corrosion current density of the steel sample with the polyurea coating containing 2% silver nanoparticles can be reduced by increasing the time of immersion, thereby corrosion resistance increased. This result shows that the high immersion time has not only had any negative effect on the corrosion protection performance of the mentioned nanocomposite coating but also improved the performance of the corrosion protection.
Improvement of the corrosion behavior in samples with silver nanoparticles can be attributed to the arming of the polyurea coating with silver nanoparticles and the emergence of a composite network of metal and polymer on the steel substrate, the creation of a physical barrier of nanoparticles against the corrosive species, chemical reactions which cause chlorine consumption, changing the nature of corrosive species, trapping and preventing them from movement as well as increasing the occupied volume by nanoparticles in coating as a result of the reaction to chlorine and chemical change of nanoparticles nature from metal to salt or a metal oxide which can change the dielectric coefficient of coating.
Figure 3 shows the images of SEM for ASTM A194 steel samples with polyurea coating containing 2 wt% silver nanoparticles at four different magnifications. In all images, it is clear that the nanoparticles distribution in the polyurea coating layer was well done and there were no agglomerated silver nanoparticles on the surface of the coating. SEM images show that there were no cavities or foreign impurities on the surface of the samples. Additionally, no porosity, cracks, or holes were found on the surface. Due to the fact that silver nanoparticles were well mixed with polyurea resin. In all images and analyzes, a mixture of resin and silver metal nanoparticles has been revealed.
Figure 4 shows the SEM image of the ASTM A194 steel sample coated with polyurea containing 2wt% silver nanoparticles along with its EDS analysis. The presence of Ag peaks in the EDS analyzes confirms the presence of silver nanoparticles on the surface of the coating and the absence of deposition on the substrate, chemical reaction or deterioration of silver nanoparticles. In addition to the Ag peak, the peaks of some elements such as C, O, Au, and Ba are also visible. The observation of different elemental peaks is due to the mixing of the silver nanoparticles with the resin. Furthermore, C and O on the surface of the sample are found due to the abundance of these materials in the atmosphere.
The addition of silver nanoparticles has two obvious effects on coating behavior. Firstly, it fills spaces, holes, and generally possible defects of the coating, and secondly, the anti-corrosion nature of silver nanoparticles. Silver is a noble metal and inherently resistant to corrosive environments. Hence, by adding it to the coating, it shifts the potentiodynamic polarization curves to more positive potential values. Silver nanoparticle has very high wetting properties in contact with polyurea resin and distributed homogeneously across the resin matrix. Silver nanoparticles help to modify the coating structure and eliminate some defects. Silver nanoparticles also have a significant mechanical influence on the structure of the polyurea coating [38]. The addition of metallic particles in nanoscale with high wettability reinforces the coating. Silver nanoparticle-reinforced polyurea coatings provide a semi-composite network of metal and polymer on steel substrates that improve the both corrosion behavior and mechanical properties of the coating. The improvement in the corrosion behavior of polyurea coatings by adding silver nanoparticles can be attributed to the physical and chemical behavior of silver nanoparticles in contact with corrosive electrolytes [38,39,40,41]. The addition of silver nanoparticles creates a physical barrier against the penetration of corrosive species. The higher the coating resistance to penetration of corrosive species, the better the corrosion behavior of the substrate steel. Adding silver nanoparticles to polyurea coating creates physical barriers to the penetration of corrosive species such as chloride ions. The chemical reaction of silver nanoparticles with aggressive ions such as chloride can also alter the nature of the aggressive ion and prevent its destructive activity.
4 Conclusion
In this study, the protective behavior of the polyurea coating containing various concentrations of silver nanoparticles for corrosion control of ASTM A194 alloy was experimentally investigated by using the potentiodynamic polarization test. The corrosion properties of the coated samples were assessed in the chloride-containing electrolyte. The following results were achieved:
By applying polyurea coating on the surface of ASTM A194 steel, the corrosion current density decreased while the potential increased. This result indicated that the corrosion resistance of the polyurea-coated steel was improved compared to the non-coated steel.
By examining the results of the potentiodynamic polarization test of the ASTM A194 steel specimen with polyurea/silver nanocomposite coating, it was observed that by increasing the percentage of silver nanoparticles to the polyurea coating, the corrosion current density of the sample decreased; hence, the corrosion resistance increased. The best sample in terms of corrosion resistance, among the tested specimens, was the ASTM A194 steel specimen with a polyurea coating containing 2 wt% silver nanoparticles.
At 3-, 7-, 12-, 18-, and 30-day intervals, the potentiodynamic polarization test for polyurea coating 2 wt% silver nanoparticles showed that by increasing immersion time, the corrosion current density of the samples decreased. Therefore, the corrosion resistance of the coated sample was improved.
References
Roberge PR (2008) Corrosion engineering: principles and practice, vol 2. McGraw-Hill, New York
Yabuki A, Fathona IW (2019) Recent trends in nanofiber-based anticorrosion coatings. In: Barhoum A, Bechelany M, Makhlouf ASH (eds) Handbook of nanofibers. Springer International Publishing, Cham, pp 905–936. https://doi.org/10.1007/978-3-319-53655-2_38
Sapkota R, Zou J, Dawka S, Bobak JE, Papadopoulos C (2018) Multi-functional thin film coatings formed via nanogrinding. Appl Nanosci 8(6):1437–1444. https://doi.org/10.1007/s13204-018-0812-y
Sai Pavan AS, Ramanan SR (2016) A study on corrosion resistant graphene films on low alloy steel. Appl Nanosci 6(8):1175–1181. https://doi.org/10.1007/s13204-016-0530-2
Ashraf PM, Anuradha R (2018) Corrosion resistance of BIS 2062-grade steel coated with nano-metal-oxide mixtures of iron, cerium, and titanium in the marine environment. Appl Nanosci 8(1):41–51. https://doi.org/10.1007/s13204-018-0650-y
Li DG, Wang JD, Chen DR, Liang P (2015) Influence of molybdenum on tribo-corrosion behavior of 316L stainless steel in artificial saliva. J Bio Tribo Corros 1(2):14. https://doi.org/10.1007/s40735-015-0014-z
Manoj A, Ramachandran R, Menezes PL (2020) Self-healing and superhydrophobic coatings for corrosion inhibition and protection. Int J Adv Manuf Technol 106(5):2119–2131. https://doi.org/10.1007/s00170-019-04758-z
McCafferty E (2010) Introduction to corrosion science. Springer Science & Business Media, Berlin
Indira K, Nishimura T (2017) In situ study of effect of chromium content and epoxy coating on localized corrosion behavior of low-alloy steel using localized electrochemical impedance spectroscopy. J Bio Tribo Corros 3(3):28. https://doi.org/10.1007/s40735-017-0088-x
Hemmati AR, Soltanieh SM, Masoudpanah SM (2018) On the interaction between erosion and corrosion in chromium carbide coating. J Bio Tribo Corros 4(1):10. https://doi.org/10.1007/s40735-018-0128-1
Vijayasarathi P (2019) Characterization and corrosion studies of TiAlN PVD coating by using the polarization test method. J Bio Tribo Corros 5(1):29. https://doi.org/10.1007/s40735-019-0220-1
Ma Y, Zhang Y, Liu J, Ge Y, Yan X, Sun Y, Wu J, Zhang P (2020) GO-modified double-walled polyurea microcapsules/epoxy composites for marine anticorrosive self-healing coating. Mater Des 189:108547. https://doi.org/10.1016/j.matdes.2020.108547
Feng L, Iroh JO (2014) Corrosion resistance and lifetime of polyimide-b-polyurea novel copolymer coatings. Prog Org Coat 77(3):590–599
Orlov V (2016) Computer simulation of optimal thickness of polyurea coating using for trenchless renovation of potable water pipes. Proc Eng 165:1168–1175
Gauch E, LeBlanc J, Shukla A (2018) Near field underwater explosion response of polyurea coated composite cylinders. Compos Struct 202:836–852. https://doi.org/10.1016/j.compstruct.2018.04.048
Beiki H, Keramati M (2019) Improvement of methane production from sugar beet wastes using TiO2 and Fe3O4 nanoparticles and chitosan micropowder additives. Appl Biochem Biotechnol 189(1):13–25. https://doi.org/10.1007/s12010-019-02987-2
Talbert R (2007) Paint technology handbook. CRC Press, Boca Raton
Alarcon EI, Griffith M, Udekwu KI (2015) Silver nanoparticle applications. Springer, New York
Qian X, Song L, Tai Q, Hu Y, Yuen RKK (2013) Graphite oxide/polyurea and graphene/polyurea nanocomposites: a comparative investigation on properties reinforcements and mechanism. Compos Sci Technol 74:228–234
Nantasetphong W, Jia Z, Amirkhizi AV, Nemat-Nasser S (2016) Dynamic properties of polyurea-milled glass composites Part I: experimental characterization. Mech Mater 98:142–153
Zhao Z, Meng F, Tang J, Liu H, Liu H, Yang L, Wang J, Xiong T (2019) A novel method of fabricating an antibacterial aluminum-matrix composite coating doped graphene/silver-nanoparticles. Mater Lett 245:211–214. https://doi.org/10.1016/j.matlet.2019.02.121
Torrico RFAO, Harb SV, Trentin A, Uvida MC, Pulcinelli SH, Santilli CV, Hammer P (2018) Structure and properties of epoxy-siloxane-silica nanocomposite coatings for corrosion protection. J Colloid Interface Sci 513:617–628. https://doi.org/10.1016/j.jcis.2017.11.069
Rahmani S, Omrani A, Hosseini SR (2019) Effects of silica nanoparticles content on the properties and corrosion behavior of electroless Ni-Ba-B alloy coatings. Silicon. https://doi.org/10.1007/s12633-019-00162-0
Manjumeena R, Venkatesan R, Duraibabu D, Sudha J, Rajendran N, Kalaichelvan PT (2016) Green nanosilver as reinforcing eco-friendly additive to epoxy coating for augmented anticorrosive and antimicrobial behavior. Silicon 8(2):277–298. https://doi.org/10.1007/s12633-015-9327-2
Kundan N, Parida B, Keshri AK, Soni PR (2019) Synthesis and characterization of the nanostructured solid solution with extended solubility of graphite in nickel by mechanical alloying. Int J Miner Metall Mater 26(8):1031–1037. https://doi.org/10.1007/s12613-019-1816-7
Coutinho TC, Tardioli PW, Farinas CS (2019) Phytase immobilization on hydroxyapatite nanoparticles improves its properties for use in animal feed. Appl Biochem Biotechnol. https://doi.org/10.1007/s12010-019-03116-9
Jiang L, Chen D, Wang Z, Zhang Z, Xia Y, Xue H, Liu Y (2019) Preparation of an electrically conductive graphene oxide/chitosan scaffold for cardiac tissue engineering. Appl Biochem Biotechnol 188(4):952–964. https://doi.org/10.1007/s12010-019-02967-6
Fard MM, Beiki H (2017) Experimental measurement of solid solutes solubility in nanofluids. Heat Mass Transfer 53(4):1257–1263. https://doi.org/10.1007/s00231-016-1894-2
Shahmohammadi P, Beiki H (2016) A numerical investigation of γ-Al2O3-water nanofluids heat transfer and pressure drop in a shell and tube heat exchanger. Transp Phenom Nano Micro Scales 4(1):29–35
Manouchehrian Fard M, Beiki H (2016) Experimental investigation of benzoic acid diffusion coefficient in γ-Al2O3 nanofluids at different temperatures. Heat Mass Transfer 52(10):2203–2211. https://doi.org/10.1007/s00231-015-1734-9
Hojjat M, Nayebzadeh H, Khadangi-Mahrood M, Rahmani-Vahid B (2017) Optimization of process conditions for biodiesel production over CaO–Al2O3/ZrO2 catalyst using response surface methodology. Chem Pap 71(3):689–698. https://doi.org/10.1007/s11696-016-0096-1
Nasehi P, Mahmoudi B, Abbaspour SF, Moghaddam MS (2019) Cadmium adsorption using novel MnFe2O4-TiO2-UIO-66 magnetic nanoparticles and condition optimization using a response surface methodology. RSC Adv 9(35):20087–20099. https://doi.org/10.1039/C9RA03430G
Beiki H, Soukhtanlou E (2019) Improvement of salt gradient solar ponds’ performance using nanoparticles inside the storage layer. Appl Nanosci 9(2):243–254. https://doi.org/10.1007/s13204-018-0906-6
Abdel-Gaber AM, Awad R, Rahal HT, Moussa D (2019) Electrochemical behavior of composite nanoparticles on the corrosion of mild steel in different media. J Bio Tribo Corros 5(2):49. https://doi.org/10.1007/s40735-019-0241-9
Chintada VB, Koona R (2018) Influence of SiC nano particles on microhardness and corrosion resistance of electroless Ni–P coatings. J Bio Tribo Corros 4(4):68. https://doi.org/10.1007/s40735-018-0186-4
El-Nour KMMA, Eftaiha AA, Al-Warthan A, Ammar RAA (2010) Synthesis and applications of silver nanoparticles. Arab J Chem 3(3):135–140
ASTM G1–03 (2003) Standard practice for preparing, cleaning, and evaluating corrosion test specimens. ASTM International, West Conshohocken, PA
Boomadevi Janaki G, Xavier JR (2019) Evaluation of mechanical properties and corrosion protection performance of surface modified nano-alumina encapsulated epoxy coated mild steel. J Bio Tribo Corros 6(1):20. https://doi.org/10.1007/s40735-019-0316-7
Jain P, Patidar B, Bhawsar J (2020) Potential of nanoparticles as a corrosion inhibitor: a review. J Bio Tribo Corros 6(2):1–12
Verma S, Mohanty S, Nayak SK (2019) A review on protective polymeric coatings for marine applications. J Coat Technol Res 16(2):307–338. https://doi.org/10.1007/s11998-018-00174-2
Abioye OP, Loto CA, Fayomi OSI (2019) Evaluation of anti-biofouling progresses in marine application. J Bio Tribo Corros 5(1):22
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Beiki, H., Mosavi, S.J. Silver Nanoparticles-Polyurea Composite Coatings on ASTM A194 Steel: A Study of Corrosion Behavior in Chloride Medium. J Bio Tribo Corros 6, 66 (2020). https://doi.org/10.1007/s40735-020-00364-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-020-00364-9