Abstract
Reverse osmosis concentrate (ROC) from wastewater reclamation in water reuse retains concentrated toxic bio-refractory organics, and developing technologies for their removal is essential. This paper reviews innovative treatment technologies for organic contaminants in the ROC, and treatment options for applications are proposed. To adequately manage ROC, volume reduction and quality improvement are important. Forward osmosis (FO) can reduce the ROC volume. Advanced oxidation processes (AOPs) result in degrading organic contaminants and producing biodegradable organics, but the reduction of energy consumption is required. Coagulation is an effective option as a pre-treatment of AOPs and can improve the biodegradability of ROC. Partial use of short-time AOPs can transform high molecular weight organics into relatively biodegradable organics. Among AOPs, a rotating advanced oxidation contactor (RAOC) can be an energy-saving technique for removing bio-refractory organics from ROC using solar light irradiation. Post-biological treatment can significantly save energy and efficiently eliminate biodegradable organics that are produced by AOPs. Microalgae cultivation is also an effective option for resource recovery from ROC. Considering the techniques, an integrated process comprising FO, pre-coagulation, short-time and/or solar-driven AOPs (e.g., RAOC), and post-biological treatment is proposed as an energy-saving and cost-effective technology for ROC treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Reverse osmosis (RO) is a well-established technology for water desalination, the production of potable water, and tertiary wastewater treatment [1,2,3,4]. The global market for RO system components (e.g., RO membrane and pressure vessels) for water treatment reached nearly $6.6 billion in 2016. By 2019, the market for RO system components is projected to reach $8.8 billion with a compound annual growth rate of 10.5%, primarily driven not only by desalination for municipal water supply but also by process water treatment (pharmaceutical, power plant, and microelectronics) and water reuse [5]. With increasing global water demand, it is predicted that the global market value of RO system components will reach $11.0 billion by 2021 [6].
To solve global water scarcity, RO has been widely used for water reclamation and has been rapidly spreading since around 1970. Expanding the scale of plants has progressed and has exceeded 100,000 m3 day-1 since 2000 (corresponding to the amount of water necessary for about 400,000 people on average in developed countries) [7]. The largest recent wastewater reclamation plant is in Sulaibiya (Kuwait) where RO and ultrafiltration (UF) membrane–based water purification is used for wastewater reuse. With an initial capacity of up to 375,000 m3 day-1 and designed for extension to 600,000 m3 day-1 in the future, Sulaibiya treats wastewater to produce potable quality water for non-potable uses in agriculture, industry, and aquifer recharge.
In addition to membrane fouling, concentrate management is a crucial problem of pressurized membrane systems for water treatment [2]. The RO concentrate (ROC) from wastewater reclamation in water reuse is about 15–20% of the influent volume and contains concentrated levels of rejected pollutants [8]. Therefore, suitable and cost-effective technology is necessary for treating ROC.
This manuscript reviews innovative treatment technologies for organic contaminants in ROC from wastewater reclamation in water reuse and proposes treatment options for future applications.
Contaminants in RO Concentrate and Their Potential Impact on the Environment
The characteristics of ROC vary significantly depending on feed–water quality, RO process operation parameters, type of pre-treatment technology, and the properties of chemicals used as antiscalants and biocides used to prevent the formation of biofilm on the membrane surface in some cases [1, 9,10,11]. The successful rejection of emerging pollutants contained in the feed water by the RO membrane has resulted in their elevated levels in the ROC.
Emerging contaminants persistent in sewage effluent have raised awareness of the environmental risk of ROC [2, 12, 13]. Emerging organic contaminants can be classified as pharmaceuticals and personal care products (PPCPs) (e.g., drugs, sunscreens, cosmetics), endocrine-disrupting chemicals (EDCs) (e.g., estrogens), persistent organic contaminants (e.g., pesticides), and nanomaterials (e.g., C60 fullerene) [2]. In line with the continuous development of analysis detection technology, a variety of PPCPs are being detected in the environment resulting from the wide consumption by humans or from the use for animal production [14, 15]. A comparison of the concentration of the different pharmaceuticals in ROC shows an average concentration factor of 3- to 4-fold in municipal wastewater treatment plant effluent [16]. Table 1 shows a summary of the concentration of detected micropollutants in ROC [16,17,18,19,20,21,22].
PPCPs detected in the environment typically originate from hospitals, pharmaceutical manufacturing plants, agricultural practices, and wastewater treatment facilities [23]. Many pharmaceuticals used to treat humans and livestock are excreted in urine and feces [24]. PPCPs are transported to the aqueous environment through domestic wastewater, industrial wastewater, runoff, and landfill leachate [25]. Treatment plants for sewage and industrial wastewater were found to be the largest sources of PPCPs [26].
Some PPCPs have been reported to have toxic effects in the environment at concentrations of micrograms per liter [27]. Pharmaceutical pollution has been verified to induce endocrine or hormonal disruption problems, causing abnormal reproduction of fish [28]. Studies have found that concentrations of diclofenac as low as 5 μg L-1 can accumulate inside the bodies of rainbow trout [29], and a mixture of acetaminophen, carbamazepine, gemfibrozil, and venlafaxine (0.5 μg L-1 each) can cause significant effects in zebrafish including tissue degeneration, a decline in embryo production, and increased embryo mortalities [30]. Pharmaceutical residues in the aquatic environment are reported to have ecotoxic effects [28, 31]. In addition, the antibiotic tetracycline has been shown to be toxic to plants and the early growth stage of aquatic organisms, and it also negatively affects sewage sludge bacteria by inhibiting protein synthesis [32]. Studies have shown antibiotics and antibiotic resistance genes in sewage treatment could significantly affect the bacteria involved in the sewage treatment process in removing carbon and nitrogen in the wastewater through nitrogen assimilation [33].
Genotoxicity evaluation using the SOS/umu test has provided direct evidence that ROC has a much higher toxicological risk than RO influent [34]. Sun et al. studied the characteristics and biotoxicity of different fractions of dissolved organic matter in ROC from a municipal wastewater reclamation system [35]. Using the SOS/umu assay with Salmonella typhimurium TA1535/pSK1002, it was found that the hydrophilic neutral (HIN), hydrophobic acid (HOA), and hydrophobic base (HOB) contributed to the genotoxicity of the ROC, and the HIN has the highest genotoxic level. The HOA, HON, and HIN lead to the total antiestrogenic activity of the ROC, and HOA occupied approximately 60% of the total.
The aim of treating ROC is to increase the overall yield of reclaimed water and to minimize its negative effect on discharge to the environment. In particular, with the increase in the scale of RO processes, it is necessary to develop suitable technology to treat ROC before discharging it into receiving water or recycling for other purposes [1]. A number of treatment processes have been proposed for treating ROC, including physico-chemical and biological processes. The combination of different treatment technologies has also been studied. The following sections introduce treatment processes for ROC.
Treatment Processes for RO Concentrate
Adsorption
High-silica zeolite has been found to be effective in removing organic micropollutants. The adsorption capacity of high-silica zeolite is related to surface hydrophobicity/hydrophilicity and structural features (micropore volume and pore size of high-silica zeolite), as well as the properties of pollutants. By using high-silica zeolite, the undesired competitive adsorption of background organic matters in real wastewater could potentially be prevented [36].
Activated carbon (AC) has been widely utilized for removing organic pollutants from wastewater through adsorption. Lin et al. investigated the factors affecting the use of biochar as an alternative adsorbent to treat pharmaceuticals from ROC compared with commercial granular activated carbon (GAC) [37]. The adsorption capacity of W-biochar obtained from Wakefield Agricultural Carbon LLC. is comparable to GAC, where the biochar achieved 20% higher removal for sulfamethoxazole but 10% lower for ibuprofen than GAC. Adsorption capacity is related not only to the properties of adsorbent and adsorbate but also to the water chemistry of concentrate. Multi-step pre-treatment using activated carbon (AC) for microfiltration (MF) was suggested by Wang [38], who compared single-, two-, and four-stage adsorption integrated with microfiltration, and the four-stage adsorption–MF process showed highest chemical oxygen demand (COD) and dissolved organic carbon (DOC) removal based on the same AC dosage. Jamil et al. used a GAC fixed bed reactor, which removed 15 micropollutants in ROC from a water purification plant, but negatively charged and hydrophilic pollutants were not removed because of the low interaction with the GAC surface [39,40,41]. Shanmuganathan et al. suggested a GAC/MF hybrid system to remove organics and 19 micropollutants in ROC from a water treatment plant [42, 43]. A submerged membrane was adopted to recover GAC, and DOC removal, trans-membrane pressure (TMP), and the adsorption of micropollutants on GAC were evaluated. Not only recalcitrant organics such as humic-like and fulvic-like compounds but also 19 micropollutants were successfully removed by the GAC/MF treatment. GAC also contributed to decreasing TMP by reducing fouling through the adsorption of organics. Adsorption techniques permanently transfer the contaminants to the adsorbent but do not destroy the contaminants, and saturation of the adsorbent is an important problem. Coupling the oxidizing agent (e.g., ozone and photocatalyst) with the adsorbent was applied to restore the adsorption capacity of the adsorbent and prevent the toxic residues from re-entering the environment [36]. Zhang et al. evaluated the effect of ozonation on performance of zeolite for trichlorophenol adsorption and found the relationship between the ozone dosage and the mass of trichlorophenol adsorbed on the zeolite [44]. Increasing the ozone dosage resulted in the regeneration of the zeolite, and the mass ratio of ozone to trichlorophenol adsorbed on the zeolite was estimated to be 1.2 ± 0.3 g O3 g trichlorophenol-1. In our previous publication, we proposed a synergistic model, in which a portion of the adsorbed SMT transfers to the surface of the TiO2 in the TiO2/high-silica zeolite HSZ-385 composite powder and is subsequently photocatalytically decomposed [45].
Advanced Oxidation Processes
Advanced oxidation processes (AOPs) generate highly reactive hydroxyl radicals at ambient temperature and pressure, and they are significantly useful for destructing a wide variety of recalcitrant organic compounds. Various AOPs applicable to ROC treatment are introduced in the following sections.
Ozonation and Ozone-Based Advanced Oxidation Processes
Ozonation and ozone-based AOPs such as ozone coupled with hydrogen peroxide, ultraviolet irradiation, sonolysis, and their combinations have been widely recognized as promising technologies to improve water quality in drinking water treatment and wastewater treatment. In terms of the application of ozone technologies to membrane concentrate, the main focuses are removing total organic carbon (TOC) and COD as the overall organic matter, PPCPs as trace organic chemicals, and the toxicity of the parent and by-product compounds. In addition, the organic components in membrane concentrate before and after oxidation have been characterized with molecular weight (MW) distribution and fractions based on acid–base and hydrophobic–hydrophilic properties [13, 46,47,48,49,50].
Zhou et al. investigated several AOPs including ozonation and ozone-based AOPs for treating ROC collected from a water reclamation plant in Singapore [50]. Some of the water quality parameters are as follows: pH 6.9 ± 0.2, TOC 18.0 ± 2.0 mg L-1, COD 60.0 ± 5 mg L-1, total dissolved solids (TDS) 1129 ± 40 mg L-1, and color (Pt-Co) 144 ± 10. Ozonation achieved a removal efficiency of 21.7 and 14.4% in DOC and COD, respectively, along with almost 90% in color removal. Because several AOPs with ozone, i.e., ultraviolet A irradiation/ozone (UVA/O3), sonolysis/ozone (US/O3), ultraviolet A irradiation/hydrogen peroxide/ozone (UVA/H2O2/O3), and sonolysis/hydrogen peroxide/ozone (US/H2O2/O3), could not effectively enhance DOC removal over that exhibited by ozonation, they explained that molecular ozone selective oxidation exhibits better efficiency than hydroxyl radical non-specific oxidation in removing the organics in the raw ROC. Reduced specific ultraviolet absorbance showed that most aromatic contents in the ROC could be effectively degraded by the O3-based AOPs. MW analysis showed that ozonation could break down the large MW organics to smaller MW of < 1 kDa, leading to an increase in the fraction of MW < 1 kDa from 47 to 60% while the total DOC decreased. It also showed that ozonation was more favorable to decompose organics with MW of 10–100 kDa than the higher MW organics (> 100 kDa), which were less effectively decomposed. Although ozonation and ozone-based AOPs exhibited better DOC removal efficiencies among other AOPs, the removal efficiency of DOC was still low. They proposed a sequential process consisting of coagulation and AOPs, which could achieve synergistical improvement in DOC removal, an increase in biodegradability, and a decrease in ecotoxicity, MW, and aromaticity.
Weng et al. investigated the effect of ozonation on the toxicity of four organic fractions—HOA, HOB, hydrophobic neutral (HON), and HIN—of the ROC from a typical industrial park wastewater treatment plant in China [13]. They conducted systematic bioassays covering bacteria, algae, fish, and human cell lines to reveal the role of ozonation in toxicity variation in the four ROC fractions. Some of the water quality parameters in the study are as follows: pH 7.8, TOC 78.4 ± 2.6 mg L-1, and UV254 2.48 ± 0.15 cm-1. The HOA, HOB, HON, and HIN fractions of the ROC accounted for 30, 3, 28, and 39%, respectively, of the total. Ozonation significantly reduced the TOC concentration in the HOA and HON fractions but not in the HOB and HI fractions. Ozonation did not reduce the HOB fraction. The percentage of the HIN fraction in the ROC increased after ozonation. Because the UV254 value in the ROC significantly decreased to 0.58 ± 0.43 cm-1 after ozonation, the organic matter with unsaturated carbon bonds could be efficiently degraded. The UV254 value in the HOA, HON, and HIN fractions significantly decreased, with the HIN fraction contributing the highest UV254 value and percentage to the total UV254. The toxicity changes in the raw ROC and each fraction are summarized in Table 2. Basically, ozonation significantly reduced the toxicity of the raw ROC, but it should be noted that the mortality of zebrafish before and after ozonation was 100%. Of the four fractions, the HOA fraction in the raw ROC exhibited the highest toxicity, followed by the HON and HIN fractions, and their toxicities were reduced by ozonation. However, the toxicity of the HOB fraction could not be effectively reduced by ozonation. Rather, it sometimes increased in several bioassays. Correlation analysis indicated that the chemical data (TOC and UV254) of the HOA and HON fractions correlated well with their toxicities. Against these results, Weng et al. indicated that TOC reduction during ozonation could not fully reflect the toxicity issue, so they proposed that a battery of toxicity assays is necessary in conjunction with the chemical data to evaluate the effectiveness of ozonation [13]. Joo and Tansel stated that combined systems could involve hybrid processes and AOPs such as MBR (membrane bioreactor)/RO, O3/UF(/MF)/RO, MF/RO/AOP, pre-treatment/UF(/MF)/RO, and pre-treatment(/AOP)/RO [2]. ROC quantity was significantly reduced in an MBR/RO system in which ROC was returned to the MBR, and a combined MBR/RO treatment scheme with ozonation of ROC counterbalanced chlorinated and ozonated organics by biological degradation and RO rejection [51].
With respect to removing micropollutants from ROC, Acero et al. investigated the combined processes of coagulation, ozonation, and adsorption and found that coagulation was ineffective in eliminating pharmaceuticals, but it could remove high molecular weight organic compounds in ROC and indirectly contributed to decomposing micropollutants by subsequent ozonation [52]. In addition to water quality analyses of ROC after ozonation, the required amount of ozone to achieve the purposes of ozone applications needs to be evaluated quantitatively.
Fenton Oxidation
Fenton oxidation mainly consists of Fenton (Fe2+/H2O2) and Fenton-like reactions (Fe3+/H2O2), in which hydroxyl radicals are continuously produced until H2O2 disappears. Fenton oxidation has been applied to the treatment of ROC produced via filtrating secondary effluent from municipal wastewater treatment plants [17] and wastewater from a paper mill [53]. Westerhoff et al. reported that 50% of DOC (DOC0 = 40 mg L-1) in ROC was degraded by Fenton oxidation, and the residual iron can be recovered and recycled by increasing the pH of treated water to 7.5–8.0. Hermosilla et al. investigated the effect of solution pH on the treatment of ROC by Fenton oxidation and reported that the organic matter was efficiently degraded at pH < 4 because the inhibitory effects of inorganic carbon that mainly scavenges hydroxyl radicals on removing organic matter were mitigated [53]. They also calculated the optimum pH using a response surface methodology and found pH 2.8 is adequate for efficiently removing organic matter from ROC. The combination of an Fe/Cu catalytic process and Fenton reaction was studied by Ren et al., and high COD removal and biochemical oxygen demand (BOD5)/COD ratios were obtained [54]. Aouni et al. reported the integration of electrocoagulation, electro-Fenton, and electrodialysis for treating synthetic textile ROC [55]. The appropriate condition of electrocoagulation, electro-Fenton, and electrodialysis resulted in the effective removal of organics and salts and reduced treatment cost.
Photocatalysis
TiO2 photocatalysis has been extensively applied to treat PPCPs in wastewater [23, 28]. Dialynas et al. treated ROC with photocatalysis at 0.5 and 1 g L-1 TiO2, and when the suspension was irradiated with UVA light for 60 min, DOC was oxidized yielding 49 and 41% DOC removal at the high and low catalyst level, respectively [56]. Birben et al. investigated the applicability of solar photocatalysis to remove organics present in ROC comprising humic matter as well as selected emerging contaminants (sulfamethoxazole and carbamazepine) using commercially available and newly synthesized photocatalysts [57]. The photocatalysts successfully demineralized (up to 85% non-purgeable organic carbon removal in 60 min) ROC from municipal wastewater and emerging contaminants in ROC samples in the following order: TiO2 > nitrogen-doped TiO2 > TiO2/ZnO > ZnO. Westerhoff et al. reported a maximum DOC reduction of up to 95% using a UVC/TiO2 process followed by biological treatment compared with other processes (coagulation, Fenton, and O3/H2O2) for treating ROC. A UV/TiO2 AOP is the most effective both in terms of DOC reduction and energy efficiency [17]. Removing trace organics was also effective and all 16 pharmaceutical compounds monitored were reduced to below 2 ng L-1.
When a suspended catalyst is used in the UV/TiO2 AOP as slurry, as in the treatment carried out by Dialynas et al., the separation and recycling of powder materials from the treated solution can be an inconvenient, time-consuming, and expensive process [58]. Moreover, UV transmissivity decreases when the amount of suspended catalyst is high. Furthermore, when TiO2 or nano-TiO2 powder in water is exposed to UV radiation, radicals harmful to aquatic organisms are produced [59]. Therefore, effective recovery of the catalyst powders after wastewater treatment should also be considered. The immobilization of TiO2 particles on a support media would be a solution for the abovementioned problem. To mitigate the drawbacks of the adsorbent, a composite of adsorbent and photocatalyst was prepared. Xiang et al. reported on the removal of crotamiton, a scabicide and antipruritic agent, contained in ROC using a sheet-like TiO2/high-silica zeolite composite prepared using a papermaking technique [60]. The composite sheet was easy to handle, and the crotamiton in the ROC was removed through adsorption and photocatalytic decomposition. The adsorption rate of the crotamiton in the ROC was almost equal to that in ultrapure water, indicating that the crotamiton was selectively adsorbed onto the high-silica zeolite in the composite sheet, although there were large amounts of coexisting organics and salts in the ROC.
UV/H2O2
Hydroxyl radicals are produced via H2O2 degradation with UV irradiation at 254 nm, and the UV/H2O2 AOP has been used to treat ROC. Zhou et al. reported that coupling pre-coagulation with subsequent UV/H2O2 AOP achieved synergistically improved DOC removal, increased biodegradability, and decreased ecotoxicity, molecular weight, and aromaticity [50]. The organic matter and UV-absorbing agents in the ROC likely inhibit UV/H2O2 AOP. Alum coagulation was applied as a pre-treatment for UVC/H2O2 treatment by Umar et al. [61]. The recalcitrant humic-like organics were effectively removed by coagulation, and the biodegradability of the treated ROC was enhanced. Umar et al. further conducted a comparative study using aluminum and ferric-based coagulants as a pre-treatment for UVC/H2O2 treatment of ROC. Although the total removal of organic and inorganic contents after 60 min of UVC/H2O2 treatment with and without coagulation were comparable, large differences in the trends of reduction were observed, which were attributed to the different characteristics of the humic-like organic content of the samples and different initial biodegradability. Coagulation and UVC/H2O2 treatment preferentially removed humic-like compounds, thus resulting in the low reaction rates of the coagulated samples after UVC/H2O2 treatment. Likewise, greater improvement (2- to 3-fold) in biodegradability was observed during UVC/H2O2 treatment of the pre-treated samples than those without pre-treatment [61]. From the perspective of electrical energy dose, ferric chloride (FeCl3) was superior in removing UV-absorbing agents, DOC, and color, and in improving UV light transmittance than aluminum-based coagulant [62]. Lu et al. evaluated the potential of a BAC process combined with pre-oxidation using a UVC/H2O2 AOP to treat a high-salinity municipal wastewater ROC (TDS ~ 10,000 mg L-1) during 90 days of operation [63]. The combined system reduced 60% of DOC and 50% of COD, no toxicity was detected for the ROC after the combined treatment, and the trihalomethane formation potential was reduced from 3.5 to 2.8 mg L-1. Pradhan et al. investigated the effect of salinity on removing organic matter and nitrogen from ROC through a BAC system after pre-oxidation with UV/H2O2. The combined system removed considerably more total nitrogen at high salinity (TDS ~ 16 g L-1) compared with low (~ 7 g L-1) and medium salinity (~ 10 g L-1) [64].
Electrochemical Oxidation
Electrochemical treatment of ROC is a promising technique because high salinity enhances electrical conductivity, reducing energy consumption during treatment [65,66,67,68,69,70,71]. Organic and inorganic compounds contained in ROC can be decomposed by direct oxidation at the electrode surface or reaction with reactive species such as hydroxyl radicals generated at the electrode surface. However, the high cost of energy for electrochemical treatment restricts its application; therefore, various studies have been conducted to optimize reaction conditions.
Weng and Pei utilized a Co-doped PbO2 anode, and operational parameters and the degradation behavior of quinoline in ROC were investigated [66]. After 2 h of treatment, 100% of the quinoline was removed from the concentrate, and it was clarified that Co-doping significantly reduces electrical efficiency per log order (EE/O), indicating that electrochemical treatment is effective in eliminating micropollutants in ROC. Electrochemical oxidation of catechol using a Cu–graphite electrode was studied by Maharaja et al., and the effects of the inner electrode space of electrodes, pH, temperature, catechol concentration, and current density were investigated [67]. The kinetic parameters of electrochemical oxidation of catechol and apparent faradic efficiency, as well as specific energy consumption were calculated. Theoretical evaluation of current density proposed that catechol removal was regulated when it was supplied at above limiting applied current densities. Wang et al. reported on the electro-oxidation of ROC in printing and dyeing wastewater using a PbO2/Ti electrode [70]. Instead of current density, the oxidation–reduction potential (ORP) was treated as an important indicator, and the quantitative relationship among ORP, COD, and Cl− concentration was clarified. The experimental results showed ORP could be an indicator of current density, Cl− concentration, pollutants, and reaction time, so the developed constant ORP system can be a new monitoring technique to reduce operational cost. Barazesh et al. investigated the transformation of micropollutants in ROC and its degradation mechanism [68]. They revealed the composition of electrolytes (Cl−, HCO3−, NH4+) affected the formation of oxidants, and Cl− and Br− accelerated the removal of micropollutants. On the other hand, HCO3− negatively affected the degradation of electron-poor compounds. The reaction mechanism at the diffuse layer of the anode and bulk solution was proposed, and removal rates of electron-rich micropollutants were accurately predicted.
Forward Osmosis
Forward osmosis (FO) has been used to reduce the volume of ROC. The overview of ROC treatment by FO in the previous studies is summarized in Table 3. Kazner et al. investigated a membrane fouling mechanism, and the primary organic foulant in the concentrate was specified as humic acid, whereas the inorganic substances mainly contributed to the membrane fouling with scaling [72]. Jamil et al. also examined the volume reduction of ROC from a water purification plant with five FO treatment steps using a 2–3 M NaCl solution as a draw solution (DS) [39]. The concentrate volume finally decreased from 6.00 to 0.47 L, whereas the organic and inorganic substances in the concentrate caused membrane fouling. To mitigate the membrane fouling, they proposed pre-treatment of ROC such as adjusting the pH and adsorbing organic matter in the concentrate using a fixed-bed GAC column. Maintaining the concentrate pH at 5 improved the permeate flux (PF) and possibly the dissolution of carbonate precipitates. The PF was greatly improved by GAC pre-treatment, and 0.14–0.31 mg C cm-1 of organic matter was captured on the GAC. By combining GAC pre-treatment and FO treatment, 17 pharmaceuticals (e.g., atenolol, carbamazepine, and ibuprofen) were retained in the concentrate. Jamil et al. developed a pressure-assisted FO for treating ROC to enhance the rate of water recovery [40]. Potassium chloride solutions (0.25 and 0.4 M) were used as DSs. The PF did not change as the KCl concentration increased, and the reverse solute flux increased with the increase in DS concentration. As reported in their previous work [39], GAC pre-treatment was effective in mitigating membrane fouling, and target organic pollutants (e.g., diuron, sulfamethoxazole, and triclosan) were rejected by the pressure-assisted FO treatment combined with GAC pre-treatment. Jamil et al. used an FO membrane as a nanofiltration membrane to treat the ROC because of the similarity of water permeability and structural property between those membranes [41]. The nanofiltration of the concentrate by using the FO membrane was more effective than the original nanofiltration membrane from the viewpoint of rejecting inorganic substances. Maltos et al. examined FO-RO treatment of Denver–Julesburg (D–J) basin, and the ROC was treated by FO [73]. The PF was 3.1 L m-2 h-1 at the beginning of treatment and decreased by 1.0 L m-2 h-1 owing to the membrane fouling. The reverse salt flux (RSF) decreased by 4 g m-2 h-1, but it increased until reaching ~ 12 g m-2 h-1 because of slow release of salt accumulated in the fouling layer. The FO treatment resulted in the removal of 14 polycyclic aromatic hydrocarbons (> 93.6 ± 1.7%). In FO treatment using a high saline solution, the recovery of DS is important. Kim et al. examined the treatment of coal seam gas ROC using FO by using fertilizer as a DS to directly apply the diluted fertilizer solution to the irrigation [74]. FO treatment was effective from the viewpoint of favorable composition of nutrients in diluted fertilizer. The initial PF was significantly higher than other previous studies. However, the reverse transportation of DS caused the decrease in the osmotic pressure difference, resulting in the decrease in the PF. The RSF of (NH4)2SO4 was lowest among DS used in their study. They investigated the mechanism of membrane fouling with scaling. The Ca3(PO4)2, Mg3(PO4)2, and MgNH4PO4 struvite were observed on the FO membrane when using (NH4)2HPO4 and Ca(NO3)2 as DS, and the struvite was mainly formed on FO membrane when using (NH4)2HPO4 DS. They proposed a strategy for controlling membrane fouling and found that the membrane fouling could be controlled by chemical cleaning using citric acid.
As mentioned above, FO is an attractive process for decreasing the volume of ROC. However, the concentrated FS still retains the organic contaminants, and the previous studies reported that managing the concentrate including its disposal is important to reduce the ecological risk [75,76,77]. Treatment options for contaminants in the concentrated FS should be considered before discharging it to receiving water bodies to mitigate potential environmental risk.
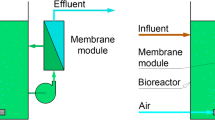
Biological Treatment
Biological treatment has been investigated to treat ROC derived from various types of wastewater. Jia et al. applied a membrane bioreactor (MBR) with intermittent aeration to treat ROC from coal gasification wastewater [78]. They found that an aeration cycle of 6 h:6 h is adequate for efficiently removing organic matter (48.35%), nitrites (36.05%), and nitrates (64.34%). Yao et al. examined the on-site recovery of phosphorus and the removal of nitrogen from a mixture of fresh urine taken from a male toilet and ROC from a wastewater reclamation facility using MBR [79]. Considering the concentration of divalent cations (Ca2+ and Mg2+) in ROC and phosphorus in fresh urine, an adequate mixture ratio was determined at pH 9 for effective phosphorus precipitation, and 99% of the phosphorus was recovered in the ratio of ROC:fresh urine = 3:2. Justo et al. used biological activated carbon (BAC) filter for eliminating the trace organic contaminants including pharmaceuticals from ROC. BAC filter could remove 11 pharmaceuticals (e.g., naproxen, gemfibrozil, and atenolol), and β-proteobacteria was identified as main bacteria phylum [80].
Badia-Fabregat et al. investigated the removal of pharmaceuticals in ROC from an urban wastewater treatment plant using the white-rot fungus Trametes versicolor [22]. The fungal treatment with and without externally adding nutrients was conducted, and nutrient loading at 221–278 mg C g fungus-1 day-1 and 0.2 mg N g fungus-1 day-1 was effective in enhancing the treatment performance. Some of the pharmaceuticals (e.g., acetaminophen, diclofenac, and sulfamethoxazole) were completely removed, but their destruction resulted in the production of other pharmaceuticals (e.g., salicylic acid, tetracycline, and ketoprofen) as metabolites. Llorca et al. used T. versicolor for destructing benzotriazoles (BTs) in ROC from a municipal wastewater treatment plant [81]. They found that T. versicolor has enough potential to degrade BTs, and biotransformation by-products were generated by conjugation with some sugars via the methylation of the triazole group. Badia-Fabregat et al. also treated ROC from a municipal wastewater treatment plant and veterinary hospital wastewater with and without inoculating with T. versicolor [82].
Miranda et al. isolated microalgal biofilms from a saline lake (Biofilm #52) and used them to remove nutrients in ROC from a wastewater reclamation plant [83]. Biofilm #52 contained five biofilm-associated photosynthetic species such as unicellular microalgae, diatoms, and filamentous cyanobacteria. During 9 days of treatment, 99% of NO3− and PO43− were removed from the ROC, and the uptake rate of NO3− and PO43− was 21 ± 5.1 mg N L -1day-1 and 13 ± 1.2 mg P L-1 day-1, respectively. Ikehata et al. isolated Pseudostaurosira trainorii E. Morales PEWL001 from agricultural drainage water and used a photobioreactor containing the strain to remove nutrients and trace organic contaminants in ROC from a groundwater replenishment system (GWRS) [84]. NH4+ was preferentially removed compared with NO3−, and NH4+ (C0 = 8.2 ± 0.3 mg N L-1) and PO43− (C0 = 6.7 ± 0.3 mg P L-1) were completely removed within 5 days. Twelve pharmaceuticals (e.g., benzotriazole, atenolol, and trimethoprim) were also degraded by the strain.
Proposed Treatment Options
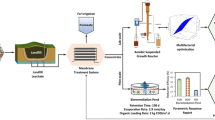
Volume reduction and quality improvement are essential for adequate management of ROC. Although FO is promising for reducing the volume of ROC as mentioned above, decomposition of the concentrated toxic recalcitrant organics is essential before discharging into public water bodies. AOPs destroy a wide variety of bio-refractory organics in ROC and produce assimilable organic carbon [85]. However, reduction of energy consumption for AOPs is urgently required. Simple pre-treatment techniques before the AOPs should be considered for a more cost-effective treatment scheme. We propose an integrated process comprising FO, pre-coagulation, AOP, and post-biological treatment as a better option for ROC treatment at lower cost and using energy (Fig. 1).
Coagulation is one useful option as a pre-treatment of AOPs to remove organics and inorganics from ROC. Long et al. applied coagulation to remove TOC in ROC from landfill leachate [86]. FeCl3 coagulates relatively high molecular weight organics hindering AOP [50] and shows more effective coagulation of almost all fractions of DOC in ROC than polyaluminum chloride and aluminum chlorohydrate [87]. Adding NaOH and polyacrylamide to ROC results in the removal of Ca2+ and Mg2+ and reduced membrane fouling by subsequent MBR treatment because of coagulation of humic-like organics and divalent metal ions [88]. Coagulation improves the biodegradability of ROC and enhances the performance of biological treatment of recalcitrant organics [89,90,91,92].
Partial use of short-time AOPs can break down high molecular weight organics into relatively biodegradable organics [61]. Of the AOPs, solar-driven photocatalysis saves energy. We have also confirmed that UVA/TiO2 AOP is effective in destructing organic contaminants [93, 94]. We synthesized a composite powder containing TiO2 and high silica zeolite, which can selectively adsorb hydrophobic organics via hydrophobic interaction [95,96,97]. The synergetic effect of the composite powder enhances their degradation [45], and the composite powder selectively removes them from actual wastewater treatment including secondary effluent from a municipal wastewater treatment plant [98,99,100]. We fabricated an HSZ-385 zeolite/TiO2 composite sheet using a papermaking technique to simply recover functional powder materials from treated wastewater. We also showed that the composite sheet can remove crotamiton from ROC [60]. The crotamiton was selectively adsorbed onto the zeolite in the composite sheet and thereafter was degraded, despite the presence of coexisting organics and inorganics at significantly higher concentrations than crotamiton. Furthermore, we have developed a novel rotating advanced oxidation contactor (RAOC) equipped with the zeolite/TiO2 composite sheets and have published details of the removal mechanism of target contaminants and its transformation by-products from wastewater by using the RAOC [101,102,103]. The bottom part of the RAOC disk is immersed in water for adsorption, and the top part of the disk is irradiated with UVA light for photocatalysis with a view to using solar light irradiation for future application. By only rotating the disk, the adsorption in water and photocatalysis in the thin water film occurs continuously. Moreover, the RAOC can simultaneously remove sulfamethazine and recover ureaform from synthetic urine containing coexisting substances at extremely high concentrations [103]. The adsorbent and photocatalyst can be changed in response to the characteristics of the target contaminant, and the RAOC can remove 1,4-dioxane from landfill leachate using less energy than a TiO2 slurry reactor [104]. A pilot RAOC has been developed, and operational and design parameters are now under investigation. Operational cost can be contained by using sunlight as the light source. We anticipate that the RAOC will become a better option for simultaneously removing contaminants and recovering resources at lower cost and using less energy.
Hybrid processes involving AOPs coupled to pre-coagulation and post-biological treatment might be promising to save energy. Photocatalysis prior to biological treatment improves the biodegradability of tetracycline and tylosin and reduces their toxicity [32]. Pre-coagulation enhances the performance of UV/H2O2 treatment, and biodegradable by-products are produced as a result. Post-biological treatment is therefore critical for efficient degradation of the by-products [61, 62]. A UVC/H2O2 AOP combined with pre-coagulation and post-biological treatment reduces the energy required by 55–83% for pre-treatment and the UV/H2O2 AOP [62]. The effluent quality of the BOD level from the post-biological treatment system should be equivalent to that in the secondary effluent from sewage treatment plant. Therefore, the target BOD level should be less than effluent standard (e.g., less than 15 mg L-1 in Japan). This work shows that post-biological treatment is a significant energy-saving approach for efficiently eliminating organic matter from ROC.
From the viewpoint of resource recovery from ROC, microalgae cultivation is an effective approach. Microalgae can be used for recovering nutrients, and lipids accumulated inside the algae are useful for producing energy. Wang et al. isolated Chlorella sp. ZTY4 and Scenedesmus sp. LX1 from a domestic wastewater treatment plant and used them to treat ROC from a wastewater reclamation plant [105]. Both algae removed more than 90% of total nitrogen and total phosphorus from the concentrate and about 30% of their bodies consisted of lipids after 16 days of cultivation. Maeng et al. attempted to remove pharmaceuticals (e.g., diclofenac, carbamazepine, and ketoprofen) from synthetic ROC using the microalga Scenedesmus quadricauda and found the S. quadricauda removed the pharmaceuticals by supplying 10% (v/v) CO2 under continuous irradiation [106]. They also used S. quadricauda to remove polymeric organic matter, which are bio-refractory, from synthetic ROC with high salinity and revealed that reactive oxygen species released from S. quadricauda induced degradation of the bio-refractory matter [107]. Chakraborti et al. created a pilot-scale subsurface-flow wetland, comprising a mature stand of bulrush (Schoenoplectus californicus, 2 m) and soil (11.9 m3) to treat ROC from a wastewater treatment plant [108]. The bulrush captured 23% of NH4+ (C0 = 146.2 mg N L-1) and 23% of PO43− (17.9 mg P L-1) under 2.5 days of hydraulic retention time during 6 months of treatment and 58% of NO3− (7.2 mg N L-1) and 51% of organic matter (10.0 mg C L-1) were removed by microorganisms in the soil. The wetland could easily be applied to the local environment and might be an effective approach to improve the water quality of ROC.
Conclusion
In this manuscript, innovative treatment technologies for organic contaminants in RO concentrate from water reclamation in water reuse was reviewed, and treatment options for future applications were proposed. Numerous treatment technologies for ROC including adsorption, AOPs, FO, and biological treatment have been studied. Volume reduction and quality improvement are essential for adequate management of ROC. FO is promising for reducing the volume of ROC, but decomposing the concentrated toxic recalcitrant organics is essential before discharging into public water bodies. Although AOPs destroy a wide variety of bio-refractory organics in ROC and produce biodegradable organics, the reduction of energy consumption is required. We propose an integrated process comprising FO, pre-coagulation, short-time and/or solar-driven AOPs (e.g., RAOC), and post-biological treatment as an energy-saving and cost-effective technology for ROC treatment.
References
Subramani A, Jacangelo JG. Treatment technologies for reverse osmosis concentrate volume minimization: a review. Sep Purif Technol. 2014;122:472–89. https://doi.org/10.1016/j.seppur.2013.12.004.
Joo SH, Tansel B. Novel technologies for reverse osmosis concentrate treatment: a review. J Environ Manag. 2015;150:322–35. https://doi.org/10.1016/j.jenvman.2014.10.027.
Wang Y, Yang Q, Dong J, Huang H. Competitive adsorption of PPCP and humic substances by carbon nanotube membranes: effects of coagulation and PPCP properties. Sci Total Environ. 2018;619–620:352–9. https://doi.org/10.1016/j.scitotenv.2017.11.117.
Morillo J, Usero J, Rosado D, El Bakouri H, Riaza A, Bernaola F-J. Comparative study of brine management technologies for desalination plants. Desalination. 2014;336:32–49. https://doi.org/10.1016/j.desal.2013.12.038.
Cumming S. Global markets for reverse osmosis (RO) membranes and components to reach $8.1 billion by 2018: BCC Research, 2014; http://www.prweb.com/pdfdownload/10959343.pdf.
Ramamurthy S. Major reverse osmosis system components for water treatment: the global market, 2017. https://globenewswire.com/news-release/2017/07/17/1047263/0/en/Global-Market-for-R-O-System-Components-to-See-Double-Digit-CAGR-11.html. Accessed 9 July 2019
Kurihara M, Takeuchi H. SWRO-PRO system in “mega-ton water system” for energy reduction and low environmental impact. Water. 2018;10:1–15. https://doi.org/10.3390/w10010048.
Umar M, Roddick F, Fan L. Assessing the potential of a UV-based AOP for treating high-salinity municipal wastewater reverse osmosis concentrate. Water Sci Technol. 2013;68:1994–9. https://doi.org/10.2166/wst.2013.417.
Umar M, Roddick F, Fan L. Recent advancements in the treatment of municipal wastewater reverse osmosis concentrate—an overview. Crit Rev Environ Sci Technol. 2015;45:193–248. https://doi.org/10.1080/10643389.2013.852378.
Birben NC, Uyguner-Demirel CS, Bekbolet M. Organic matrix in reverse osmosis concentrate: composition and treatment alternatives. Curr Org Chem. 2017;21:1084–97. https://doi.org/10.2174/1385272821666170102151901.
Feiner M, Beggel S, Jaeger N, Geist J. Increased RO concentrate toxicity following application of antiscalants - acute toxicity tests with the amphipods Gammarus pulex and Gammarus roeseli. Environ Pollut. 2015;197:309–12. https://doi.org/10.1016/j.envpol.2014.11.021.
Rodriguez-Mozaz S, Ricart M, Köck-Schulmeyer M, Guasch H, Bonnineau C, Proia L, et al. Pharmaceuticals and pesticides in reclaimed water: efficiency assessment of a microfiltration–reverse osmosis (MF–RO) pilot plant. J Hazard Mater. 2015;282:165–73. https://doi.org/10.1016/j.jhazmat.2014.09.015.
Weng J, Jia H, Wu B, Pan B. Is ozonation environmentally benign for reverse osmosis concentrate treatment? Four-level analysis on toxicity reduction based on organic matter fractionation. Chemosphere. 2018;191:971–8. https://doi.org/10.1016/j.chemosphere.2017.10.054.
Wang J, Wang S. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: a review. J Environ Manag. 2016;182:620–40. https://doi.org/10.1016/j.jenvman.2016.07.049.
Tamura I, Yasuda Y, Kagota K, Yoneda S, Nakada N, Kumar V, et al. Contribution of pharmaceuticals and personal care products (PPCPs) to whole toxicity of water samples collected in effluent-dominated urban streams. Ecotoxicol Environ Saf. 2017;144:338–50. https://doi.org/10.1016/j.ecoenv.2017.06.032.
Benner J, Salhi E, Ternes T, von Gunten U. Ozonation of reverse osmosis concentrate: kinetics and efficiency of beta blocker oxidation. Water Res. 2008;42:3003–12. https://doi.org/10.1016/j.watres.2008.04.002.
Westerhoff P, Moon H, Minakata D, Crittenden J. Oxidation of organics in retentates from reverse osmosis wastewater reuse facilities. Water Res. 2009;43:3992–8. https://doi.org/10.1016/j.watres.2009.04.010.
Pérez G, Fernández-Alba AR, Urtiaga AM, Ortiz I. Electro-oxidation of reverse osmosis concentrates generated in tertiary water treatment. Water Res. 2010;44:2763–72. https://doi.org/10.1016/j.watres.2010.02.017.
Abdelmelek SB, Greaves J, Ishida KP, Cooper WJ, Song W. Removal of pharmaceutical and personal care products from reverse osmosis retentate using advanced oxidation processes. Environ Sci Technol. 2011;45:3665–71. https://doi.org/10.1021/es104287n.
Justo A, González O, Aceña J, Pérez S, Barceló D, Sans C, et al. Pharmaceuticals and organic pollution mitigation in reclamation osmosis brines by UV/H2O2 and ozone. J Hazard Mater. 2013;263:268–74. https://doi.org/10.1016/j.jhazmat.2013.05.030.
Wei X, Gu P, Zhang G, Huang J. Occurrence of emerging and priority pollutants in municipal reverse osmosis concentrates. Environ Sci Process Impacts. 2015;17:488–94. https://doi.org/10.1039/C4EM00205A.
Badia-Fabregat M, Lucas D, Gros M, Rodríguez-Mozaz S, Barceló D, Caminal G, et al. Identification of some factors affecting pharmaceutical active compounds (PhACs) removal in real wastewater. Case study of fungal treatment of reverse osmosis concentrate. J Hazard Mater. 2015;283:663–71. https://doi.org/10.1016/j.jhazmat.2014.10.007.
Yang Y, Ok YS, Kim K-H, Kwon EE, Tsang YF. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: a review. Sci Total Environ. 2017;596–597:303–20. https://doi.org/10.1016/j.scitotenv.2017.04.102.
Oulton RL, Kohn T, Cwiertny DM. Pharmaceuticals and personal care products in effluent matrices: a survey of transformation and removal during wastewater treatment and implications for wastewater management. J Environ Monit. 2010;12:1956–78. https://doi.org/10.1039/c0em00068j.
Awfa D, Ateia M, Fujii M, Johnson MS. Photodegradation of pharmaceuticals and personal care products in water treatment using carbonaceous-TiO2 composites: a critical review of recent literature. Water Res. 2018;142:26–45. https://doi.org/10.1016/j.watres.2018.05.036.
Chen Y, Vymazal J, Březinová T, Koželuh M, Kule L, Huang J, et al. Occurrence, removal and environmental risk assessment of pharmaceuticals and personal care products in rural wastewater treatment wetlands. Sci Total Environ. 1660–1669;2016:566–7. https://doi.org/10.1016/j.scitotenv.2016.06.069.
Kyzas GZ, Fu J, Lazaridis NK, Bikiaris DN, Matis KA. New approaches on the removal of pharmaceuticals from wastewaters with adsorbent materials. J Mol Liq. 2015;209:87–93. https://doi.org/10.1016/j.molliq.2015.05.025.
Lee CM, Palaniandy P, Dahlan I. Pharmaceutical residues in aquatic environment and water remediation by TiO2 heterogeneous photocatalysis: a review. Environ Earth Sci. 2017;76:611. https://doi.org/10.1007/s12665-017-6924-y.
Schwaiger J, Ferling H, Mallow U, Wintermayr H, Negele RD. Toxic effects of the non-steroidal anti-inflammatory drug diclofenac. Part I: histopathological alterations and bioaccumulation in rainbow trout. Aquat Toxicol. 2004;68:141–50. https://doi.org/10.1016/j.aquatox.2004.03.014.
Galus M, Jeyaranjaan J, Smith E, Li H, Metcalfe C, Wilson JY. Chronic effects of exposure to a pharmaceutical mixture and municipal wastewater in zebrafish. Aquat Toxicol. 2013;132–133:212–22. https://doi.org/10.1016/j.aquatox.2012.12.016.
Fent K, Weston AA, Caminada D. Ecotoxicology of human pharmaceuticals. Aquat Toxicol. 2006;76:122–59. https://doi.org/10.1016/j.aquatox.2005.09.009.
Yahiat S, Fourcade F, Brosillon S, Amrane A. Removal of antibiotics by an integrated process coupling photocatalysis and biological treatment - case of tetracycline and tylosin. Int Biodeterior Biodegrad. 2011;65:997–1003. https://doi.org/10.1016/j.ibiod.2011.07.009.
Grabert R, Boopathy R, Nathaniel R, LaFleur G. Effect of tetracycline on ammonia and carbon removal by the facultative bacteria in the anaerobic digester of a sewage treatment plant. Bioresour Technol. 2018;267:265–70. https://doi.org/10.1016/j.biortech.2018.07.061.
Tang F, Hu HY, Wu QY, Tang X, Sun YX, Shi XL, et al. Effects of chemical agent injections on genotoxicity of wastewater in a microfiltration-reverse osmosis membrane process for wastewater reuse. J Hazard Mater. 2013;260:231–7. https://doi.org/10.1016/j.jhazmat.2013.05.035.
Sun YX, Gao Y, Hu HY, Tang F, Yang Z. Characterization and biotoxicity assessment of dissolved organic matter in RO concentrate from a municipal wastewater reclamation reverse osmosis system. Chemosphere. 2014;117:545–51. https://doi.org/10.1016/j.chemosphere.2014.09.024.
Jiang N, Shang R, Heijman SGJ, Rietveld LC. High-silica zeolites for adsorption of organic micro-pollutants in water treatment: a review. Water Res. 2018;144:145–61. https://doi.org/10.1016/j.watres.2018.07.017.
Lin L, Jiang W, Xu P. Comparative study on pharmaceuticals adsorption in reclaimed water desalination concentrate using biochar: impact of salts and organic matter. Sci Total Environ. 2017;601–602:857–64. https://doi.org/10.1016/j.scitotenv.2017.05.203.
Wang W. Characterisation and removal of organic matter from a reverse osmosis concentrate by a PAC accumulative countercurrent four-stage adsorption-MF hybrid process. Sep Purif Technol. 2017;189:425–32. https://doi.org/10.1016/j.seppur.2017.08.002.
Jamil S, Loganathan P, Kazner C, Vigneswaran S. Forward osmosis treatment for volume minimisation of reverse osmosis concentrate from a water reclamation plant and removal of organic micropollutants. Desalination. 2015;372:32–8. https://doi.org/10.1016/j.desal.2015.06.013.
Jamil S, Jeong S, Vigneswaran S. Application of pressure assisted forward osmosis for water purification and reuse of reverse osmosis concentrate from a water reclamation plant. Sep Purif Technol. 2016;171:182–90. https://doi.org/10.1016/j.seppur.2016.07.036.
Jamil S, Vigneswaran S, Jeong S. Application of forward osmosis membrane in nanofiltration mode to treat reverse osmosis concentrate from wastewater reclamation plants. Water Sci Technol. 2018;77:1990–7. https://doi.org/10.2166/wst.2018.087.
Shanmuganathan S, Nguyen TV, Jeong S, Kandasamy J, Vigneswaran S. Submerged membrane - (GAC) adsorption hybrid system in reverse osmosis concentrate treatment. Sep Purif Technol. 2015;146:8–14. https://doi.org/10.1016/j.seppur.2015.03.017.
Shanmuganathan S, Loganathan P, Kazner C, Johir MAH, Vigneswaran S. Submerged membrane filtration adsorption hybrid system for the removal of organic micropollutants from a water reclamation plant reverse osmosis concentrate. Desalination. 2017;401:134–41. https://doi.org/10.1016/j.desal.2016.07.048.
Zhang Y, Prigent B, Geißen SU. Adsorption and regenerative oxidation of trichlorophenol with synthetic zeolite: ozone dosage and its influence on adsorption performance. Chemosphere. 2016;154:132–7. https://doi.org/10.1016/j.chemosphere.2016.03.079.
Fukahori S, Fujiwara T. Modeling of sulfonamide antibiotic removal by TiO2/high-silica zeolite HSZ-385 composite. J Hazard Mater. 2014;272:1–9. https://doi.org/10.1016/j.jhazmat.2014.02.028.
Mendret J, Azaïs A, Petit E, Brosillon S, Cazals G. Ozonation as a pretreatment process for nanofiltration brines: monitoring of transformation products and toxicity evaluation. J Hazard Mater. 2017;338:381–93. https://doi.org/10.1016/j.jhazmat.2017.05.045.
Li A, Chen Z, Wu QY, Huang MH, Liu ZY, Chen P, et al. Study on the removal of benzisothiazolinone biocide and its toxicity: the effectiveness of ozonation. Chem Eng J. 2016;300:376–83. https://doi.org/10.1016/j.cej.2016.04.021.
Azaïs A, Mendret J, Petit E, Brosillon S. Influence of volumetric reduction factor during ozonation of nanofiltration concentrates for wastewater reuse. Chemosphere. 2016;165:497–506. https://doi.org/10.1016/j.chemosphere.2016.09.071.
Wang H, Wang YN, Li X, Sun Y, Wu H, Chen D. Removal of humic substances from reverse osmosis (RO) and nanofiltration (NF) concentrated leachate using continuously ozone generation-reaction treatment equipment. Waste Manag. 2016;56:271–9. https://doi.org/10.1016/j.wasman.2016.07.040.
Zhou T, Lim T-T, Chin S-S, Fane AG. Treatment of organics in reverse osmosis concentrate from a municipal wastewater reclamation plant: feasibility test of advanced oxidation processes with/without pretreatment. Chem Eng J. 2011;166:932–9. https://doi.org/10.1016/j.cej.2010.11.078.
Joss A, Baenninger C, Foa P, Koepke S, Krauss M, McArdell CS, et al. Water reuse: >90% water yield in MBR/RO through concentrate recycling and CO2 addition as scaling control. Water Res. 2011;45:6141–51. https://doi.org/10.1016/j.watres.2011.09.011.
Acero JL, Benitez FJ, Real FJ, Teva F. Micropollutants removal from retentates generated in ultrafiltration and nanofiltration treatments of municipal secondary effluents by means of coagulation, oxidation, and adsorption processes. Chem Eng J. 2016;289:48–58. https://doi.org/10.1016/j.cej.2015.12.082.
Hermosilla D, Merayo N, Ordóñez R, Blanco Á. Optimization of conventional Fenton and ultraviolet-assisted oxidation processes for the treatment of reverse osmosis retentate from a paper mill. Waste Manag. 2012;32:1236–43. https://doi.org/10.1016/j.wasman.2011.12.011.
Ren Y, Yuan Y, Lai B, Zhou Y, Wang J. Treatment of reverse osmosis (RO) concentrate by the combined Fe/Cu/air and Fenton process (1stFe/Cu/air-Fenton-2ndFe/Cu/air). J Hazard Mater. 2016;302:36–44. https://doi.org/10.1016/j.jhazmat.2015.09.025.
Aouni A, Altinay AD, Ilhan F, Koseoglu-Imer DY, Avsar Y, Hafiane A, et al. The applicability of combined physico-chemical processes for treatment and reuse of synthetic textile reverse osmosis concentrate. Desalin Water Treat. 2018;111:111–24. https://doi.org/10.5004/dwt.2018.22244.
Dialynas E, Mantzavinos D, Diamadopoulos E. Advanced treatment of the reverse osmosis concentrate produced during reclamation of municipal wastewater. Water Res. 2008;42:4603–8. https://doi.org/10.1016/j.watres.2008.08.008.
Cemre Birben N, Bekbolet M. Role of emerging contaminants on solar photocatalytic treatment of organic matter in reverse osmosis concentrate. Catal Today. 2019;326:101–7. https://doi.org/10.1016/j.cattod.2018.10.048.
Goedecke C, Sojref R, Nguyen TY, Piechotta C. Immobilization of photocatalytically active TiO2 nanopowder by high shear granulation. Powder Technol. 2017;318:465–70. https://doi.org/10.1016/j.powtec.2017.06.025.
Haynes VN, Ward JE, Russell BJ, Agrios AG. Photocatalytic effects of titanium dioxide nanoparticles on aquatic organisms—current knowledge and suggestions for future research. Aquat Toxicol. 2017;185:138–48. https://doi.org/10.1016/j.aquatox.2017.02.012.
Xiang Q, Fukahori S, Yamashita N, Tanaka H, Fujiwara T. Removal of crotamiton from reverse osmosis concentrate by a TiO2/zeolite composite sheet. Appl Sci. 2017;7:778. https://doi.org/10.3390/app7080778.
Umar M, Roddick F, Fan L. Effect of coagulation on treatment of municipal wastewater reverse osmosis concentrate by UVC/H2O2. J Hazard Mater. 2014;266:10–8. https://doi.org/10.1016/j.jhazmat.2013.12.005.
Umar M, Roddick F, Fan L. Comparison of coagulation efficiency of aluminium and ferric-based coagulants as pre-treatment for UVC/H2O2 treatment of wastewater RO concentrate. Chem Eng J. 2016;284:841–9. https://doi.org/10.1016/j.cej.2015.08.109.
Lu J, Fan L, Roddick FA. Potential of BAC combined with UVC/H2O2 for reducing organic matter from highly saline reverse osmosis concentrate produced from municipal wastewater reclamation. Chemosphere. 2013;93:683–8. https://doi.org/10.1016/j.chemosphere.2013.06.008.
Pradhan S, Fan L, Roddick FA, Shahsavari E, Ball AS. Impact of salinity on organic matter and nitrogen removal from a municipal wastewater RO concentrate using biologically activated carbon coupled with UV/H2O2. Water Res. 2016;94:103–10. https://doi.org/10.1016/j.watres.2016.02.046.
Lütke Eversloh C, Schulz M, Wagner M, Ternes TA. Electrochemical oxidation of tramadol in low-salinity reverse osmosis concentrates using boron-doped diamond anodes. Water Res. 2015;72:293–304. https://doi.org/10.1016/j.watres.2014.12.021.
Weng M, Pei J. Electrochemical oxidation of reverse osmosis concentrate using a novel electrode: parameter optimization and kinetics study. Desalination. 2016;399:21–8. https://doi.org/10.1016/j.desal.2016.08.002.
Maharaja P, Boopathy R, Karthikeyan S, Mahesh M, Komal AS, Gupta VK, et al. Advanced oxidation of catechol in reverse osmosis concentrate generated in leather wastewater by Cu–graphite electrode. Int J Environ Sci Technol. 2016;13:2143–52. https://doi.org/10.1007/s13762-016-1044-x.
Barazesh JM, Prasse C, Sedlak DL. Electrochemical transformation of trace organic contaminants in the presence of halide and carbonate ions. Environ Sci Technol. 2016;50:10143–52. https://doi.org/10.1021/acs.est.6b02232.
Lan Y, Coetsier C, Causserand C, Groenen SK. On the role of salts for the treatment of wastewaters containing pharmaceuticals by electrochemical oxidation using a boron doped diamond anode. Electrochim Acta. 2017;231:309–18. https://doi.org/10.1016/j.electacta.2017.01.160.
Wang J, Zhang T, Mei Y, Pan B. Treatment of reverse-osmosis concentrate of printing and dyeing wastewater by electro-oxidation process with controlled oxidation-reduction potential (ORP). Chemosphere. 2018;201:621–6. https://doi.org/10.1016/j.chemosphere.2018.03.051.
Wohlmuth da Silva S, Venzke C, Bitencourt Welter J, Schneider D, Zoppas Ferreira J, Siqueira Rodrigues M, et al. Electrooxidation using Nb/BDD as post-treatment of a reverse osmosis concentrate in the petrochemical industry. Int J Environ Res Public Health. 2019;16:816. https://doi.org/10.3390/ijerph16050816.
Kazner C, Jamil S, Phuntsho SK, Shon H, Wintgens T, Vigneswaran S. Forward osmosis for the treatment of reverse osmosis concentrate from water reclamation: process performance and fouling control. Water Sci Technol. 2014;69:2431–7. https://doi.org/10.2166/wst.2014.138.
Maltos RA, Regnery J, Almaraz N, Fox S, Schutter M, Cath TJ, et al. Produced water impact on membrane integrity during extended pilot testing of forward osmosis – reverse osmosis treatment. Desalination. 2018;440:99–110. https://doi.org/10.1016/j.desal.2018.02.029.
Kim Y, Woo YC, Phuntsho S, Nghiem LD, Shon HK, Hong S. Evaluation of fertilizer-drawn forward osmosis for coal seam gas reverse osmosis brine treatment and sustainable agricultural reuse. J Membr Sci. 2017;537:22–31. https://doi.org/10.1016/j.memsci.2017.05.032.
Xu P, Cath TY, Robertson AP, Reinhard M, Leckie JO, Drewes JE. Critical review of desalination concentrate management, treatment and beneficial use. Environ Eng Sci. 2013;30:502–14. https://doi.org/10.1089/ees.2012.0348.
Tong T, Elimelech M. The global rise of zero liquid discharge for wastewater management: drivers, technologies, and future directions. Environ Sci Technol. 2016;50:6846–55. https://doi.org/10.1021/acs.est.6b01000.
Arola K, Van der Bruggen B, Mänttäri M, Kallioinen M. Treatment options for nanofiltration and reverse osmosis concentrates from municipal wastewater treatment: a review. Crit Rev Environ Sci Technol. 2019:1–68. https://doi.org/10.1080/10643389.2019.1594519.
Jia S, Han Y, Zhuang H, Han H, Li K. Simultaneous removal of organic matter and salt ions from coal gasification wastewater RO concentrate and microorganisms succession in a MBR. Bioresour Technol. 2017;241:517–24. https://doi.org/10.1016/j.biortech.2017.05.158.
Yao S, Chen L, Guan D, Zhang Z, Tian X, Wang A, et al. On-site nutrient recovery and removal from source-separated urine by phosphorus precipitation and short-cut nitrification-denitrification. Chemosphere. 2017;175:210–8. https://doi.org/10.1016/j.chemosphere.2017.02.062.
Justo A, González O, Sans C, Esplugas S. BAC filtration to mitigate micropollutants and EfOM content in reclamation reverse osmosis brines. Chem Eng J. 2015;279:589–96. https://doi.org/10.1016/j.cej.2015.05.018.
Llorca M, Badia-Fabregat M, Rodríguez-Mozaz S, Caminal G, Vicent T, Barceló D. Fungal treatment for the removal of endocrine disrupting compounds from reverse osmosis concentrate: identification and monitoring of transformation products of benzotriazoles. Chemosphere. 2017;184:1054–70. https://doi.org/10.1016/j.chemosphere.2017.06.053.
Badia-Fabregat M, Lucas D, Tuomivirta T, Fritze H, Pennanen T, Rodríguez-Mozaz S, et al. Study of the effect of the bacterial and fungal communities present in real wastewater effluents on the performance of fungal treatments. Sci Total Environ. 2017;579:366–77. https://doi.org/10.1016/j.scitotenv.2016.11.088.
Miranda AF, Ramkumar N, Andriotis C, Höltkemeier T, Yasmin A, Rochfort S, et al. Applications of microalgal biofilms for wastewater treatment and bioenergy production. Biotechnol Biofuels. 2017;10:1–23. https://doi.org/10.1186/s13068-017-0798-9.
Ikehata K, Zhao Y, Kulkarni HV, Li Y, Snyder SA, Ishida KP, et al. Water recovery from advanced water purification facility reverse osmosis concentrate by photobiological treatment followed by secondary reverse osmosis. Environ Sci Technol. 2018;52:8588–95. https://doi.org/10.1021/acs.est.8b00951.
Volk CJ, Lechevallier MW. Effects of conventional treatment on AOC and BDOC levels. J Am Water Works Assoc. 2002;94:112–23. https://doi.org/10.1002/j.1551-8833.2002.tb09494.x.
Long Y, Xu J, Shen D, Du Y, Feng H. Effective removal of contaminants in landfill leachate membrane concentrates by coagulation. Chemosphere. 2017;167:512–9. https://doi.org/10.1016/j.chemosphere.2016.10.016.
Ho JS, Ma Z, Qin J, Sim SH, Toh CS. Inline coagulation-ultrafiltration as the pretreatment for reverse osmosis brine treatment and recovery. Desalination. 2015;365:242–9. https://doi.org/10.1016/j.desal.2015.03.018.
Shi J, Dang Y, Qu D, Sun D. Effective treatment of reverse osmosis concentrate from incineration leachate using direct contact membrane distillation coupled with a NaOH/PAM pre-treatment process. Chemosphere. 2019;220:195–203. https://doi.org/10.1016/j.chemosphere.2018.12.110.
Yuan Z, He C, Shi Q, Xu C, Li Z, Wang C, et al. Molecular insights into the transformation of dissolved organic matter in landfill leachate concentrate during biodegradation and coagulation processes using ESI FT-ICR MS. Environ Sci Technol. 2017;51:8110–8. https://doi.org/10.1021/acs.est.7b02194.
Qian F, He M, Wu J, Yu H, Duan L. Insight into removal of dissolved organic matter in post pharmaceutical wastewater by coagulation-UV/H2O2. J Environ Sci (China). 2019;76:329–38. https://doi.org/10.1016/j.jes.2018.05.025.
Huang J, Chen J, Xie Z, Xu X. Treatment of nanofiltration concentrates of mature landfill leachate by a coupled process of coagulation and internal micro-electrolysis adding hydrogen peroxide. Environ Technol (United Kingdom). 2015;36:1001–7. https://doi.org/10.1080/09593330.2014.971882.
Shah TM, Ramaswami S, Behrendt J, Otterpohl R. Simultaneous removal of organics and ammonium-nitrogen from reverse osmosis concentrate of mature landfill leachate. J Water Process Eng. 2017;19:126–32. https://doi.org/10.1016/j.jwpe.2017.07.024.
Fukahori S, Fujiwara T, Ito R, Funamizu N. Photocatalytic decomposition of crotamiton over aqueous TiO2 suspensions: determination of intermediates and the reaction pathway. Chemosphere. 2012;89:213–20. https://doi.org/10.1016/j.chemosphere.2012.04.018.
Fukahori S, Fujiwara T. Photocatalytic decomposition behavior and reaction pathway of sulfamethazine antibiotic using TiO2. J Environ Manag. 2015;157:103–10. https://doi.org/10.1016/j.jenvman.2015.04.002.
Fukahori S, Fujiwara T, Ito R, Funamizu N. pH-dependent adsorption of sulfa drugs on high silica zeolite: modeling and kinetic study. Desalination. 2011;275:237–42. https://doi.org/10.1016/j.desal.2011.03.006.
Fukahori S, Fujiwara T, Funamizu N, Matsukawa K, Ito R. Adsorptive removal of sulfonamide antibiotics in livestock urine using the high-silica zeolite HSZ-385. Water Sci Technol. 2013;67:319–25. https://doi.org/10.2166/wst.2012.513.
Chen X, Fujiwara T, Fukahori S, Ishigaki T. Factors affecting the adsorptive removal of bisphenol A in landfill leachate by high silica Y-type zeolite. Environ Sci Pollut Res. 2015;22:2788–99. https://doi.org/10.1007/s11356-014-3522-3.
Nomura Y, Fukahori S, Fukada H, Fujiwara T. Removal behaviors of sulfamonomethoxine and its degradation intermediates in fresh aquaculture wastewater using zeolite/TiO2 composites. J Hazard Mater. 2017;340:427–34. https://doi.org/10.1016/j.jhazmat.2017.07.034.
Xiang Q, Fukahori S, Nomura Y, Fujiwara T. Removal of crotamiton and its degradation intermediates from secondary effluent using TiO2–zeolite composites. Water Sci Technol. 2018;77:788–99. https://doi.org/10.2166/wst.2017.578.
Ito M, Fukahori S, Fujiwara T. Adsorptive removal and photocatalytic decomposition of sulfamethazine in secondary effluent using TiO2-zeolite composites. Environ Sci Pollut Res. 2014;21:834–42. https://doi.org/10.1007/s11356-013-1707-9.
Fukahori S, Ito M, Fujiwara T. Removal mechanism of sulfamethazine and its intermediates from water by a rotating advanced oxidation contactor equipped with TiO2–high-silica zeolite composite sheets. Environ Sci Pollut Res. 2018;25:29017–25. https://doi.org/10.1007/s11356-018-2909-y.
Nomura Y, Fukahori S, Fujiwara T. Removal of sulfamonomethoxine and its transformation byproducts from fresh aquaculture wastewater by a rotating advanced oxidation contactor equipped with zeolite/TiO2 composite sheets (in preparation). J Hazard Mater 2019.
Fukahori S, Fujiwara T, Ito R, Funamizu N. Sulfonamide antibiotic removal and nitrogen recovery from synthetic urine by the combination of rotating advanced oxidation contactor and methylene urea synthesis process. Water Sci Technol. 2015;72:238–44. https://doi.org/10.2166/wst.2015.182.
Nomura Y, Fukahori S, Fujiwara T. Removal of 1,4-dioxane from landfill leachate by a rotating advanced oxidation contactor equipped with activated carbon/TiO2 composite sheets (under review). J Hazard Mater. 2019.
Wang X-X, Wu Y-H, Zhang T-Y, Xu X-Q, Dao G-H, Hu H-Y. Simultaneous nitrogen, phosphorous, and hardness removal from reverse osmosis concentrate by microalgae cultivation. Water Res. 2016;94:215–24. https://doi.org/10.1016/j.watres.2016.02.062.
Maeng SK, You SH, Nam JY, Ryu H, Timmes TC, Kim HC. The growth of Scenedesmus quadricauda in RO concentrate and the impacts on refractory organic matter, Escherichia coli, and trace organic compounds. Water Res. 2018;134:292–300. https://doi.org/10.1016/j.watres.2018.01.029.
Maeng SK, Khan W, Park JW, Han I, Yang HS, Song KG, et al. Treatment of highly saline RO concentrate using Scenedesmus quadricauda for enhanced removal of refractory organic matter. Desalination. 2018;430:128–35. https://doi.org/10.1016/j.desal.2017.12.056.
Chakraborti RK, Bays JS, Ng T, Balderrama L, Kirsch T. A pilot study of a subsurface-flow constructed wetland treating membrane concentrate produced from reclaimed water. Water Sci Technol. 2015;72:260–8. https://doi.org/10.2166/wst.2015.201.
Acknowledgements
We thank Prof. Dennis Murphy of The United Graduate School of Agricultural Sciences, Ehime University for editing a draft of this manuscript.
Funding
This work was financially supported by JSPS KAKENHI Grant Number 16H02372.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
On behalf of all the authors, Taku Fujiwara states that there is no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Water Pollution
Rights and permissions
About this article
Cite this article
Xiang, Q., Nomura, Y., Fukahori, S. et al. Innovative Treatment of Organic Contaminants in Reverse Osmosis Concentrate from Water Reuse: a Mini Review. Curr Pollution Rep 5, 294–307 (2019). https://doi.org/10.1007/s40726-019-00119-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40726-019-00119-2