Abstract
Deterioration of metals in the form of corrosion is the major problem faced in almost all industries. Annual cost of corrosion world-wide has been estimated to exceed 3% of world’s GDP. Corrosion control of metals is an activity of economic, environmental, aesthetic, and technical importance. Zinc, one of the most important non-ferrous metals, has a wide range of applications in industry as well as in domestic sectors. It is used as a coating material on steel and iron. Corrosion of zinc and galvanized articles can be prevented by using chemical inhibitors. Even though they are proven to be effective for industrial applications, environmental hazards resulted as a consequence cannot be ignored. Over the last decades, research is more focussed towards the use of environmental friendly green inhibitors. In this regard, plant extracts have become important as an environmentally acceptable, readily available, and renewable source for a wide range of inhibitors. This review consolidates and documents an overview of the application of plant products as corrosion inhibitors for the corrosion mitigation of zinc.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Corrosion of metals and its alloys employed in various sectors is an unstoppable but a controlled process. Zinc is currently the fourth most widely consumed metal in the world after iron, aluminium, and copper. It has strong anticorrosive properties [1]. Major application of zinc is in the process of galvanization, which is the process of coating thin layers of zinc to iron or steel to prevent rusting. Zinc is combined with copper (to form brass) and with other metals to form materials that are used in automobiles, electrical components, and household fixtures. It is also used in batteries, in toys, as pigments, activator, and catalyst and also has a wide range of applications in pharmaceuticals [2]. Numerous field and laboratory studies of zinc in atmospheric environments have resulted in the ISO classification system, which is used for practical purposes in order to predict the corrosion rates of zinc in different corrosive mediums. Zinc has been extensively studied for a better fundamental understanding and is today probably the most investigated metal from an atmospheric corrosion perspective [3]. Zinc owes its high degree of resistance to atmospheric corrosion to the formation of insoluble basic carbonate films. The corrosion of zinc in water is largely controlled by the impurities present in the water. The foreign substances in natural waters affect the structure and composition of the resulting films and corrosion products on the surface, which in turn control the corrosion of zinc. In distilled water, which cannot form a protective scale to reduce the access of oxygen to the zinc surface, the attack is more severe than in most types of domestic or river water, which do contain some scale-forming salts. Zinc in contact with acid and strong alkaline solutions causes rapid corrosion. Certain salts, such as the dichromate, borates, and silicates, act as inhibitors to the aqueous corrosion of zinc. Moisture content stimulates corrosion action. SO2 and chlorides have a corrosive action because of the presence of water-soluble and hygroscopic salts [4].
Proper corrosion prevention regulations enable to abolish severe damages which include loss of economy, water resources, ecological pollution, and lack of human values [5]. In industries, acidic solutions are used for pickling, descaling, cleaning, and drilling operations in oil and gas explorations. These processes not only clean the surface but also dissolve it to a certain extent leading to considerable material loss and are prone to corrosion [6]. Inorganic inhibitors like phosphate, chromates, and heavy metal containing compounds are restricted because of the toxicity of these compounds to the human health and the surrounding. The disposal of these compounds into the marine environment can also pose a threat to the aquatic life [7]. Synthetic organic compounds are extensively used and provide a promising efficiency against corrosion of the metal. However due to high manufacturing cost and toxicity concern, their usage is becoming less and the focus is turning towards the usage of eco-friendly green inhibitors. There are wide classes of green inhibitors. They include plant products, amino acids, biopolymers, surfactants, ionic liquids, essential oil, etc [8].
2 Need of Green Inhibitors for Corrosion Control
Synthetic compounds containing multiple bonds and heteroatoms are effective inhibitors. However, some of the organic inhibitors are reported to be either costly or toxic. Toxicity may arise either during the preparation or during the applications. Sometimes toxicity of the inhibitor can cause jeopardizing effects on human beings or on the environment. In additions to this, some inorganic compounds like chromates reported to exhibit extremely good anticorrosive property, but are highly toxic to both human beings and the environment. The safety of human health and environment should be of prime importance. In this regard, there is continuous urge to replace toxic inhibitors by environmental friendly inhibitors [9]. Over the last one decade, research is more focussed towards the environmentally benign, eco-friendly inhibitors. These are known as green inhibitors.
Green corrosion inhibitors are eco-friendly compounds which do not contain heavy metals or other poisonous compounds. They are known as site-blocking elements or adsorption site blockers, due to their adsorptive properties. The term “green inhibitor” or “eco-friendly inhibitor” refers to the substances that have biocompatibility with the natural environment [10]. Also, an increasing research is going on for the effective utilization of natural products, such as plant extracts, essential oils, surfactants, and biopolymers as environmentally friendly corrosion inhibitors [11].
Among various classes of green inhibitors, plant products emerged as effective and efficient corrosion inhibitors [12]. Plant extracts are regarded as green inhibitors with biological origin. Over last decades, they are replacing the synthetic organic and inorganic inhibitors. These extracts contain large amount of tannins, alkaloids, flavonoids, etc., which are rich sources of organic compounds.
Every tropical region has its own cultivation and tons of waste is being produced from the agricultural waste. The biowaste material left after utilization of nutritious compounds will serve as effective inhibitors for protection of the metal surfaces [13,14,15,16,17]. These can be used as corrosion inhibitors at a considerable low cost. Some of the examples, include rice husks [18], solid waste from fresh leaves of banana, sugarcane, and water melon hard core [15], watermelon rind, seeds, and peel [16], tomato peel waste [17], glycolipids produced from sunflower oil cakes and pineapple waste [19], and the seeds of Phoenix dactylifera [20] which are thrown as waste, are used as corrosion inhibitors. The effective utilization and extraction of the phytochemicals from these sources do not require huge financial assistance and work can be carried out at effective low cost with good inhibition efficiency. Thus, plant extracts can be considered as effective green inhibitors with environmental and economic benefits.
Reports are available for diverse applications of plant products in industries where the non-hazardous behaviour is mentioned. A few examples being the use of seeds of Phoenix dactylifera as corrosion inhibitor for steel [20], sweet potato stems, and lettuce flower stalks (Chinese patent) [21]. The plant products that are used served as an effective tool in material saving because most of them showed promising inhibition efficiencies [22,23,24,25].
Even though few review papers are available for using various plant products for corrosion control of mild steel, stainless steel, aluminium, and its alloys, copper no comprehensive literature is available for the use of plant products as corrosion inhibitor for zinc. Zinc being an important non-ferrous metal finds a wide range of applications. This short review gives vivid account of application of plant products for corrosion mitigation of zinc in various acidic, alkaline, and neutral medium. This enables the corrosion engineers and material scientists to easy access the early literature, its content, and recent updates contributing to sustainable and green manufacturing.
In this overview, initially a brief account of corrosion behaviour of zinc and corrosion control of zinc using chemical inhibitors is reported. It is followed by a detailed account for the use of plant products as green inhibitors for corrosion mitigation of zinc in various acidic, alkaline, and nearly neutral mediums.
3 Corrosion Behaviour of Zinc
Zinc undergoes intense corrosion at pH below 6 and above 12. In between 6 and 12 pH, it undergoes very slow corrosion. Zinc is highly susceptible to acid corrosion. Acids are used for pickling [26], descaling [27], and cleaning the metal surface [28]. Cleaning of metals with dilute mineral acids is a mandatory protocol in all industries before they are used for applications. Corrosion behaviour of zinc and its inhibition studies in various acid mediums such as hydrochloric acid, sulphuric acid, phosphoric acid, sulphamic acid has been extensively studied [29]. In alkaline solutions, zinc dissolves slowly, forming zincate ions and evolving hydrogen [30]. Different types of electrolyte had been tested on zinc such as sodium hydroxide and potassium hydroxide. Each of alkaline media can produced corrosion product such as zinc hydroxide carbonate, zinc oxide, and zinc hydroxide [31, 32]. The combination of moisture, oxygen, and salt, especially sodium chloride, damages metal worse than rust does. This corrodes, or eats away, the metal, weakening it and causing it to fall apart [33]. Saltwater attacks the metal leading to its corrosion. Various methods have been employed for the effective inhibition of corrosion in salt water [34].
4 Corrosion Inhibition of Zinc with Organic Inhibitors
Organic inhibitors generally have hetero atoms. O, N, S, and P are found to have higher basicity and electron-donor abilities and thus act as corrosion inhibitor. O, N, S, and P are the active centres for the process of adsorption on the metal surface. The use of inhibitors is one of the best options of protecting metals against corrosion [35]. These inhibitors build up a protective hydrophobic film adsorbed molecule on the metal surface, which provides a barrier to the dissolution of the metal in the electrolyte [36].
The inhibiting effect of some of synthetic organic compounds like ethoxylated fatty acids, organic phosphonium and ammonium compounds, m-substituted aniline-N-salicylidene, ziprasidone, semicarbazide, ethylenediamine N, N′-di (p-methoxybenzylidene), 2-[4-(methylthio) phenyl] acetohydrazide, and ethoxylated fatty alcohols on the corrosion of zinc has been recently reported by several authors [37,38,39,40,41,42,43,44].
5 Corrosion Inhibition of Zinc with Plant Products
The mechanism of action of green inhibitors depends on the structure of the active ingredient that is present and many theories have been put forward to explain it [45]. The active constituents of natural inhibitors vary from one plant species to another but their structures are closely related to their organic counterparts. For example, pepper contains an alkaloid piperine [46], garlic contains allyl propyl disulphide [47], fennel seeds contain limonene (20.8%) and pinene (17.8%) followed by myrcene (15%) and fenchone (12.5%), mustard seeds contain an alkaloid berberine [48], soya bean contains tannins, pectins, flavonoids, steroids, and glycosides, carrot contains pyrrolidine, and castor seed contains the alkaloid ricinine [47]. In eucalyptus oil, the active ingredient is monomtrene-1,8-cineole. Gum exudate has a variety of active ingredients like volatile monoterpenes, hexuronic acid, neutral sugar residues, canaric and triterpene acids. It also contains reducing and non-reducing sugars. Garcinia kola seed extract possess bioflavonoids along with unsaturated fatty acids, primary and secondary amines. The extract of calyx has the presence of ascorbic acid, pigments, amino acids, flavonoids, and carotene [9].
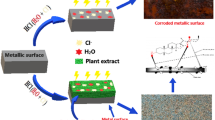
Plant extracts contain polar or hetero atoms such as S, N, O, P, and aromatic rings, favouring the adsorption by donor–acceptor interaction between π electrons of donor atoms S, N, O, P, and aromatic rings of inhibitors, and the vacant d-orbitals of metal surface atoms. Because of adsorption of inhibitor molecules on the metal surface, protective film is formed that isolates the metal from the aggressive environment [49]. Thus, corrosion is controlled. The schematic representation of the action of plant products is given in Fig. 1.
In the present short review, a detailed account of green inhibitors which are used exclusively to control the corrosion of zinc are documented.
5.1 Achillea fragrantissima (Lavender Cotton)
Achillea fragrantissima is a small perennial herb belongs to the family Asteraceae. Ali et al. studied the corrosion of zinc in 0.5 M HCl using aqueous extract of Achillea fragrantissima as the inhibitor. The corrosion rates were calculated using weight loss, hydrogen evolution, and polarization measurements. The inhibition efficiency was found to increase with increase in concentration and decrease with increase in temperature. The adsorption of Achillea fragrantissima constitutes on a zinc surface followed Langmuir adsorption isotherm. Maximum efficiency of 82% was observed for 800 ppm inhibitor concentration. α-Thujone (60.9%), β-thujone (9.1%), sabinene (4.1%), and camphor (3.7%) were characterized as the main constitutes (Fig. 2). The extract acts as inhibitor due to adsorption of its components on the metal surface. Therefore, one can conclude that the major component; Thujone may be responsible for the inhibitive action of the extract. The other components may act in synergism with the major one [50].
5.2 Aloe vera
Abiola et al. studied the effect of the extract of Aloe vera leaves on the corrosion of zinc in 2 M HCl solution using weight loss technique. Aloe vera extract inhibited the corrosion of zinc in 2 M HCl solution and the inhibition efficiency increased with increase in concentration of the extract but decreased with increase in temperature. An efficiency of 67.1% was observed for 10 v/v inhibitor concentration at 30 °C. The adsorption of the inhibitor molecules was consistent with Langmuir adsorption isotherm. Aloe vera contains several biologically active compounds aloin A, aloin B, and aloe emodins. The molecular structure of aloin reveals an anthraquinone link to a pentose with 5 oxygen atoms, and aloe emodins show heterocyclic organic compounds with nitrogen or oxygen atoms (Fig. 3). The inhibition effect of A. vera may be due to the presence of these organic compounds in the extract. Since A. vera contains several compounds, synergistic and antagonistic effects may play an important role on the inhibition efficiency of A. vera as an inhibitor [51].
5.3 Ocimum tenuiflorum (Tulsi)
Sanjay et al. studied the corrosion inhibition properties of Ocimum tenuiflorum leave extract as a potential green inhibitor of zinc corrosion in H2SO4. The results showed that different concentrations of the O. tenuiflorum extract have inhibited zinc corrosion and that the inhibition efficiency varied with the concentration of extract and temperature in H2SO4 medium at 0.5 N, 1.0 N, and 2.0 N at 30 °C and 60 °C. A maximum of 86.2% efficiency was observed for 333 K. It was found that the content of the main constituents was β-bisabolene (13–20%), 1,8-cineole (9–33%), and methyl chavicol (2–12%), which varied during development of the plant [52].
5.4 Trigonella foenum graecum (Fenugreek)
Abdel-Gaber studied the inhibitive effect of fenugreek (Trigonella foenum graecum) seed extract on the corrosion of zinc in aqueous solution of 0.5 M sulphuric acid at 30, 35, 40, and 45 °C. Fenugreek seed extract works as anodic type inhibitor and was used as effective inhibitor for the corrosion of zinc in sulphuric acid media. Inhibition was found to increase with increase in concentration of the extract but decreases with increasing temperature. The corrosion resistance is due to the formation and adsorption of organozinc complex onto the metal surface. The chemical constituents responsible for corrosion control are lysine and l-tryptophan (alkaloid), diosgenin (steroidal saponin), and 4-hydroxyisoleucine (an amino acid) (Fig. 4). Fenugreek seed extract worked as an anodic type inhibitor and could effectively inhibit the corrosion of zinc in sulphuric acid environment [53].
5.5 Cannabis (Hemp)
El-Housseiny studied the corrosion inhibition of zinc in 0.5 M sulphuric acid using the extract of cannabis plant. Inhibition efficiency was found to increase with the increase in inhibitor concentration. Cannabis extract containing 20% ethanol was used for the studies. The maximum inhibition efficiency was found to be 90% for 10 ppm of the inhibitor in 0.5 M sulphuric acid. The adsorption of the inhibitor molecules was consistent with Flory–Huggins isotherm [54].
5.6 Moringa oleifera (Drumstick Tree)
Dass et al. studied the corrosion of zinc in 0.5 M hydrochloric acid medium using the leaf extract of Moringa oleifera. The inhibition efficiency was increased with the increase in the extract concentration. The maximum inhibition efficiency was found to be 67.50%. The functional groups such as –C=O, C=N, C–OH, C=C, Ph–OH present in the organic compounds of the leaf extracts mainly contributed to the inhibition effect. The presence of organic compounds like alkaloids, saponins, tannins, and phenols were responsible for the corrosion inhibition activity [55].
5.7 Mansoa alliacea (Garlic Vine)
Suedile et al. studied the corrosion inhibition of zinc in 3% NaCl using ethanol extract of Mansoa alliacea. Studies were carried out using linear polarization and electrochemical impedance spectroscopy. Potentiodynamic polarization curves indicated that the plant extract behaves as mixed-type inhibitor. The adsorption behaviour followed the Langmuir’s adsorption isotherm. The extract obtained gave inhibition around 90%. The inhibition activity was due to the presence of flavonoids in the crude extract [56]. Schematic representation of various active constituents is shown in Fig. 5.
5.8 Nypa fruticans Wurmb (Mangrove)
Orubite et al. studied the corrosion inhibition of zinc in hydrochloric acid by extract of Nypa Fruticans Wurmb. Optimum inhibition efficiency for zinc in the presence of Nypa fruticans Wurmb extract was 36.43%. Methanolic extract of the Fresh leaves of Nypa fruticans Wurmb was used for the studies. It was suggested that the inhibition was due to the presence of bulky nitrogen containing groups or tannin. The adsorption of constitutents of Nypa fruticans on zinc surface followed Langmuir adsorption isotherm [57].
5.9 Mangifera indica (Mango)
Ugi et al. studied the adsorption characteristics and the inhibition efficiency of flavonoids, alkaloids, and tannins extracts of Mangifera indica leaves on the corrosion control of zinc sheets in 5 M sulphuric acid solution. At 30 °C, the extract offered an inhibition efficiency of 96.2%, 85.3%, and 70.6% in alkaloids, flavonoids, and tannins, respectively. The functional groups such as –C=O, C=N, C–OH, C=C, Ph–OH present in the organic compounds of the leaf extracts mainly contributed to the inhibition effect. Dass et al. studied the corrosion of zinc in 0.5 M hydrochloric acid medium using the leaf extract of Mangifera indica. The inhibition efficiency increased with the increase in the extract concentration. Maximum inhibition efficiency was found to be 58.75%. The presence of organic compounds like alkaloids, saponins, tannins, and phenols were responsible for the corrosion inhibition activity [55, 58].
5.10 Allium cepa (Red Onion)
James et al. studied the corrosion of zinc in 2 M hydrochloric acid solution by using acetone extract of red onion skin (Allium cepa). Quercetin is one of the flavonoid compounds found in red onion skin. It is a compound with conjugated system and contains heteroatoms and carbonyl groups that are electron rich which can serve as a good adsorption site onto the metal surface thereby inhibiting the corrosion of the zinc. Inhibition efficiency of the extract increased with the concentration and temperature [59]. Structure of quercetin is shown in Fig. 6.
5.11 Lupinus (Lupin)
Abd-El-Naby et al. studied the corrosion mitigation of zinc by using lupine seed (Lupinus) extracts 0.5 M sodium chloride medium at 30 °C. A maximum efficiency of 89.1% was obtained for 40 ppm concentration of the inhibitor. The adsorption of the inhibitor molecules was consistent with Langmuir adsorption isotherm. Lupine seeds contain up to 5% quinolizidine alkaloids; the important chemical constituents responsible for the inhibition are lupanine, multiflorane, and sparteine and the schematic representation is shown in Fig. 7 [60].
5.12 Damssisa
Abd-El-Naby et al. studied the corrosion activity of zinc by using Damssisa extracts in 0.5 M sodium hydroxide and 0.5 M sodium chloride medium at 30 °C. A maximum efficiency of 59.5% and 90.7% was obtained for Damssisa at 1000 and 15 ppm in 0.5 M sodium hydroxide and 0.5 M sodium chloride medium, respectively. The adsorption of the inhibitor molecules was consistent with Flory–Huggins isotherm. The important chemical constituents of Damssisa include lactones, damsin, ambrosin, and coumarins (Fig. 8) [60, 61].
5.13 Hlfabar
Abd-El-Naby et al. studied the corrosion activity of zinc by using Hlfabar extracts in 0.5 M sodium hydroxide and 0.5 M sodium chloride medium at 30 °C. A maximum efficiency of 60.2% and 94.7% was obtained for Hlfabar at 1400 and 40 ppm in 0.5 M sodium hydroxide and 0.5 M sodium chloride medium, respectively. The adsorption of the inhibitor molecules was consistent with Langmuir adsorption isotherm. The important constituent responsible for the inhibition activity are hydroxyl-α-eudesmol derivatives (Fig. 9) [60, 61].
5.14 Origanum majorana (Water Marjoram)
Sobhi evaluated the effect of water marjoram (Origanum majorana L.) for the corrosion inhibition of zinc 1.0 M HCl solution. Inhibition efficiency increased with the concentration of the inhibitor. Maximum efficiency of 92.5% was obtained for 500 ppm inhibitor concentration. The important chemical component responsible for corrosion control is thymol and cis-sabinene hydrate (Fig. 10) [62].
5.15 Azadirachta indica
Sanjay et al. studied the corrosion of zinc using Neem (Azadirachta indica (AZI)) leave extract as a green inhibitor in sulphuric acid medium. The results revealed that the inhibition efficiency varied with the concentration of the inhibitor and the temperature. Maximum efficiency of 83.58% and 81.25% was obtained at 30 °C and 60 °C, respectively. The important constituents of the extract include alkaloids, fatty acids, and nitrogen and oxygen-containing compounds [63].
5.16 Tagetes (Marigold)
Chauhan et al. studied the corrosion behaviour of zinc using the plant product of marigold (Tagetes) that is Nyctanthes in 0.5 M HCl. Studies were carried out using Cyclic-Voltameter and potentiometry technique and the data obtained showed that the inhibition efficiency increased with the increase in extract concentration. Schematic representation of various active constituents of nyctanthes is shown in Fig. 11 [64].
5.17 Coriandrum sativum (Coriander)
Neha et al. evaluated the corrosion inhibition studies of zinc in 0.5 M HCl using extracts of coriander seeds and also the incorporation of zinc oxide nanoparticles with coriander seed extract. The results revealed that Np-natural inhibitor was more effective than natural inhibitor. A large number of organic compounds found in the extract were responsible for corrosion inhibition. Maximum efficiency of 93.01% and 89.41% was obtained for Np-natural inhibitor and natural inhibitor, respectively [65].
5.18 Brown Seaweed
Wang et al. studied the corrosion behaviour of zinc in natural sea water using different concentrations of Fucoidan. Potentiodynamic polarization and electrochemical impedance studies were conducted. Maximum efficiency of 92.9% was observed for 120 mg/l of the extract. Fucoidan is extracted mainly from brown seaweeds and is a heteropolysaccharide containing fucose units and sulphate groups (Fig. 12). The adsorption followed Langmuir adsorption isotherm [66].
6 Summary and Future Recommendation
Corrosion inhibition capacity of different plant extracts is discussed in detail. In all the reported literature, corrosion and inhibition studies were done both by classical method and electrochemical methods. Weight loss method and hydrogen evolution methods are most preferred classical methods. Among electrochemical methods, potentiodynamic polarization method and electrochemical impedance spectroscopy methods are widely adopted. In some of the literature, it was obvious that there is an effort to isolate the single most active constituent which is responsible for corrosion inhibition process. Unless and otherwise stated, in all literature, synergistic effect of all the constituents is reported to be responsible for corrosion inhibition process. In all the studies, kinetic and thermodynamic parameters were evaluated and discussed in detail. Surface studies were done by SEM and EDX techniques. Adsorption of the inhibitor on the metal surface was further confirmed by taking FT-IR spectrum of the inhibitor and corrosion product (Table 1).
Even though many references are available for using plant products as corrosion inhibitor, the use of other classes of green inhibitors like surfactants, amino acids, ionic liquids, and biopolymers for corrosion control of zinc is highly limited. Umoren et al. [67] have reviewed and reported utility of variety of biopolymers for the corrosion behaviour of different metals. In our group, Charitha et al. [68,69,70,71] have carried out corrosion inhibition studies on aluminium alloy and aluminium composite material by using novel carbohydrate biopolymers like pullulan, inulin, starch, and dextran. Further these biopolymers were also used to enhance the coating characteristics of epoxy resin [72]. Biopolymers emerged as effective eco-friendly inhibitors because of their structure containing large number of electron donating groups, non-toxicity, biodegradability, and eco-friendliness. The use of these biopolymers as corrosion inhibitors for zinc is not much explored. In addition to this, there is a wide scope for using different classes of green inhibitors like surfactants, amino acids, and ionic liquids for the corrosion control of zinc and galvanized articles.
References
Zhang XG (1996) Corrosion and electrochemistry of zinc. Springer, New York
Narayana H, Praveen BM, Prasanna BM, Venkatesha TV (2015) Anticorrosion potential of a pharmaceutical intermediate Floctafenine for zinc in 0.1 M HCl solution. Int J Ind Chem 6:221–231
Odnevall WI, Leygraf C (2017) A critical review on corrosion and runoff from zinc and zinc-based alloys in atmospheric environments. Corros 73:1060–1061
Peter M, Peter P (2011) Corrosion and corrosion protection-handbook of hot-dip galvanization. Wiley, Germany
Pandian BR, Ismail M, Seyedmojtaba G, Jahangir M, Mokthar CI, Saeid K, Afidah AR (2016) Reviews on corrosion inhibitors—a short view. Chem Eng Commun 203:1145–1156
Fiori-Bimbi MV, Alvarez PE, Vaca H, Gervasi CA (2015) Corrosion inhibition of mild steel in HCl solution by pectin. Corros Sci 92:192–199
Roy P, Karfa P, Adhikari U, Sukul D (2014) Corrosion inhibition of mild steel in acidic medium by polyacrylamide grafted Guar gum with various grafting percentage: effect of intramolecular synergism. Corros Sci 88:246–253
Sigircik G, Tuken T, Erbil (2016) Assessment of the inhibition efficiency of 3,4-diamino benzonitrile against the corrosion of steel. Corros Sci 102:437–445
Chigondo M, Chigondo F (2016) Recent natural corrosion inhibitors for mild steel: an overview. J Chem 2016:1–7
Sanjay KS, Ackmez M, Essam K, Gargi J (2008) Green corrosion inhibitors: an overview of recent research. Int J Corros Sci Eng 11:1–32
Znini M, Majidi L, Bouyanzer L, Paolini J, Desjobert JM, Costa J, Hammouti B (2012) Essential oil of Salvia aucheri mesatlantica as a green inhibitor for the corrosion of steel in 0.5 M H2SO4. Arab J Chem 5:467–474
Deepa P, Padmalatha R (2013) Coriandrum sativum L-A novel green inhibitor for the corrosion inhibition of aluminium in 1.0 M phosphoric acid solution. J Environ Chem Eng 1:676–683
Stefania M, Luisella V, Stefano PT (2019) Green corrosion inhibitors from natural sources and biomass wastes. Molecules 2019:24–48
Hofer R, Bigorra J (2008) Biomass-based green chemistry: sustainable solutions for modern economies. Green Chem Lett Rev 1:79–97
Ismail M, Abdulrahman AS, Hussain MS (2011) Solid waste as environmental benign corrosion inhibitors in acidic medium. Int J Eng Sci 3:1742–1748
Odewunmi NA, Umoren SA, Gasem ZM (2015) Watermelon waste products as green corrosion inhibitors for mild steel in HCl solution. J Environ Chem Eng 3:286–296
Grassino AN, Halambek J, Djakovic S, Rimac Brncic S, Dent M, Grabaric Z (2016) Utilization of tomato peel waste from canning factory as a potential source for pectin production and application as tin corrosion inhibitor. Food Hydrocoll 52:265–274
Pramudita M, Sukirno Nasikin M (2018) Rice husk extracts ability to reduce the corrosion rate of mild steel. Int J Chem Eng App 9:143–146
Amr AK, Samy S, Mohamed N, Mona R (2018) Effect of fungal glycolipids produced by a mixture of sunflower oil cake and pineapple waste as green corrosion inhibitors. J Environ Sci Technol 11:119–131
Rexin Thusnavis G, Vinod Kumar KP (2014) Green corrosion inhibitor for steel in acid medium. Application No. 6278/CHE/2014 A, 12 December 2014
Extract Corrosion Inhibitor of Sweet Potato Stems and Lettuce Flower Stalks and Preparation Method Thereof. Patent No. CN102492948B, 31 July 2013
Indian Oil Corporation Limited. Naturally derived corrosion inhibitors composition, process for preparing the same and use thereof 2008. Patent Application 10 March 2008
Ponciano Gomes JA, Cardoso Rocha J, D’Elia E (2015) Use of fruit skin extracts as corrosion inhibitors and process for producing same. U.S. Patent US8926867B2, 6 January 2015
Raja PB, Sethuraman MG (2008) Natural products as corrosion inhibitor for metals in corrosive media—a review. Mater Lett 62:113–116
Sangeetha M, Rajendran S, Muthumegala TS, Krishnaveni A (2011) Green corrosion inhibitors—an overview. Zastita Materijala 52:1–19
Hassane L, Subrahmanya BK, Rachid S, Shubhalaxmi C, Shehdeh J, Manuel A, Belkheir H, Ismat HA, Azzouz E (2017) Insights into corrosion inhibition behavior of three chalcone derivatives for mild steel in hydrochloric acid solution. J Mol Liq 225:271
Lgaz H, Salghi R, Jodeh S (2016) Corrosion inhibition potentiality of some benzimidazole derivatives for mild steel in hydrochloric acid: electrochemical and weight loss studies. Int J Corros Scale Inhib 5:347–359
Lgaz H, Toumiat K, Jodeh S, Salghi R (2016) Inhibition of mild steel corrosion in 1 M HCl medium by tangeretin. Appl J Environ Eng Sci 2:72–77
Selvakumar P, Balanaga KB, Thangavelu C (2013) Corrosion inhibition study of stainless steel in acidic medium—an overview. Res J Chem Sci 3:87–95
Binder L, Kordesch K (1984) Corrosion of zinc electrode mixtures in alkaline media. J Electroanal Chem Interfacial Electrochem 180:495–510
Ismail WMIM, Zulkefeli NSW, Masri MN (2016) A sight of zinc corrosion in various alkaline media. J Trop Resour Sustain Sci 4:95–97
Tuan KAH, Nam LD, Kyung EKS, Chen P (2015) Corrosion chemistry and protection of zinc & zinc alloys by polymer-containing materials for potential use in rechargeable aqueous batteries. RSC Adv 5:41677–41691
Lindström R, Svensson JE, Johansson LG (2000) The atmospheric corrosion of zinc in the presence of NaCl the influence of carbon dioxide and temperature. J Electrochem Soc 147:1751
Mouanga M, Berçot P, Rauch JY (2010) Comparison of corrosion behaviour of zinc in NaCl and in NaOH solutions. Part I: corrosion layer characterization. Corros Sci 52:3984–3992
Anjali P, Obot IB, Sanjay KS (2015) Use of natural gums as green corrosion inhibitors: an overview. Int J Ind Chem 6:153–164
Yıldırım A, Çetin M (2008) Synthesis and evaluation of new long alkyl side chain acet- amide, isoxazolidine and isoxazoline derivatives as corrosion inhibitors. Corros Sci 50:155–165
Foad El-Sherbini E, Abdel Wahaab ESM, Deyab M (2005) Ethoxylated fatty acids as inhibitors for the corrosion of zinc in acid media. Mater Chem Phys 89:183–191
Morad MS (1999) Inhibition of phosphoric acid corrosion of zinc by organic onium compounds and their adsorption characteristics. J Appl Electrochem 29:619–626
Talati JD, Desai MN, Shah NK (2005) meta-Substituted aniline-N-salicylidenes as corrosion inhibitors of zinc in sulphuric acid. Mater Chem Phys 93:54–64
Shylesha BS, Venkatesha TV, Praveen BM (2011) Ziprasidone as a corrosion inhibitor for zinc in different acid medium. J Chem Pharm Res 3:501–507
Fouda AS, Madkour LH, Shafei AAE, Abd El-Maksoud SA (1995) Corrosion inhibitors for zinc in 2 M HCl solution. Bull Korean Chem Soc 16:454–458
Agrawal Y, Talati K, Shah JD, Desai MD, Shah NK (2004) Schiff bases of ethylenediamine as corrosion inhibitors of zinc in sulphuric acid. Corros Sci 46:633–651
Shylesha BS, Venkatesha TV, Praveen BM (2011) New electroactive compounds as corrosion inhibitors for zinc in acidic medium. Adv Appl Sci Res 2:333–341
Abdallah M (2003) Ethoxylated fatty alcohols as corrosion inhibitors for dissolution of zinc in hydrochloric acid. Corros Sci 45:2705–2716
Yadav M, Gope L, Kumari N, Yadav P (2016) Corrosion inhibition performance of pyranopyrazole derivatives for mild steel in HCl solution: gravimetric, electrochemical and DFT studies. J Mol Liq 216:78–86
Quraishi MA, Yadav DK, Ahamad I (2009) Green approach to corrosion inhibition by black pepper extract in hydrochloric acid solution. Open Corros J 2:56–60
Rani BEA, Basu BBJ (2012) Green inhibitors for corrosion protection of metals and alloys: an overview. Int J Corros 2012:1–15
Lahhit N, Bouyanzer A, Desjobert JM (2011) Fennel (Foeniculum vulgare) essential oil as green corrosion Inhibitor of carbon steel in hydrochloric acid solution. Port Electrochim Acta 29:127–138
Emran KM, Ali SM, Lehaibi HAA (2018) Green methods for corrosion control. Corros Inhib Princ Recent Appl 3:61–78
Ali AI, Megahed HE, Mona AE, Ismail MN (2013) Zinc corrosion in HCl in the presence of aqueous extract of Achillea fragrantissima. J Mater Environ Sci 5:923–930
Abiola OK, James AO (2010) The effects of Aloe vera extract on corrosion and kinetics of corrosion process of zinc in HCl solution. Corros Sci 52:661–664
Sanjay K, Mudhoo A, Jain G, Jyoti S (2009) Inhibitory effects of Ocimum tenuiflorum (Tulsi) on the corrosion of zinc in sulphuric acid: a green approach. Rasayan J Chem 2:332–339
Abdel-Gaber AM (2007) Effect of immersion time and temperature on the inhibition of the acid corrosion of zinc by fenugreek seeds extract. Int J Appl Chem 3:161–174
El-Housseiny S (2017) Inhibition of the acid corrosion of zinc by Cannabis plant extract. Am J Chem 7:6–15
Dass PM, Onen AI, Maitera ON, Ushahemba G (2015) Corrosion inhibition of zinc in acid medium by Moringa oleifera and Mangifera indica leaves extracts. Int J Dev Sustain 4:940–950
Suedile F, Robert F, Roos C, Lebrini M (2014) Corrosion inhibition of zinc by Mansoa alliacea plant extract in sodium chloride media: extraction, characterization and electrochemical studies. Electrochim Acta 133:631–638
Orubite OK, Oforka NC (2004) Corrosion inhibition of zinc on HCl using Nypa fruticans Wurmb extract and 1,5 diphenyl carbazonen. J Appl Sci Environ Manag 8:56–61
Ugi B, Ekerete J, Ikeuba IA, Uwah IE (2015) Mangifera indica leave extracts as organic inhibitors on the corrosion of zinc sheet in 5 M H2SO4 solution. J Appl Sci Environ Manag 19:145–152
James AO, Akaranta O (2011) Inhibition of corrosion of zinc in hydrochloric acid solution by red onion skin acetone extract. Res J Chem Sci 1:31–37
Abd-El-Naby BA, Abdullatef OA, Abd-El-Gaber AM, Shaker MA, Esmail G (2014) Electrochemical studies on the inhibitive action of Damssisa and Halfabar on the alkaline corrosion of zinc. Int J Electrochem Sci 9:1163–1178
Abd-El-Naby BA, Abdullatef OA, Abd-El-Gaber AM, Shaker MA, Esmail G (2012) Effect of some natural extracts on the corrosion of zinc in 0.5 M NaCl. Int J Electrochem Sci 7:5864–5879
Sobhi M (2013) Marjoram extract as corrosion inhibitor for dissolution of zinc in 1.0 M HCl. Int J Corros 2013:1–7
Sanjay KS, Ackmez M, Gargi J, Essam K (2009) Corrosion inhibition of neem (Azadirachta indica) leaves extract as a green corrosion inhibitor for Zinc in H2SO4. Green Chem Lett Rev 2:47–51
Chauhan JS, Anita D, Gupta DK (2013) Corrosion inhibition of Zn in HCL by Nictanthes plant extract. Asian J Adv Basic Sci 1:58–61
Neha P, Divya L, Poonam W, Mukesh M, Suresh K, Nisha S (2015) Comparative study of natural inhibitor and Np-natural Inhibitor for the corrosion protection of Zinc in HCl. Nano Vision 5:295–304
Wang C, Zhang J, Chen XL, Xiang B, Duan JZ, Hou BR (2016) Inhibition of zinc corrosion by Fucoidan in natural sea water. Acta Metall Sin 30:594–600
Umoren SA, Eduok UM (2016) Application of carbohydrate polymers as corrosion inhibitors for metal substrates in different media: a review. Carbohydr Polym 140:314–341
Charitha BP, Padmalatha R (2017) Environmentally benign green inhibitor to attenuate acid corrosion of 6061Aluminum15%(v) SiC(P) composite. J Ind Eng Chem 58:357–368
Charitha BP, Padmalatha R (2017) Carbohydrate biopolymer for corrosion control of 6061 Al-alloy and 6061Aluminum-15%(v) SiC(P) composite—Green approach. Carbohydr Polym 168:337–345
Charitha BP, Padmalatha R (2018) Pullulan as a potent green inhibitor for corrosion mitigation of aluminum composite: electrochemical and surface studies. Int J Biol Macromol 112:461–472
Charitha BP, Padmalatha R (2016) An ecofriendly approach for corrosion control of 6061 Al-15%(v) SiC(P) composite and its base alloy. Chin J Chem Eng 25:363–372
Charitha BP, Padmalatha R (2017) Enhancement of surface coating characteristics of epoxy resin by dextran: an electrochemical approach. Ind Eng Chem Res (ACS publications) 56:1137–1147
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pais, M., Rao, P. Biomolecules for Corrosion Mitigation of Zinc: A Short Review. J Bio Tribo Corros 5, 92 (2019). https://doi.org/10.1007/s40735-019-0286-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-019-0286-9