Abstract
The corrosion inhibition performance of the synthesized green compound namely: 4,4′-(1,4phenylene)bis(6-amino-3-methyl-2,4 dihydropyrano[2,3-c]pyrazole-5-carbonitrile) (BPP) has been tested for mild steel (MS) in 0.5 M H2SO4. Two techniques (gravimetric and electrochemical) have been utilized to evaluate the corrosion inhibition performance of BPP. The experimental results indicate that BPP shows excellent inhibition efficiency, i.e. 98% at 150 mg L− 1. Langmuir adsorption isotherm was fitted well for the adsorption of BPP molecules on MS surface. The electrochemical technique such as electrochemical impedance spectroscopy results revealed increase in charge transfer resistance in presence of BPP and polarization study indicates mixed type of inhibitor action. The surface morphology for MS with and without BPP was studied by scanning electron microscopy and atomic force microscopy which suggested corrosion retardation in the presence of BPP.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Corrosion of metals is a matter of serious concern in industries during acid pickling, etching and descaling processes. In comparison with other metal/alloys, mild steel (MS) is highly used metal in industries as a structuring material. This is because of low cost and high mechanical properties of MS [1, 2]. But along with these properties, it is also susceptible to corrosion during surface cleaning process, which caused severe financial and industrial losses. To fight with this serious problem, the use of corrosion inhibitors is considered as the best method for corrosion control [3]. The selection preference of organic corrosion inhibitor is based on some points: non-toxic compounds, presence of heteroatoms (N, O and S) and π electrons in the moiety etc. The organic inhibitors having electron-rich centres pump more electrons to interact with vacant d-orbital of surface iron atoms [4,5,6,7]. The adsorption of inhibitor molecule creates a protecting barrier on metal surface to stop the direct contact with acid solution which prevents corrosion. These points impelled us to test bispyranopyrazole as corrosion inhibitor for MS in 0.5 M H2SO4. Pyranopyrazoles are important class of heterocyclic compounds including pharmaceuticals and biodegradable agrochemicals. Pyranopyrazoles compounds exhibit a wide range of biologically activities such as anticancer, antibacterial, antiherpetic, anti-inflammatory, and molluscicidal activity [8, 9]. Bispyranopyrazoles are highly functionalized compounds with NH2, CN as functional groups and possess four N and two O atoms in the ring, besides π electrons, through which they can get readily, adsorb on MS surface and bring down corrosion rate.
The aim of present study is to test the corrosion inhibition performance of 4,4′-(1,4phenylene)bis(6-amino-3-methyl-2,4 dihydropyrano[2,3-c]pyrazole-5-carbonitrile) (BPP) for MS in 0.5 M H2SO4 by using gravimetric method, electrochemical impedance spectroscopy (EIS) and potentiodynamic polarization (PDP) techniques. The surface studies of MS were carried out by using scanning electron microscopy (SEM) and atomic force microscopy (AFM) instruments.
2 Experimental
2.1 Materials and Solutions
The MS samples used in corrosion study have following composition (wt%): C 0.15%, Mn 1.02%, Si 0.08%, S 0.02%; and balance Fe 98.72%. For gravimetric measurements, the MS samples were mechanically cut into 3 cm × 1 cm × 0.1 cm and for electrochemical analysis cubical shaped MS samples rod samples with dimensions 4 cm × 1 cm × 1 cm were connected at one end with the copper wire and cover the entire surface with epoxy resin with only the uncovered active surface of 1 cm2. Before to each experimental, the MS surface was abraded with emery paper (600–1200 grade), washed with water, acetone and dried. The test solution of 0.5 M H2SO4 was prepared by diluting analytical grade concentrated sulphuric acid with double distilled water.
2.2 Inhibitors
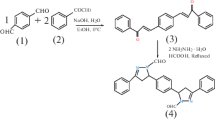
The 4,4′-(1,4phenylene)bis(6-amino-3-methyl-2,4 dihydropyrano[2,3-c]pyrazole-5-carbonitrile) (BPP) was synthesized according to scheme previously reported in the literature [9], and the schematic root is shown in Fig. 1. On completion of the reaction, the obtained precipitate was filtered off and washed thoroughly with EtOH to give the desired product. The molecular structure of BPP and analytical data is given in Table 1. The 1H NMR spectra recorded on a Bruker instrument at 500 MHz, and IR spectra recorded on an FTIR (02) [Perkin Elmer, Bruker] spectrophotometer.
2.3 Gravimetric Measurements
The gravimetric experiment was performed by using MS specimens in 0.5 M H2SO4 without and with different concentrations of BPP. The MS coupons were firstly abraded, washed, dried and the weighed accurately. These MS samples were immersed in a 100-mL conical flask for 6 h at 298 K. After that the samples were takeout washed with water and acetone then dried and weighed. By determining the difference in weight loss of MS samples, we can calculate the corrosion parameters like corrosion rate (CR), inhibition efficiency (η%) and surface coverage (θ) by using equations:
where W is the weight loss of specimen (mg), A is the area of the MS coupon (cm2), t represents the immersion time (h), and CR and CR(i) represents the corrosion rates in the absence and presence of BPP.
2.4 Electrochemical Measurements
A three-electrode cell system for electrochemical analysis (250 mL) connected to the CHI 760c (CH Instruments, Inc., USA) was used to perform electrochemical experiments. The three-electrode cell system contains MS as working, platinum as auxiliary and saturated calomel electrodes (SCEs) as reference electrodes, respectively. The SCE is fitted in a bent luggin capillary tube and filled with the prepared test solution. All the electrochemical experiments were performed by using the prepared MS samples as mentioned above with an exposed surface area of 1 cm2 at temperature 298 K. The experiments were performed after 1 h of immersion period of three-electrode cell in the absence and presence of BPP in 0.5 M H2SO4, placed in the thermostat (Julabo-F34). EIS measurements were taken in a frequency range, 100 kHz to 10 mHz, with the amplitude of 5 mV AC signal. Tafel curves were obtained by sweeping the electrode potential from − 0.9 to + 2.0 V versus open circuit potential at 0.01 mV s− 1 scan rate.
2.5 Surface Characterization
2.5.1 Scanning Electron Microscopy
Surface morphology was studied by using SEM instrument (Jeol Japan, Model No. JSM-6610LV). The abraded MS coupons having dimension 1 cm × 1 cm × 0.1 cm were used for surface analysis. In order to analyse the surface morphology of MS, it was immersed in 0.5 M H2SO4 for 6 h without and with optimum concentration of BPP at 298 K.
2.5.2 Atomic Force Microscopy
AFM is another surface analysis technique which deals with the surface roughness on MS surface in the absence and presence of BPP. AFM analysis was performed by using an instrument (AG Nanosurf NaioAFM, Switzerland) with a Si3N4 cantilever (Nanosensor, CONTR type) having a spring constant of ~ 0.1 N m− 1 and tip radius > 10 nm.
3 Results and Discussion
3.1 Gravimetric Measurements
The results obtained from the gravimetric measurements of MS in 0.5 M H2SO4 without and with BPP are listed in Table 2, which shows the addition of different concentrations of BPP; the corrosion inhibition efficiency increases, while corrosion rate decreases. This behaviour suggests that corrosion inhibition depends upon inhibitor concentration. Gravimetric method is a reliable technique to optimize the effect of inhibitor concentrations on corrosion inhibition efficiency. The trend of corrosion inhibition efficiency and retardation in corrosion rate obtained on increasing BPP concentrations are shown in Fig. 2. The highest inhibition performance is obtained for BPP, i.e. 98.52% at optimized concentration of 150 mg L− 1. This excellent inhibition for MS in the presence of BPP is credited to adsorption of BPP molecules on MS surface. The large molecule of BPP along with various hetero atoms and π electrons facilitated the adsorption process on MS surface [10,11,12].
3.2 Adsorption Isotherm
The corrosion inhibition performance of investigated inhibitor mainly depends on its adsorption trend on the metal surface. For that reason, the adsorption isotherms can help to study the interaction between the used inhibitor molecules with MS surface and which type of adsorption occur either physical or chemical type [13,14,15]. To understand the adsorption mechanism, the resultant surface coverage (θ) values, i.e. obtained by gravimetric analysis at different concentrations of BPP were fitted to various adsorption isotherms, namely Langmuir, Temkin and Freundlich. The Langmuir adsorption isotherm was found to be the best fitted adsorption isotherm among them (Fig. 3) with regression coefficient (R2) values near to 1 (0.9998), and the equation is represented as below,
In the above equation, C is the concentration of inhibitor, θ represents the surface area, and Kads is adsorption equilibrium constant. By plotting C/θ and C the Kads value can be obtained in order to obtain free energy of adsorption ΔGads. Kads is related to the ΔGads by the following equation,
where R is the gas constant and T is the absolute temperature. The value of 55.5 is the concentration of water in solution in ML− 1. By putting Kads in the above equation the ΔGads values were obtained.
It is reported as, the values of ΔGads which is about − 40 kJ M− 1 or more negative suggests chemisorption and ΔGads values around − 20 kJ M− 1 or less negative implies physisorption [16]. The ΔGads obtained using BPP inhibitor is − 37 kJ M− 1, which is near to chemisorption range. But the adsorption process can not only classified as chemisorption because chemical interactions between inhibitor molecules and metal surface are usually preceded with some form of electrostatic interactions (physisorption) [17].Thus, there is a complex mode of adsorption of both physisorption as well as chemisorption.
3.3 Electrochemical Measurements
3.3.1 Electrochemical Impedance Spectroscopy
The change in corrosion behaviour in term of capacitance as well as resistance at MS surface without and with BPP has been analysed by employing EIS technique at 298 K. The Nyquist loops, Equivalent circuit model, Bode and phase angle plots obtained by EIS are shown in Figs. 4, 5, and 6, and calculated data from Nyquist loops are listed in Table 3. Figure 4 shows that Nyquist loops exhibit a depressed capacitive semicircle loop under the real axis. This behaviour may be because of the rough MS surface, and inhomogeneity creates due to accumulation of corrosion product and frequency dispersion [16]. The semicircular loop for both conditions in the presence and absence of inhibitor is same representing the similar corrosion mechanism. The diameter of the Nyquist loops increases with effect of BPP as compared to blank 0.5 M H2SO4 acid solution which confirms adsorption of inhibitor molecules on MS surface. The relatively obtained Bode (log |Z| vs. log f) and phase angle plots (α° vs. log f) are presented in Fig. 6. This is apparent in the Bode and phase angle plots that with addition of BPP, deviation is observed from blank acid curves suggested capacitive performance and also the single peak in phase angle indicates only one time constant [17]. The analysis of obtained Nyquist loops is executed by using equivalent circuit model consisting a solution resistance (Rs), constant-phase element, which is parallel to the charge transfer resistance (Rct) (Fig. 5). The corrosion inhibition performance (η%) in the presence of BPP is calculated by using the given equation,
where Rct(i) and Rct represent the charge transfer resistance with and without BPP, respectively. The calculated data depicted in Table 3 clearly indicated increase in corrosion inhibition performance from 75 to 97% on increase in the BPP concentrations from 25 to 150 mg L− 1. The highest the Rct obtained is 61.82 Ω cm2 at 150 mg L− 1, respectively.
It is also observed from Table 3 that increases in BPP concentrations cause a decrease in Cdl values. This decrease in the Cdl values points towards more adsorption of inhibitor molecules on MS surface [18].
The double layer capacitance (Cdl) was calculated by the equation:
where fmax indicates the frequency at maxima in the Nyquist plot. The relationship between Cdl and thickness of the protective layer (d) is:
where ε represents the dielectric constant, ε0 is the permittivity of free space, and A is the surface area of the electrode.
3.3.2 Potentiodynamic Polarization Measurements
In continuation to electrochemical techniques, the PDP is another helpful method to study corrosion inhibition performance and the kinetics involve in the anodic and cathodic reactions occurring on MS surface in the absence and presence of BPP. The corresponding Tafel plot is presented in Fig. 7, and experimental results are listed in Table 4. The result analysis reveals that the addition of BPP causes changes in both the anodic and cathodic curve (Fig. 7). The Tafel slopes (βa, βc) values clearly indicate that the tested inhibitor inhibits both the process: anodic metal dissolution as well as the cathodic hydrogen evolution. The lowering of both ββa, βc values in the presence of BPP as compared to the blank 0.5 M H2SO4 acid solution confirms the suppression for both reactions acting on the MS surface [19, 20]. In addition, the other important parameter, i.e. corrosion current density (icorr), shows a decrease in values with corresponding increase in BPP concentrations supported corrosion retardation process. The lowest observed icorr is 136 µA cm−2 at 150 mg L− 1. The shift in corrosion potential (Ecorr) of BPP in comparison with blank acid solution indicated mixed type action of the inhibitor for MS [21]. BPP exhibited good inhibition performance 97.78% at 150 mg L− 1. The higher inhibition performance is due to the presence of various active sites in BPP molecules to interact with MS surface, and the more the adsorption more will be the surface coverage that forms a barrier to retard metal corrosion [22,23,24,25]. The inhibition efficiency with the help of icorr can be calculated by using the equation:
where icorr and \(i_{{{\text{corr}}}}^{ \circ }\) are the corrosion current density in the absence and presence of BPP, respectively.
3.4 Surface Characterization
3.4.1 Scanning Electron Microscopy
The surface morphological pictures obtained for MS with and without BPP at 298 K for 6 h in 0.5 H2SO4 are shown in Fig. 8a, b. The obtained SEM micrograph clearly depicts the difference between MS exposed to acid solution and with inhibitor molecules. The MS in contact with aggressive acid solution without inhibitor undergoes severe corrosion showing rough surface as shown in Fig. 8a. On the other hand, the MS immersed with inhibitor is quite smooth shown in Fig. 8b suggested the corrosion inhibition phenomenon [26, 27] in the presence of BPP.
3.4.2 Atomic Force Microscopy
The AFM is also a useful technique for surface study and also gives information about surface roughness. The AFM images of MS in the absence and presence of BPP are shown in Fig. 9a, b. The MS immersed in acid shows various up and downs with highly rough surface with average surface roughness 775 nm (Fig. 9a), while the MS dipped in acid solution with BPP is smooth and the average roughness is 111 nm (Fig. 9b). This reduction in average surface roughness indicates the better corrosion resistance in the presence of BPP [28, 29].
3.5 Mechanism of Inhibition
The explanation of corrosion inhibition phenomenon is based on adsorption phenomenon [30]. Adsorption of inhibitor molecules on MS surface blocked the active corrosion cites on metal surface and creates a barrier between MS/solution interfaces. Generally, adsorption process in case of metal depends upon the nature and surface charge on the metal and chemical structure of the inhibitor molecules [31]. MS is positively charged with respect to the potential zero charge in acid solution [32]. The inhibitor molecule may adsorb on MS surface by following ways:
(a) By the donor–acceptor type of interaction in between π electrons available of aromatic ring and vacant d-orbital of Fe. (b) Transfer of electrons from heteroatoms containing the unshared electron pairs to the empty orbital of Fe. (c) Back donation from electrons available in d-orbital of Fe to the vacant orbital of hetero atom. (d) Interaction between the protonated inhibitor molecules with the adsorbed sulphate ions on metal surface [33] (Fig. 10).
4 Conclusions
Synthesized novel bispyranopyrazole: 4,4′-(1,4phenylene)bis(6-amino-3-methyl-2,4 dihydropyrano[2,3-c]pyrazole-5-carbonitrile) showed excellent inhibition performance, i.e. 98% at 150 mg L−1, respectively. EIS analysis showed that increasing concentration of inhibitor causes increases in the Rct values for MS and decrease in Cdl observes supported corrosion inhibition for MS. PDP analysis suggests that BPP acted as mixed type inhibitors. SEM and AFM corroborated the formation of film on the MS surface in the presence of BPP.
References
Behpour M, Ghoreishi SM, Soltani N, Salavati-Niasari M, Hamadanian M, Gandomi A (2008) Electrochemical and theoretical investigation on the corrosion inhibition of mild steel by thiosalicylaldehyde derivatives in hydrochloric acid solution. Corros Sci 50:2172–2181
Fouda AS, Ellithy AS (2009) Inhibition effect of 4-phenylthiazole derivatives on corrosion of 304 L stainless steel in HCl solution. Corros Sci 51:868–875
Döner A, Solmaz R, Özcan M, Kardas G (2011) Experimental and theoretical studies of thiazoles as corrosion inhibitors for mild steel in sulphuric acid solution. Corros Sci 53:2902–2913
Shukla SK, Quraishi MA, Prakash R (2008) A self-doped conducting polymer “polyanthranilic acid”: an efficient corrosion inhibitor for mild steel in acidic solution. Corros Sci 50:2867–2872
Singh P, Quraishi MA, Gupta SL, Dandia A (2016) Investigation of the corrosion inhibition effect of 3-methyl-6-oxo-4-(thiophen-2-yl)-4,5,6,7-tetrahydro-2Hpyrazolo[3,4 b]pyridine-5-carbonitrile (TPP) on mild steel in hydrochloric acid. J Taibah Univ Sci 10:139–147
Li X, Deng S, Fu H (2011) Three pyrazine derivatives as corrosion inhibitors for steel in 1.0 M H2SO4 solution. Corros Sci 53:3241–3247
Quartarone G, Ronchin L, Vavasori A, Tortato C, Bonaldo L (2012) Inhibitive action of gramine towards corrosion of mild steel in deaerated 1.0 M hydrochloric acid solutions. Corros Sci 64:82–89
Vasuki G, Kumaravel K (2008) Rapid four-component reactions in water: synthesis of pyranopyrazoles. Tetrahedron Lett 49:5636–5638
Ablajan K, Wang LJ, Maimaiti Z, Lu YT (2017) CeCl–-promoted one-pot synthesis of multisubstituted bispyrano[2,3-c]pyrazole derivatives. Monatsh Chem 145:491–496
Srivastava V, Haque J, Verma C, Singh P, Lgaz H, Salghi R, Quraishi MA (2017) Amino acid based imidazolium zwitterions as novel and green corrosion inhibitors for mild steel: experimental, DFT and MD studies. J Mol Liq 244:340–352
Singh P, Singh A, Quraishi MA (2014) Inhibition effect of 1,3,5-tri-p-tolyl-1,3,5-triazene on the corrosion of brass in 0.5 M HCl solution. Res Chem Intermed 40:595–604
Singh P, Singh A, Quraishi MA (2015) Thiopyrimidine derivatives as new and effective corrosion inhibitors for mild steel in hydrochloric acid: electrochemical and quantum chemical studies. J Taiwan Inst Chem Eng 60:1–14
Sasikumar Y, Adekunle AS, Olasunkanmi LO, Bahadur I, Baskar R, Kabanda MM, Obot IB, Ebenso EE (2015) Experimental, quantum chemical and Monte Carlo simulation studies on the corrosion inhibition of some alkyl imidazolium ionic liquids containing tetrafluoroborate anion on mild steel in acidic medium. J Mol Liq 211:105–118
Umoren SA, Obot IB, Israel AU, Asuquo PO, Solomon MM, Eduok UM, Udoh AP (2014) Inhibition of mild steel corrosion in acidic medium using coconut coir dust extracted from water and methanol as solvents. J Ind Eng Chem 20:3612–3622
Singh P, Ebenso EE, Lukman OO, Obot IB, Quraishi MA (2016) Electrochemical, theoretical, and surface morphological studies of corrosion inhibition effect of green naphthyridine derivatives on mild steel in hydrochloric acid. J Phys Chem C 120:3408–3419
Chandrabhan V, Ebenso EE, Vishal Y, Quraishi MA (2016) Dendrimers: a new class of corrosion inhibitors for mild steel in 1 M HCl: experimental and quantum chemical studies. J Mol Liq 224:1282–1293
Ugin Inbaraj N, Venkatesa Prabhu G (2018) Corrosion inhibition properties of paracetamol based benzoxazine on HCS and Al surfaces in 1 M HCl. Prog Org Coat 115:27–40
Sasikala T, Parameswari K, Chitra S, Kiruthika A (2017) Synthesis and corrosion inhibition study of benzodiazepines on mild steel in sulphuric acid medium. Measurement 101:175–182
Saxena A, Prasad D, Haldhar R, Singh G, Kumar A (2018) Use of Sida cordifolia extract as green corrosion inhibitor for mild steel in 0.5 M H2SO4. J Environ Chem Eng 6:694–700
Haldhar R, Prasad D, Saxena A (2018) Myristica fragrans extract as an eco-friendly corrosion inhibitor for mild steel in 0.5 M H2SO4 solution. J Environ Chem Eng 6:2290–2301
Kumar R, Yadav SO, Singh G (2017) Electrochemical and surface characterization of a new eco-friendly corrosion inhibitor for mild steel in acidic media: a cumulative study. J Mol Liq 237:413 – 427
Kumar R, Chopra R, Singh G (2017) Electrochemical, morphological and theoretical insights of a new environmentally benign organic inhibitor for mild steel corrosion in acidic media. J Mol Liq 241:9–19
Baggaa MK, Gadib R, Yadav SO, Kumar R, Chopra R, Singh G (2016) Investigation of phytochemical components and corrosion inhibition property of Ficus racemosa stem extract on mild steel in H2SO4 medium. J Environ Chem Eng 4:4699–4707
Haldhar R, Prasad D, Saxena A, Singh P (2018) Valeriana wallichii root extract as a green & sustainable corrosion inhibitor for mild steel in acidic environments: experimental and theoretical study. Mater Chem Front 2:1225–1237
Dandia A, Gupta SL, Singh P, Quraishi MA (2013) Ultrasound-assisted synthesis of pyrazolo[3,4b]pyridines as potential corrosion inhibitors for mild steel in 1.0 M HCl. ACS Sustain Chem Eng 1:1303–1310
Singh P, Quraishi MA (2016) Corrosion inhibition of mild steel using Novel Bis Schiff’s Bases as corrosion inhibitors: electrochemical and Surface measurement. Measurement 86:114–124
Gupta NK, Quraishi MA, Singh P, Srivastava V, Srivastava K, Verma C, Mukherjee AK (2017) Curcumine Longa: green and sustainable corrosion inhibitor for aluminum in HCl medium. Anal Bioanal Electrochem 9:245–265
Singh P, Makowska-Janusik M, Slovensky P, Quraishi MA (2016) Nicotinonitriles as green corrosion inhibitors for mild steel in hydrochloric acid: electrochemical, computational and surface morphological studies. J Mol Liq 220:71–81
Singh P, Srivastava V, Quraishi MA (2016) Novel quinoline derivatives as green corrosion inhibitors for mild steel in acidic medium: electrochemical, SEM, AFM, and XPS studies. J Mol Liq 216:164–173
Singh P, Chauhan DS, Srivastava K, Srivastava V, Quraishi MA (2017) Expired atorvastatin drug as corrosion inhibitor for mild steel in hydrochloric acid solution. Int J Ind Chem 8:363–372
Yadav DK, Quraishi MA (2012) Application of some condensed uracils as corrosion inhibitors for mild steel: gravimetric, electrochemical, surface morphological, UV–visible, and theoretical investigations. Ind Eng Chem Res 51:14966 – 14979
Yadav DK, Quraishi MA (2012) Electrochemical investigation of substituted pyranopyrazoles adsorption on mild steel in acid solution. Ind Eng Chem Res 51:8194 – 8210
Yadav DK, Chauhan DS, Ahamad I, Quraishi MA (2013) Electrochemical behavior of steel/acid interface: adsorption and inhibition effect of oligomeric aniline. RSC Adv 3:632–646
Acknowledgements
Priyanka Singh is thankful to SERB-National Post Doctoral Fellowship (N-PDF), DST, India, for the financial assistance and University of Delhi for facilitation of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Rights and permissions
About this article
Cite this article
Singh, P., Chauhan, S.S., Singh, G. et al. Corrosion Inhibition by Green Synthesized Inhibitor: 4,4′-(1,4Phenylene)bis(6-amino-3-methyl-2,4dihydropyrano[2,3-c]pyrazole-5 carbonitrile) for Mild Steel in 0.5 M H2SO4 Solution. J Bio Tribo Corros 5, 11 (2019). https://doi.org/10.1007/s40735-018-0204-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-018-0204-6