Abstract
Purpose of Review
The purpose of this review is to briefly outline the current state of hemorrhage control and resuscitation in trauma patients with a specific focus on the role viscoelastic assays have in this complex management to include indications for use across all phases of care in the injured patient.
Recent Findings
Viscoelastic assay use to guide blood-product resuscitation in bleeding trauma patients can reduce mortality by up to 50%. Viscoelastic assays also reduce total blood products transfused, reduce ICU length of stay, and reduce costs. There are a large number of observational and retrospective studies evaluating viscoelastic assay use in the initial trauma resuscitation, but only one randomized control trial. There is a paucity of data evaluating use of viscoelastic assays in the operating room, post-operatively, and during ICU management in trauma patients, rendering their use in these settings extrapolative/speculative based on theory and data from other surgical disciplines and settings.
Summary
Both hypocoagulable and hypercoagulable states exist in trauma patients, and viscoelastic assays are better at diagnosing both relative to standard coagulation testing and can better indicate what therapy may be most appropriate. Further study is needed, particularly in the operating room and post-operative/ICU settings in trauma patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the CDC, unintentional injury and accidents are the leading cause of death in people between the ages of 1 to 44 years, accounting for over 200,000 deaths in the USA per year [1]. As such, proper and expeditious management of trauma in the hospital and even the pre-hospital setting is critical to improving health outcomes in the USA. It is estimated that 50% of trauma victims die at the scene of injury while the remaining 50% of deaths occur within the hospital [2,3,4]. Up to 40% of these deaths are due to hemorrhage [4]. Roughly on, three severely injured patients experience trauma-induced coagulopathy, which is a multifactorial biological event to that contributes to ongoing bleeding and is associated with early deaths [5]. Previous work has emphasized the importance of stratifying and categorizing trauma patients based on coagulopathy subtypes to guide goal-directed resuscitation [6]. In fact, rapid diagnosis using viscoelastic assays to guide blood product resuscitation in trauma patients requiring massive transfusion led to a 50% reduction in mortality in one recent randomized control trial [7••]. With a growing body of evidence that viscoelastic assays can improve outcomes including mortality, it is clear that appropriate use of viscoelastic assays in trauma patients is of paramount importance. While there are a large number of publications that suggest different thresholds for transfusing trauma patients with blood products based on viscoelastic assessment to treat coagulopathy and hemorrhage, there are few publications that address the ideal patient population, timing, and frequency of testing. The purpose of this review is to briefly outline the current state of hemorrhage management and resuscitation and define what role viscoelastic assays have in this complex management to include indications for use across all phases of care in the injured patient.
Trauma-Induced Coagulopathy—One Size Does Not Fit All
In order to appreciate the importance of viscoelastic assays as diagnostic tools in trauma, it is necessary to understand that various coagulopathies and fibrinolytic perturbations are captured under the umbrella term trauma-induced coagulopathy (“TIC”). Hypocoagulable TIC, where clot formation is impaired, is prevalent and can result from either the failure to form an adequate clot (inclusive of the role of platelet dysfunction) or from the rapid breakdown of forming clots. Poor clot formation on assays using platelet-poor plasma (i.e., international normalized ratio or “INR”), for example, is present in 1 of every 4 polytrauma patients and carries a mortality fourfold higher than in those without a measurable coagulopathy (46% versus 10.9%) [8]. Furthermore, hyperfibrinolysis, such that any hemostatic clot that forms is rapidly and inappropriately broken down, is present in 1 of every 5 trauma patients and carries an OR for death of 3.3 relative to physiologic fibrinolysis states [9•, 10]. The process by which these systemic hypocoagulable states result has been previously termed the “bloody vicious cycle” at the intersection of coagulopathy, hypothermia, and acidosis [11], and since Kashuk et al. published this seminal paper, much work has been done to deepen our mechanistic understanding of the coagulopathy of trauma and has highlighted the need to identify the point of failure for targeted resuscitation. In contrast to hypocoagulable TIC, hypercoagulability from overactivation/unregulated activation of clot formation or from blunted fibrinolysis (termed “fibrinolysis shutdown”) is also a highly prevalent problem in trauma patients, where viscoelastic assay use is more sensitive than standard coagulations tests such as the prothrombin time (PT/INR) and partial thromboplastin time while also providing information about the functional state of the fibrinolysis system that allows for a more detailed analysis of hypercoagulable states for targeted treatment [9•, 10, 12,13,14,15]. In fact, fibrinolysis shutdown alone is present in nearly 50% of trauma patients, and it has been shown repeatedly that such hypercoagulable states in trauma patients are a herald for organ failure and death with an OR of 1.6 for increased mortality [9•, 10, 13].
Understanding these various points of failure in coagulation and fibrinolysis to generate hypocoagulable and hypercoagulable states after trauma highlights a clear opportunity for viscoelastic assay use to guide therapy and improve trauma outcomes. For example, excessively high levels of tPA, a powerful activator of the fibrinolytic pathway following trauma, has been suggested as a probable cause for the hyperfibrinolytic state of so many trauma patients [16, 17] and such a state, if diagnosed, may benefit greatly from targeted use of antifibrinolytics like tranexamic acid (TXA) [18, 19]. Meanwhile, on the other end of the spectrum is “fibrinolysis shutdown,” which is extremely common after trauma and its diagnosis may indicate that TXA would cause an increased risk of harm or even death [18, 19]. The need for early thromboembolism chemoprophylaxis (low molecular weight or unfractionated heparin) to prevent complications such as venous thrombosis or pulmonary embolism is apparent in this setting where prolonged fibrinolysis shutdown is known to occur in patients who go on to develop VTE [20], and early initiation of chemoprophylaxis in a variety of settings including traumatic brain injury and solid organ injury is both safe and effective [21,22,23,24]. Of note, there is ongoing work including a phase II clinical trial of trauma patients evaluating the role of more aggressive and novel approaches to thromboembolism prevention that include the use of aspirin and HMG-CoA reductase inhibitors (STAT Trial, University of Colorado, ClinicalTrials.gov Identifier NCT02901067). It is also possible that further therapies for this group of patients, who are at significantly increased risk of death from organ failure that likely results from microvascular thrombosis [10, 18, 25, 26], are on the horizon. Evidence exists that pro-fibrinolytic states or therapy can help patients with medically refractory severe ARDS and organ failure [25, 26], and there is also an argument for therapeutic systemic anticoagulation to prevent microvascular thrombosis in the first place (and thus, fibrinolysis is not required to maintain microvascular patency), where agents like heparin have been known to be effective in coagulopathy-associated organ failure for decades (c.f. [27]). Of course, in that setting, the risk of bleeding would need to be carefully weighed against the risk of adverse outcomes from organ failure. Ultimately, it is clear that patient-specific interventions for trauma patient coagulation and fibrinolysis disorders may lead to improved outcomes, but doing so effectively requires a rapid and comprehensive evaluation of both the complex coagulation and fibrinolytic functional states of the patient. Viscoelastic assays are at the forefront of providing this information.
Principles of Hemorrhage Management—Resuscitation in the Era of REBOA, Modern Surgery, and Viscoelastic Assays
Principles of hemorrhage management are multifaceted and the current framework for treatment encompasses mechanical control, resuscitation, and targeted treatment of coagulopathy (if present). The latter two principles often require diagnostic tools to aid in their focused application, but an understanding of all three is required to define when and where viscoelastic assays have a role in trauma management.
While beyond the scope of this review to detail all aspects of mechanical management of hemorrhage, the primary methods include direct pressure, tourniquet use, and more recent techniques such as Resuscitative Balloon Occlusion of the Aorta (REBOA) (Mechanical Hemorrhage Control. Debose et al., 2017, Hemorrhagic Shock: Recognition, Pathophysiology and Management, pp. 285–306). Tourniquet usage has long been used in military settings and dates back to antiquity, and while their effectiveness has been debated by some, tourniquets are generally accepted as a reasonable method to rapidly stop blood loss to preserve blood volume [28,29,30]. While there are some drawbacks and risks from tourniquet use [31], the American College of Surgeons’ recent Stop the Bleed initiative includes tourniquet usage as its final step if manual application of pressure and attempts to cover the wound with a hemostatic dressing fail to control hemorrhage. Mechanical control of hemorrhage then extends from the field into the hospital setting where techniques such as REBOA or open surgical aortic occlusion to stop blood loss become options, particularly in non-compressible torso hemorrhage (NCTH), as temporary life-saving measures while working to get the patient to the operating theater for definitive surgical control of bleeding [32].
Resuscitation techniques are another key component of managing a trauma patient with hemorrhage, where multiple studies have shown that patient-specific focused interventions can have a marked impact on survival [7] and even broad-based, less focused interventions can improve survival when trauma patients meet preset criteria [33••, 34]. A common protocol taught in standard trauma management algorithms such as ATLS, which is decades old, involves immediate infusion with saline or lactated ringers solution to replenish the lost intravascular volume followed by later transfusion with blood products. This technique has come under criticism in recent years as numerous surgeons have noted that the practice worsens the patients’ coagulopathy, hypothermia, and acidosis, in part because crystalloid-based resuscitation does not adequately address the coagulation disorders already present in an injured patient on arrival [35]. In fact, the dangers of crystalloid-based resuscitation were recognized several decades ago, where during the Vietnam War surgeons called the resulting pulmonary edema and lung failure after massive crystalloid resuscitation “Da Nang Lung,” which would eventually be referred to as ARDS [36, 37•]. Instead, a newer technique coined “damage control resuscitation” has been adopted, coupling earlier administration of blood products, treatment of hypothermia and acidosis, and permissive hypotension with surgical intervention to stop blood loss [35]. Historically, coagulopathy was assumed to be primarily caused by dilution of blood with crystalloid solution, but the modern damage control technique recognizes that this is not the case [38]. The damage control approach emphasizing early blood-product based resuscitation strategies such as 1:1:1 ratios of red blood cells to fresh frozen plasma to platelets has become popular and with measurable efficacy, as evidenced in the PROPPR clinical trial [35, 38, 39]. The earlier inclusion of blood products in this resuscitation strategy naturally led to questions about whether rapid viscoelastic assays could be used to meaningfully direct targeted blood product administration for further improvement in outcomes beyond the empiric transfusion ratio strategy. A prospective randomized clinical trial examined this question and demonstrated feasibility and marked improvement in survival within a single mature level 1 trauma center, where viscoelastic assay-guided blood product administration led to a 50% reduction in mortality compared to an early standardized massive transfusion protocol and conventional coagulation assays (INR and PTT) [7••].
The last component of dealing with hemorrhage focuses on addressing any primary and/or secondary coagulopathies present in the patient, where primary coagulopathy refers to TIC phenotypes present before any resuscitation takes place while secondary coagulopathy refers to those coagulopathies that develop or are exacerbated by various resuscitation strategies, as resuscitation itself is known to produce coagulation changes that may be harmful [40]. Addressing these is critical because, as described in more detail above, a high proportion of trauma patients present with some type of coagulopathy that significantly increases their risk of mortality and requires medical management to improve outcomes [8, 41]. Furthermore, it should be noted that trauma patients’ coagulation phenotypes often change over time. For example, even in trauma patients presenting with an initial hypocoagulable state, it is important to anticipate rebound hypercoagulopathy (including fibrinolysis shutdown), a long-known phenomena originally described by Walter Cannon in World War I era studies that is associated with increased mortality [42, 43] and clearly highlights the need for ongoing surveillance with viscoelastic assays in patients initially presenting with coagulopathy. This is integral in explaining why patients after trauma have high rates of DVT and PE (the latter of which is the third most common cause of death > 24 h after trauma), and a role for viscoelastic assays in diagnosing these hypercoagulable conditions exists to guide risk mitigation of thrombotic complications and death as a result [43,44,45,46]. Suffice it to say that targeted treatment of coagulopathies is greatly aided by the use of viscoelastic assays—which are discussed in detail in the ensuing text.
Viscoelastic Assays—Thromboelastography and Rotational Thromboelastometry
Various “viscoelastic assays” that measure mechanical properties of blood clots have been developed to capture information on the formation, mechanical strength, and breakdown of clots, are becoming increasingly common in hospitals with a trauma center designation, and transfusion guidelines using these viscoelastic devices are also now included in the American College of Surgeons Trauma Quality Improvement Program (TQIP) [37•, 47]. The process of clot formation is typically divided into primary and secondary hemostasis, with the primary portion involving platelet aggregation and the secondary portion involving coagulation factors converting fibrinogen to fibrin (reviewed in [48]). Historically, platelet aggregation assays along with fibrinogen level assays helped capture some of the deficiencies in primary hemostasis, while prothrombin time [34] and partial thromboplastin time (PTT) assays revealed deficiencies in secondary hemostasis. The PT and PTT assays have long been used in hospitals to examine coagulopathies affecting the intrinsic and extrinsic pathways of the coagulation cascade, respectively [49]. Despite their common usage, there are several drawbacks to using these tests, including the following: (1) both assays taking roughly an hour to give only limited information (see below), meaning that critical intervention time is lost [37•]; (2) they are measuring the clotting of platelet-poor plasma without the presence of blood cells (i.e., in the absence of platelets, erythrocytes, leukocytes), making functional meaning difficult to interpret; (3) the readout from either assay is a clotting time, so neither assay indicates which specific coagulation factors are deficient in a patient; and (4) no information about fibrinolysis is obtained, which ignores half of the biology of the global human hemostatic system. Viscoelastic assays such as thromboelastography (TEG®) and rotational thromboelastometry (ROTEM®) aim to address these inadequacies of the commonly used PT and PTT assays, providing functional clotting and fibrinolysis information using whole blood (instead of platelet-poor plasma) in real time [50,51,52].
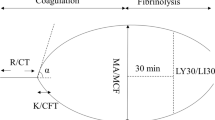
TEG® and ROTEM®, two commonly used viscoelastic assays, operate on similar principles with slight variations in their exact mechanism of action, and while the variables they generate are similar, they are not exact equivalents [53]. Traditional thromboelastography works by placing 360 μl of whole blood in a cylindrical plastic cup that is pre-heated to 37 °C with a pin suspended down in to the blood-containing cup by a torsion wire, and the cup then oscillates at 4° 45′ (0.1 Hz) [50]. If blood is anticoagulated with 3.2% citrate, then 340 μl of citrate-anticoagulated whole blood is mixed with a standard 20 μl quantity of 0.2 M calcium chloride to reverse this and initiate contact activation within the cup. Other activators such as kaolin (“kaolin TEG”) or kaolin + tissue factor + phospholipids (“Rapid-TEG” or “R-TEG”) can also be used to speed assay results. As the clot forms and then dissolves, the deflections of the pin and torsion wire are measured to give the TEG® tracing typically observed (Fig. 1a). The variables reported and what part of coagulation they reflect are depicted in Fig. 1a and are as follows: Reaction Time (R-time) (*not applicable to R-TEG) = time (seconds) until 2 mm deflection of pin, as seen on TEG tracing, represents coagulation factor activity; Activated Clotting Time (ACT) (*R-TEG only) = derived value (seconds) that is rapidly available and also represents coagulation factor activity; α-Angle = angle (degrees) formed by the tangent line drawn from the split point of the initial TEG® curve to the arcing of the lengthening TEG® tracing, which reflects the propagation phase of coagulation factor activity and fibrinogen cleavage and correlates well with fibrinogen function [54] such that this is its primary clinical interpretation; Maximal Amplitude (MA) = maximal deflection of the pin (in millimeters) that reflects the maximal clot strength, primarily a reflection of not only platelet function (and used as such clinically) but also fibrinogen activity to some extent; and LY30, LY60 = percent (%) of the clot that has undergone fibrinolysis 30 and 60 min after achieving MA and thus represent fibrinolytic activity. In the case of the new TEG® 6S, the same parameters are reported but the mechanism of measurement is novel, using vibration with optical detection of resonant frequencies in microfluidic chambers instead of a mechanical torsion pin [55, 56]. Rotational thromboelastometry (ROTEM®) was developed as a derivative of TEG® and works essentially in an inverse fashion, where whole blood is placed in a fixed pre-heated cup and a disposable pin suspended into the blood rotates back and forth by 4.75° at 0.2 Hz (i.e., 12 cycles per minute) and provides a similar tracing to TEG® (Fig. 1b). There are several variations of the test, similar to TEG®, that use different activators and inhibitors to allow for dissecting out various pathways including intrinsic and extrinsic coagulation pathway function (INTEM and EXTEM assays), several platelet function assays, and fibrinogen function (FIBTEM assay) [37•]. The typical parameters used clinically and what part of coagulation they reflect are depicted in Fig. 1b and are as follows: Coagulation Time (CT) = time (seconds) until 2 mm alteration of pin rotation on ROTEM® tracing, which represents coagulation factor activity similar to TEG R-time; α-Angle = angle (degrees) from baseline formed by the tangent line drawn through the 2 mm point on the ROTEM® curve, clinically used as metric of fibrinogen function similar to TEG α-Angle; Maximum Clot Firmness (MCF) = self-descriptive, which reflects the maximal clot strength similar to TEG MA (in millimeters); and Clot Lysis Index 30 (LI30 or CL30), Clot Lysis Index 60 (LI60 or CL60) = percent (%) of MCF remaining at 30 and 60 min after CT is reach (i.e., an inverse readout of TEG® LY30 and also measured from a different start time). Several other parameters exist for both TEG® and ROTEM®, and while a couple are used with some frequency in trauma (e.g., A5 and A10 to guide platelet transfusions with ROTEM®), most are not commonly used in the clinic and an exhaustive list can be found elsewhere [37•].
Given their ability to rapidly return results using whole blood and capture numerous variables influencing both clot formation and breakdown, these viscoelastic assays present physicians with the ability to expeditiously deduce where perturbations in the process of clot formation and breakdown are occurring that includes the critical role of platelets and other constituent blood cells [37•]. Previously published and validated transfusion protocols guided by R-TEG, for example, clearly demonstrate the utility of viscoelastic assays due to their short time and specificity, in this case taking advantage of the R-TEG’s speed to measure the ACT, angle, MA, and LY30 in order to determine whether to administer FFP, cryoprecipitate, platelets, and tranexamic acid, respectively [57••]. Using such methods has demonstrated mortality benefit, reduced hospital and ICU length of stay, reduced costs, and reduced total blood products transfused, making a strong case that viscoelastic assays should be implemented by all medical centers caring for trauma patients [7••, 58,59,60]. A discussion of indications and timing of use for viscoelastic assays in trauma across all phases of care follows and is summarized in Fig. 2.
Viscoelastic Assay Use in Trauma Patients in the Emergency Department, Pre-operative and Non-operative Settings
Viscoelastic assays are powerful tools due to their ease of use, rapid return of results, and information they provide regarding specific perturbations in the coagulation and fibrinolytic pathways, as detailed previously. Because bleeding accounts for such a high proportion of preventable trauma deaths and implementation of viscoelastic assay-guided resuscitation has demonstrated significant mortality benefit in trauma [7••], it is prudent to use viscoelastic assays on patients who are clearly hemorrhaging upon presentation to the emergency department and requiring the attention of trauma surgeons [61]. Aside from those patients clearly hemorrhaging on clinical evaluation, there are several surrogates that have been validated as predictors of hemorrhage and should prompt immediate viscoelastic assay evaluation, where doing so at the time standard trauma labs are drawn would allow for immediate targeted blood-component resuscitation if it becomes clear on further workup that the patient had or continues to have significant ongoing internal hemorrhage. Trauma patients with hypotension and/or tachycardia are high-risk for hemorrhage, and a reasonable criteria as a screening tool for ongoing bleeding can be drawn from two recent randomized controlled trials PAMPer and COMBAT, which are HR > 108 and SBP < 90, or SBP < 70 regardless of HR, at any time before or upon presentation to the ED [33••, 62]. Alternatively, instead of using raw HR and/or SBP numbers, a shock index (SI) can be used to trigger need for viscoelastic assay evaluation, as a SI ≥ 1 has been shown to predict ongoing hemorrhage with need for massive transfusion (MT) in several studies (SI = HR divided by SBP) (c.f. [63, 64]). A penetrating mechanism of trauma is another surrogate that is predictive of hemorrhage with an odds ratio of 2.6 for needing MT, and thus, penetrating trauma patients should be evaluated with viscoelastic assay upon arrival [65]. A positive Focused Abdominal Sonography in Trauma (FAST) exam should also prompt viscoelastic assay evaluation given it is highly predictive of traumatic hemorrhage [66, 67]. Other parameters that are available relatively quickly in the trauma bay to determine who is at risk for MT and thus would benefit from viscoelastic assay evaluation include Hgb ≤ 11 g/dL, hematocrit < 32%, pH < 7.25, or base deficit ≥ 6 [65, 68, 69]. Temperature < 35.5 °C has also been shown to predict need for MT and would be a reasonable trigger to prompt viscoelastic assay evaluation [69].
Aside from patients who are (or are suspected of) actively bleeding and those receiving blood products as noted above, the final major indication for viscoelastic assay usage involves patients suspected of suffering a high-risk injury for bleeding or clotting diatheses. Injuries of note that fall into this category include head trauma, blunt cerebrovascular injury, pelvic fracture, and liver injury. Head trauma can result in a variety of bleeding related conditions including hematomas or subdural and subarachnoid hemorrhages, and traumatic brain injury frequently results in development of coagulopathy that is critical to correct to prevent worsening neurotrauma or remote injury site complications [70,71,72]. Although the mechanisms underlying why brain injury results in an exceptionally high rate of coagulopathy remain to be fully explained, the increased rate of coagulopathy and high co-incidence of major hemorrhage from other injury sites is sufficient to warrant viscoelastic assay usage to guide targeted resuscitation in these trauma patients, particularly given the need to maintain tight control of volume status in patients with neurotrauma [73,74,75,76].
Blunt cerebrovascular injury (BCVI) involving the carotid and vertebral arteries also warrants viscoelastic assay evaluation. Trauma in these vessels may dislodge existing atherosclerotic plaques or result in the formation of thrombi in a region whose anatomic layout predisposes it to cause ischemic stroke, and anticoagulant and antiplatelet therapies have shown protective benefit in BCVI patients [77,78,79,80]. Increased α-angle and clot strength (MA) on TEG® have been shown to predict stroke in BCVI patients with an odds ratio of ~ 3, and as previously discussed, TBI also contributes to bleeding diatheses after trauma, so possession of viscoelastic assay data to weigh the risks of bleeding against the benefits of preventing ischemic cerebrovascular thrombotic complications using anticoagulant or antiplatelet therapy is critical [81].
Liver injuries and pre-existing liver disease (i.e., cirrhosis) have the potential to seriously impair the body’s ability to reach hemostasis due to the liver’s role in producing coagulation factors and its large vascular structures. Severe liver injuries can bleed audibly, with the mean blood loss in AAST grade IV–V liver injuries being greater than 9 l and a 33% mortality rate in AAST grade III–V liver injuries that is primarily due to exsanguination [82, 83]. Outside a small number of key components in the coagulation cascade such as tissue factor and FVIII, the rest is produced in the liver [84]. The same is true for fibrinolysis, where the critical fibrinolytic enzyme plasmin is formed from its precursor zymogen plasminogen, which is primarily produced in the liver [85]. Furthermore, the liver produces proteins C and S, which participate in slowing the coagulation cascade [86]. Thus, pre-existing liver disease renders a baseline coagulopathy and inability to properly achieve hemostasis, and while INR values are useful for following liver synthetic function, as previously discussed, they do not reveal a significant amount of information about a patients hemostatic balance so suggestions to use viscoelastic assays in the context of cirrhotic patients has already been proposed [87, 88]. Cirrhotic patients who suffer major traumatic injury have mortality rates that are markedly higher than for those without liver disease, and if an abdominal operation is required, mortality can exceed 50% [89,90,91,92]. For all of these reasons, it is clear that trauma patients with liver injury and/or pre-existing liver disease should undergo prompt viscoelastic assay evaluation to manage their resuscitation.
Finally, patients with pelvic fractures presenting in the emergency department warrant viscoelastic assay usage. Falls represent a significant source of morbidity and mortality amongst the elderly in particular, and pelvic fractures are a noticeable consequence of these accidents [93]. Hemorrhage is one of the significant drivers of mortality in pelvic fracture patients and a common concern when elderly patients present with such injuries [94,95,96,97]. As bleeding and subsequent coagulopathy is a significant concern in these patients, early performance of viscoelastic assays has been recommended to ascertain the coagulation/fibrinolytic state of these trauma patients to guide their trauma resuscitation [98].
In general, trauma patients in the emergency department with a high-risk mechanism of injury or signs of active bleeding may benefit from early viscoelastic assay testing to attenuate ongoing hemorrhage (summarized in Fig. 2). In our experience, we run thrombelastography on all trauma patients meeting our activation criteria upon presentation to the hospital and selectively on lower level trauma alert or consult patients based on injury pattern, imaging findings, or clinical decompensation. The routine use of viscoelastic assays on all trauma patients is not indicated and a waste of resources. Trauma systems should have a well-defined patient population in which viscoelastic assessment is targeted for early use. It is important for all trauma team members to be cognizant of the time delay in the results of viscoelastic testing and to not delay blood product-based resuscitation in patients with overt signs of hemorrhagic shock when test results are not yet available. Red blood cells and plasma should be given at a minimum of a 2:1 ratio until viscoelastic assay results are available, and as above, one reasonable approach is to follow a 1:1:1 transfusion ratio based on the results of the PROPPR trial while waiting [39]. If not already given, transfusion of platelets and cryoprecipitate to reduce the risk/exacerbation of dilutional coagulopathy should also be considered after 6 units of RBC have been transfused if test results are still not back.
Viscoelastic Assay Use in the Operating Room
Support for viscoelastic assay usage in the operating room is already present within a significant number of surgical subspecialties, including trauma, where ongoing operative blood loss is common. In cardiac and liver surgery, for example, which both carry a significant risk of bleeding and frequent presence of coagulopathy, multiple benefits have been found to using viscoelastic assays in the operating room including less total blood product use, lower MT rate, reduced costs, and reduced postoperative complications including mortality [59, 99,100,101,102,103,104,105,106,107,108]. Viscoelastic assays are also generating increased proposals for use in neurosurgery [76]. These developments in various surgical fields point to the utility of viscoelastic assays in operative settings, where the rapid return of data along with the previously described transfusion protocols that utilize TEG® variables provide a feasible and effective workflow for intraoperative bleeding management that includes viscoelastic assays. When in the operating theater with a trauma patient who has ongoing hemorrhage or an expectation of it, utilizing the 1:1:1 ratio of blood product transfusion from the PROPPR trial [39] or whole blood (if available) [109,110,111] until subsequent data from a Rapid-TEG test is obtained is one reasonable course of action. Once the results return, following a TEG® directed transfusion strategy such as those previously proposed [57] and repeatedly sampling the patients’ blood with Rapid-TEG at defined short intervals is appropriate (e.g., every 30–60 min, discussed in greater detail below). Exact protocols between trauma centers vary widely, and to date, no study publishing efficacy of an exact protocol for continuously re-evaluating the patient in the operating theater exists in trauma. While efficacy is implicit based on the improved outcomes in MT using viscoelastic assays to guide trauma resuscitation as well as improved outcomes with intraoperative use in other surgical specialties, the paucity of intraoperative trauma data with a specific focus on best practices for ongoing monitoring and resuscitation of the bleeding trauma patient in the OR is a clear opportunity for future study. Ultimately, once surgical hemostasis is achieved and the patient has been resuscitated the need for serial Rapid-TEG studies at near intervals is obviated, and a transition to post-operative trauma and critical care management of risk for re-bleeding and thromboembolic complications becomes paramount.
As a general guideline, almost all trauma patients that make their way to the operating room should have a baseline assessment of their coagulation if they did not otherwise already meet criteria for viscoelastic testing. However, viscoelastic testing is not warranted in all patients, particularly stable patients with isolated penetrating injuries. An INR can serve as a simple assay to predict risk of coagulopathy in such settings. While not published, we have appreciated that patients with an INR less than 1.2 are highly unlikely to harbor coagulopathy and require blood product resuscitation. In the remainder of the population, clear communication between the trauma surgeons and anesthesiologists is key for ongoing coagulation and bleeding management. At Denver Health Medical Center, we utilize the TACTIC coagulopathy score to help communicate with our anesthesiologists [112]. The score is based on a range of 1–5, with 1 being normal expected bleeding versus 5 in which the patient is bleeding from non-injured sites. A score of 3 or greater is an indication for ongoing viscoelastic testing, while a score of 2 or less conveys to the anesthesiologist that the bleeding is most likely to be related to mechanical control and resuscitation efforts should be focused on volume.
The frequency of coagulation assessment and directed testing is not standardized but is an important area of future research. Results from viscoelastic testing to guide platelets, fibrinogen, and plasma can be achieved within 30 min. There is also the delay from result to transfusion, and the patient’s coagulation profile may have changed during that interval. This critical gap in trauma-based resuscitation requires attention, as just having a viscoelastic assay device does not necessarily help reduce bleeding or mortality if potential coagulopathy is not frequently assessed via clinical exam and laboratory testing when indicated. We propose that this should be done at a minimum of once an hour in a stable patient and with shorter intervals in those patients with active bleeding (i.e., every 30 min).
Viscoelastic Assay Use in the Post-operative Phase and ICU
Beyond the obvious concern for ongoing (coagulopathic) bleeding after major trauma and operations that would benefit from viscoelastic assay-guided resuscitation until hemostasis is achieved (as discussed above), venous thromboembolism (VTE) is a major concern following traumatic injury, particularly in ICU patients given their more severe injury burdens. Rates of VTE can be as high as 58% in polytrauma patients and even higher in certain injury patterns such as pelvic and lower extremity trauma, and of more concern is the proximal lower extremity VTE rate which approaches 20%, where half of these lead to pulmonary embolism [113]. This observed increase in VTE risk arises from a combination of (patho)physiologic responses to trauma and bleeding, including the anticipated rebound hypercoagulability and fibrinolysis shutdown [42, 43], as well as the immobility that often results after severe injury such as those to the lower extremities [44, 114]. Furthermore, microvascular thrombosis resulting from these same hypercoagulable phenomena is likely a major driver of organ failure and delayed death seen after trauma and is potentially treatable through VTE chemoprophylaxis, therapeutic anticoagulation, or even pro-fibrinolytic administration [10, 18, 25, 26]. It is known that patients with more severe injury burdens are more likely to develop VTE complications, are more likely to develop organ failure, are more likely to require an operation, and are also more likely to be admitted to the ICU [114,115,116]. Given this, it is clear that an opportunity exists to define when and how to initiate interventions to prevent such complications in critically ill trauma patients, and viscoelastic assays are a logical tool given their known superiority in diagnosing hypercoagulable states after trauma compared to standard coagulation testing (INR and PTT) [14].
Striking the balance between bleeding risk/hemostasis and complications from hypercoagulable states after trauma is delicate. Similar to the paucity of intraoperative trauma data, there is no direct study establishing evidence-based protocols for viscoelastic assays to monitor trauma patients in the post-operative and ICU settings. As such, their use is somewhat speculative and depends on surrogate data and institutional experience. It has been shown, for example, that viscoelastic assays can detect a hypercoagulable state in BCVI patients that predicts stroke and may be useful as an indicator to start antithrombotic therapy [81]. In another example, viscoelastic assays have been shown to be predictive of VTE risk in cancer patients [117]. By inference, such thromboembolic predictions are likely feasible after trauma with viscoelastic assay screening, but data to date is lacking. At Denver Health Medical Center, it is routine to perform thromboelastography upon ICU admission for all trauma patients. Patients undergo viscoelastic testing for the following 5 days. Those patients with an elevated MA (> 70 mm) on TEG® are started on a daily aspirin and undergo venous duplex to rule out deep vein thrombosis. Currently, there is no direct evidence for the utility of this approach to make evidence-based recommendations. While it is clear that viscoelastic assays may provide a route to preemptively identify at-risk trauma patients for both bleeding and thromboembolic complications in the post-operative and ICU settings, further study is needed to establish evidence-based protocols for frequency, timing, and action indicators using viscoelastic assays to monitor trauma patients in these settings.
Conclusions
The rapid results and volume of information presented by viscoelastic assays should motivate their increased usage in institutions routinely caring for trauma patients. These platforms allow clinicians to gain a better handle on the variables driving persistent bleeding from traumatic coagulopathy as well as hypercoagulable states following injury and have been shown in a variety of settings to reduce mortality, volume of blood products necessary to stabilize patients, and costs. While there is a large amount of evidence for viscoelastic assay-guided resuscitation in the pre-operative/non-operative setting in the bleeding trauma patient, there is a large knowledge-gap and lack of quality studies on how to use viscoelastic assays effectively in the intra-operative, post-operative, and ICU settings after trauma and future studies are needed to better define and standardize their role in these settings.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Centers for Disease Control NCfIPaC. Web-based Injury Statistics Query and Reporting System (WISQARS) [online] Accessed February 14, 2017. [Available from: https://www.cdc.gov/injury/wisqars/leadingcauses.html.
Sobrino J, Shafi S. Timing and causes of death after injuries. Proc (Bayl Univ Med Cent). 2013;26(2):120–3.
Sauaia A, Moore FA, Moore EE, Moser KS, Brennan R, Read RA, et al. Epidemiology of trauma deaths: a reassessment. J Trauma. 1995;38(2):185–93.
Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60(6 Suppl):S3–11.
Maegele M, Paffrath T, Bouillon B. Acute traumatic coagulopathy in severe injury: incidence, risk stratification, and treatment options. Dtsch Arztebl Int. 2011;108(49):827–35.
Gonzalez E, Moore EE, Moore HB. Management of trauma-induced coagulopathy with thrombelastography. Crit Care Clin. 2017;33(1):119–34.
•• Gonzalez E, Moore EE, Moore HB, Chapman MP, Chin TL, Ghasabyan A, et al. Goal-directed hemostatic resuscitation of trauma-induced coagulopathy: a pragmatic randomized clinical trial comparing a viscoelastic assay to conventional coagulation assays. Ann Surg. 2016;263(6):1051–9 This manuscript reports outcomes from a randomized controlled trial of trauma patients undergoing massive transfusion, demonstrating a 50% reduction in 28-day mortality using thromboelastography to guide massive transfusion in bleeding trauma patients relative to conventional coagulation assays (INR, platelet count, fibrinogen level).
Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma. 2003;54(6):1127–30.
• Moore HB, Moore EE, Liras IN, Gonzalez E, Harvin JA, Holcomb JB, et al. Acute fibrinolysis shutdown after injury occurs frequently and increases mortality: a multicenter evaluation of 2,540 severely injured patients. J Am Coll Surg. 2016;222(4):347–55 This was a large retrospective study of trauma patients that confirmed and clearly defined the U-shaped mortality curve associated with the spectrum of fibrinolysis (patho)phenotypes previously described an earlier and smaller report by the same authors’, demonstrating an increased risk of death when trauma patients had too little fibrinolysis (fibrinolysis shutdown) or too much fibrinolysis (hyperfibrinolysis). This work has formed the basis for many of the current arguments behind selective use of TXA in trauma patients.
Moore HB, Moore EE, Gonzalez E, Chapman MP, Chin TL, Silliman CC, et al. Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: the spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. J Trauma Acute Care Surg. 2014;77(6):811–7 discussion 7.
Kashuk JL, Moore EE, Millikan JS, Moore JB. Major abdominal vascular trauma--a unified approach. J Trauma. 1982;22(8):672–9.
Schreiber MA. Coagulopathy in the trauma patient. Curr Opin Crit Care. 2005;11(6):590–7.
Gando S, Nanzaki S, Morimoto Y, Kobayashi S, Kemmotsu O. Systemic activation of tissue-factor dependent coagulation pathway in evolving acute respiratory distress syndrome in patients with trauma and sepsis. J Trauma. 1999;47(4):719–23.
Park MS, Martini WZ, Dubick MA, Salinas J, Butenas S, Kheirabadi BS, et al. Thromboelastography as a better indicator of hypercoagulable state after injury than prothrombin time or activated partial thromboplastin time. J Trauma. 2009;67(2):266–75 discussion 75-6.
Kaufmann CR, Dwyer KM, Crews JD, Dols SJ, Trask AL. Usefulness of thrombelastography in assessment of trauma patient coagulation. J Trauma. 1997;42(4):716–20 discussion 20-2.
Chapman MP, Moore EE, Moore HB, Gonzalez E, Gamboni F, Chandler JG, et al. Overwhelming tPA release, not PAI-1 degradation, is responsible for hyperfibrinolysis in severely injured trauma patients. J Trauma Acute Care Surg. 2016;80(1):16–23 discussion −5.
Cardenas JC, Matijevic N, Baer LA, Holcomb JB, Cotton BA, Wade CE. Elevated tissue plasminogen activator and reduced plasminogen activator inhibitor promote hyperfibrinolysis in trauma patients. Shock. 2014;41(6):514–21.
Moore EE, Moore HB, Gonzalez E, Chapman MP, Hansen KC, Sauaia A, et al. Postinjury fibrinolysis shutdown: rationale for selective tranexamic acid. J Trauma Acute Care Surg. 2015;78(6 Suppl 1):S65–9.
Moore HB, Moore EE, Chapman MP, Hansen KC, Cohen MJ, Pieracci FM, et al. Does tranexamic acid improve clot strength in severely injured patients who have elevated fibrin degradation products and low fibrinolytic activity, measured by thrombelastography? J Am Coll Surg. 2019;229(1):92–101.
McCully BH, Connelly CR, Fair KA, Holcomb JB, Fox EE, Wade CE, et al. Onset of coagulation function recovery is delayed in severely injured trauma patients with venous thromboembolism. J Am Coll Surg. 2017;225(1):42–51.
Byrne JP, Mason SA, Gomez D, Hoeft C, Subacius H, Xiong W, et al. Timing of pharmacologic venous thromboembolism prophylaxis in severe traumatic brain injury: a propensity-matched cohort study. J Am Coll Surg. 2016;223(4):621–31 e5.
Joseph B, Pandit V, Harrison C, Lubin D, Kulvatunyou N, Zangbar B, et al. Early thromboembolic prophylaxis in patients with blunt solid abdominal organ injuries undergoing nonoperative management: is it safe? Am J Surg. 2015;209(1):194–8.
Rostas JW, Manley J, Gonzalez RP, Brevard SB, Ahmed N, Frotan MA, et al. The safety of low molecular-weight heparin after blunt liver and spleen injuries. Am J Surg. 2015;210(1):31–4.
Murphy PB, Sothilingam N, Charyk Stewart T, Batey B, Moffat B, Gray DK, et al. Very early initiation of chemical venous thromboembolism prophylaxis after blunt solid organ injury is safe. Can J Surg. 2016;59(2):118–22.
Asakura H, Ontachi Y, Mizutani T, Kato M, Saito M, Kumabashiri I, et al. An enhanced fibrinolysis prevents the development of multiple organ failure in disseminated intravascular coagulation in spite of much activation of blood coagulation. Crit Care Med. 2001;29(6):1164–8.
Hardaway RM, Harke H, Tyroch AH, Williams CH, Vazquez Y, Krause GF. Treatment of severe acute respiratory distress syndrome: a final report on a phase I study. Am Surg. 2001;67(4):377–82.
Tanaka T, Tsujinaka T, Kambayashi J, Higashiyama M, Yokota M, Sakon M, et al. The effect of heparin on multiple organ failure and disseminated intravascular coagulation in a sepsis model. Thromb Res. 1990;60(4):321–30.
Richey SL. Tourniquets for the control of traumatic hemorrhage: a review of the literature. World J Emerg Surg. 2007;2:28.
Smith ER, Shapiro GL. Totally tourniquets. The facts & details about different types of tourniquets. JEMS. 2013;38(11):48 50, 2.
King DR, van der Wilden G, Kragh JF Jr, Blackbourne LH. Forward assessment of 79 prehospital battlefield tourniquets used in the current war. J Spec Oper Med. 2012;12(4):33–8.
Lee C, Porter KM, Hodgetts TJ. Tourniquet use in the civilian prehospital setting. Emerg Med J. 2007;24(8):584–7.
Ribeiro Junior MAF, Feng CYD, Nguyen ATM, Rodrigues VC, Bechara GEK, de-Moura RR, et al. The complications associated with Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA). World J Emerg Surg. 2018;13:20.
•• Sperry JL, Guyette FX, Brown JB, Yazer MH, Triulzi DJ, Early-Young BJ, et al. Prehospital plasma during air medical transport in trauma patients at risk for hemorrhagic shock. N Engl J Med. 2018;379(4):315–26 This was a 501 patient randomized controlled trial evaluating the use of fresh frozen plasma (FFP) versus standard crystalloid-based resuscitation during air medical transport of trauma patients at high risk for ongoing hemorrhage. The authors found significantly reduced 30-day mortality in the group treated with FFP, and importantly, a survival difference was noted as early as 3 h after randomization. The FFP group also had lower INR values upon arrival to the trauma center. This study population had notably longer pre-hospital transport times (median 39 min) relative to the COMBAT study (less than 20 min), which did not find a survival benefit from FFP when compared to standard crystalloid-based resuscitation.
collaborators C-t, Shakur H, Roberts I, Bautista R, Caballero J, Coats T, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376(9734):23–32.
Beekley AC. Damage control resuscitation: a sensible approach to the exsanguinating surgical patient. Crit Care Med. 2008;36(7 Suppl):S267–74.
Lewin I, Weil MH, Shubin H, Sherwin R. Pulmonary failure associated with clinical shock states. J Trauma. 1971;11(1):22–35.
• EMH G, Moore EE, editors. Trauma induced coagulopathy. New York: Springer Scientific; 2016. This is the first textbook dedicated to comprehensively reviewing trauma induced coagulopathy as well as the underlying principles behind hemostasis, coagulation, and fibrinolysis in trauma. This text includes expansive sections explaining the TEG and ROTEM mechanisms as well as interpretation of the output variables.
Ball CG. Damage control resuscitation: history, theory and technique. Can J Surg. 2014;57(1):55–60.
Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the randomized clinical trial. JAMA. 2015;313(5):471–82.
Sheppard FR, Schaub LJ, Cap AP, Macko AR, Moore HB, Moore EE, et al. Whole blood mitigates the acute coagulopathy of trauma and avoids the coagulopathy of crystalloid resuscitation. J Trauma Acute Care Surg. 2018;85(6):1055–62.
MacLeod JB, Lynn M, McKenney MG, Cohn SM, Murtha M. Early coagulopathy predicts mortality in trauma. J Trauma. 2003;55(1):39–44.
Cannon WBFJ, Cowell E. The preventive treatment of wound shock. JAMA. 1918;70:618–21.
Moore HB, Moore EE, Liras IN, Wade C, Huebner BR, Burlew CC, et al. Targeting resuscitation to normalization of coagulating status: hyper and hypocoagulability after severe injury are both associated with increased mortality. Am J Surg. 2017;214(6):1041–5.
Knudson MM, Ikossi DG, Khaw L, Morabito D, Speetzen LS. Thromboembolism after trauma: an analysis of 1602 episodes from the American College of Surgeons National Trauma Data Bank. Ann Surg. 2004;240(3):490–6 discussion 6–8.
Martini WZ. Coagulation complications following trauma. Mil Med Res. 2016;3:35.
O’Malley KF, Ross SE. Pulmonary embolism in major trauma patients. J Trauma. 1990;30(6):748–50.
Subramanian M, Kaplan LJ, Cannon JW. Thromboelastography-guided resuscitation of the trauma patient. JAMA Surg. 2019.
Gale AJ. Continuing education course #2: current understanding of hemostasis. Toxicol Pathol. 2011;39(1):273–80.
Eisenberg JM, Clarke JR, Sussman SA. Prothrombin and partial thromboplastin times as preoperative screening tests. Arch Surg. 1982;117(1):48–51.
Hartert H., Klin Wochenschr. 1948;26(37–38):577–583 [Not Available].
De Nicola P, Mazzetti GM. Evaluation of thrombelastography. Am J Clin Pathol. 1955;23(4):447–52.
Von Kaulla KN, Weiner M. Studies of coagulation and fibrinolysis by new technic of continuous recording. Blood. 1955;10(4):362–9.
Sankarankutty A, Nascimento B, Teodoro da Luz L, Rizoli S. TEG(R) and ROTEM(R) in trauma: similar test but different results? World J Emerg Surg. 2012;7(Suppl 1):S3.
Harr JN, Moore EE, Ghasabyan A, Chin TL, Sauaia A, Banerjee A, et al. Functional fibrinogen assay indicates that fibrinogen is critical in correcting abnormal clot strength following trauma. Shock. 2013;39(1):45–9.
Dias JD, Haney EI, Mathew BA, Lopez-Espina CG, Orr AW, Popovsky MA. New-generation thromboelastography: comprehensive evaluation of citrated and heparinized blood sample storage effect on clot-forming variables. Arch Pathol Lab Med. 2017;141(4):569–77.
Meledeo MA, Peltier GC, McIntosh CS, Voelker CR, Bynum JA, Cap AP. Functional stability of the TEG 6 s hemostasis analyzer under stress. J Trauma Acute Care Surg. 2018;84(6S Suppl 1):S83–S8.
•• Einersen PM, Moore EE, Chapman MP, Moore HB, Gonzalez E, Silliman CC, et al. Rapid thrombelastography thresholds for goal-directed resuscitation of patients at risk for massive transfusion. J Trauma Acute Care Surg. 2017;82(1):114–9 R-TEG® data was analyzed from 190 trauma patients to determine the predictive power of ACT, angle, MA, and LY30 in relation to outcomes including whether massive transfusion was required, 24-h mortality, ICU-, and ventilator-free days. ROC curves were generated and guided the authors to suggest the values at which the different R-TEG® variables reasonably suggested the need for massive transfusion as well as R-TEG® variable cutoffs for individual component transfusions. Of the R-TEG® variables, angle and MA were found to be the strongest predictors of requiring massive transfusion (AUROC ≥ 80%).
Tapia NM, Chang A, Norman M, Welsh F, Scott B, Wall MJ Jr, et al. TEG-guided resuscitation is superior to standardized MTP resuscitation in massively transfused penetrating trauma patients. J Trauma Acute Care Surg. 2013;74(2):378–85 discussion 85–6.
Fahrendorff M, Oliveri RS, Johansson PI. The use of viscoelastic haemostatic assays in goal-directing treatment with allogeneic blood products - a systematic review and meta-analysis. Scand J Trauma Resusc Emerg Med. 2017;25(1):39.
Mohamed M, Majeske K, Sachwani GR, Kennedy K, Salib M, McCann M. The impact of early thromboelastography directed therapy in trauma resuscitation. Scand J Trauma Resusc Emerg Med. 2017;25(1):99.
Yeung MC, Tong SY, Tong PY, Cheung BH, Ng JY, Leung GK. Use of viscoelastic haemostatic assay in emergency and elective surgery. Hong Kong Med J. 2015;21(1):45–51.
Moore HB, Moore EE, Chapman MP, McVaney K, Bryskiewicz G, Blechar R, et al. Plasma-first resuscitation to treat haemorrhagic shock during emergency ground transportation in an urban area: a randomized trial. Lancet. 2018;392(10144):283–91.
Schroll R, Swift D, Tatum D, Couch S, Heaney JB, Llado-Farrulla M, et al. Accuracy of shock index versus ABC score to predict need for massive transfusion in trauma patients. Injury. 2018;49(1):15–9.
Zhu CS, Cobb D, Jonas RB, Pokorny D, Rani M, Cotner-Pouncy T, et al. Shock index and pulse pressure as triggers for massive transfusion. J Trauma Acute Care Surg. 2019.
Schreiber MA, Perkins J, Kiraly L, Underwood S, Wade C, Holcomb JB. Early predictors of massive transfusion in combat casualties. J Am Coll Surg. 2007;205(4):541–5.
Rozycki GS, Ochsner MG, Schmidt JA, Frankel HL, Davis TP, Wang D, et al. A prospective study of surgeon-performed ultrasound as the primary adjuvant modality for injured patient assessment. J Trauma. 1995;39(3):492–8 discussion 8–500.
Rozycki GS, Ballard RB, Feliciano DV, Schmidt JA, Pennington SD. Surgeon-performed ultrasound for the assessment of truncal injuries: lessons learned from 1540 patients. Ann Surg. 1998;228(4):557–67.
McLaughlin DF, Niles SE, Salinas J, Perkins JG, Cox ED, Wade CE, et al. A predictive model for massive transfusion in combat casualty patients. J Trauma. 2008;64(2 Suppl):S57–63 discussion S.
Callcut RA, Johannigman JA, Kadon KS, Hanseman DJ, Robinson BR. All massive transfusion criteria are not created equal: defining the predictive value of individual transfusion triggers to better determine who benefits from blood. J Trauma. 2011;70(4):794–801.
Zhang J, Jiang R, Liu L, Watkins T, Zhang F, Dong JF. Traumatic brain injury-associated coagulopathy. J Neurotrauma. 2012;29(19):2597–605.
Maegele M. Coagulopathy after traumatic brain injury: incidence, pathogenesis, and treatment options. Transfusion. 2013;53(Suppl 1):28S–37S.
Laroche M, Kutcher ME, Huang MC, Cohen MJ, Manley GT. Coagulopathy after traumatic brain injury. Neurosurgery. 2012;70(6):1334–45.
Gennarelli TA, Champion HR, Copes WS, Sacco WJ. Comparison of mortality, morbidity, and severity of 59,713 head injured patients with 114,447 patients with extracranial injuries. J Trauma. 1994;37(6):962–8.
McMahon CG, Yates DW, Campbell FM, Hollis S, Woodford M. Unexpected contribution of moderate traumatic brain injury to death after major trauma. J Trauma. 1999;47(5):891–5.
van der Jagt M. Fluid management of the neurological patient: a concise review. Crit Care. 2016;20(1):126.
Kvint S, Schuster J, Kumar MA. Neurosurgical applications of viscoelastic hemostatic assays. Neurosurg Focus. 2017;43(5):E9.
Foreman PM, Harrigan MR. Blunt traumatic extracranial cerebrovascular injury and ischemic stroke. Cerebrovasc Dis Extra. 2017;7(1):72–83.
Shafafy R, Suresh S, Afolayan JO, Vaccaro AR, Panchmatia JR. Blunt vertebral vascular injury in trauma patients: ATLS((R)) recommendations and review of current evidence. J Spine Surg. 2017;3(2):217–25.
Dewan MC, Ravindra VM, Gannon S, Prather CT, Yang GL, Jordan LC, et al. Treatment practices and outcomes after blunt cerebrovascular injury in children. Neurosurgery. 2016;79(6):872–8.
Brommeland T, Helseth E, Aarhus M, Moen KG, Dyrskog S, Bergholt B, et al. Best practice guidelines for blunt cerebrovascular injury (BCVI). Scand J Trauma Resusc Emerg Med. 2018;26(1):90.
Sumislawski JJ, Moore HB, Moore EE, Swope ML, Pieracci FM, Fox CJ, et al. Not all in your head (and neck): stroke after blunt cerebrovascular injury is associated with systemic hypercoagulability. J Trauma Acute Care Surg. 2019;87(5):1082–7.
Asensio JA, Roldan G, Petrone P, Rojo E, Tillou A, Kuncir E, et al. Operative management and outcomes in 103 AAST-OIS grades IV and V complex hepatic injuries: trauma surgeons still need to operate, but angioembolization helps. J Trauma. 2003;54(4):647–53 discussion 53–4.
Doklestic K, Stefanovic B, Gregoric P, Ivancevic N, Loncar Z, Jovanovic B, et al. Surgical management of AAST grades III-V hepatic trauma by damage control surgery with perihepatic packing and definitive hepatic repair-single centre experience. World J Emerg Surg. 2015;10:34.
Palta S, Saroa R, Palta A. Overview of the coagulation system. Indian J Anaesth. 2014;58(5):515–23.
Raum D, Marcus D, Alper CA, Levey R, Taylor PD, Starzl TE. Synthesis of human plasminogen by the liver. Science. 1980;208(4447):1036–7.
Walker FJ. Protein C deficiency in liver disease. Ann Clin Lab Sci. 1990;20(2):106–12.
Harrison MF. The misunderstood coagulopathy of liver disease: a review for the acute setting. West J Emerg Med. 2018;19(5):863–71.
Davis JPE, Northup PG, Caldwell SH, Intagliata NM. Viscoelastic testing in liver disease. Ann Hepatol. 2018;17(2):205–13.
Georgiou C, Inaba K, Teixeira PG, Hadjizacharia P, Chan LS, Brown C, et al. Cirrhosis and trauma are a lethal combination. World J Surg. 2009;33(5):1087–92.
Christmas AB, Wilson AK, Franklin GA, Miller FB, Richardson JD, Rodriguez JL. Cirrhosis and trauma: a deadly duo. Am Surg. 2005;71(14):996–1000.
Demetriades D, Constantinou C, Salim A, Velmahos G, Rhee P, Chan L. Liver cirrhosis in patients undergoing laparotomy for trauma: effect on outcomes. J Am Coll Surg. 2004;199(4):538–42.
Wahlstrom K, Ney AL, Jacobson S, Odland MD, Van Camp JM, Rodriguez JL, et al. Trauma in cirrhotics: survival and hospital sequelae in patients requiring abdominal exploration. Am Surg. 2000;66(13):1071–6.
Boufous S, Finch C, Lord S, Close J. The increasing burden of pelvic fractures in older people, New South Wales. Australia Injury. 2005;36(13):1323–9.
Cryer HM, Miller FB, Evers BM, Rouben LR, Seligson DL. Pelvic fracture classification: correlation with hemorrhage. J Trauma. 1988;28(7):973–80.
Rothenberger D, Velasco R, Strate R, Fischer RP, Perry JF Jr. Open pelvic fracture: a lethal injury. J Trauma. 1978;18(3):184–7.
Henry SM, Pollak AN, Jones AL, Boswell S, Scalea TM. Pelvic fracture in geriatric patients: a distinct clinical entity. J Trauma. 2002;53(1):15–20.
Ben-Menachem Y, Coldwell DM, Young JW, Burgess AR. Hemorrhage associated with pelvic fractures: causes, diagnosis, and emergent management. AJR Am J Roentgenol. 1991;157(5):1005–14.
Coccolini F, Stahel PF, Montori G, Biffl W, Horer TM, Catena F, et al. Pelvic trauma: WSES classification and guidelines. World J Emerg Surg. 2017;12:5.
Colson PH, Gaudard P, Fellahi JL, Bertet H, Faucanie M, Amour J, et al. Active bleeding after cardiac surgery: a prospective observational multicenter study. PLoS One. 2016;11(10):e0162396.
Shen L, Tabaie S, Ivascu N. Viscoelastic testing inside and beyond the operating room. J Thorac Dis. 2017;9(Suppl 4):S299–308.
Curry NS, Davenport R, Pavord S, Mallett SV, Kitchen D, Klein AA, et al. The use of viscoelastic haemostatic assays in the management of major bleeding: a British Society for Hematology Guideline. Br J Haematol. 2018;182(6):789–806.
Erdoes G, Schloer H, Eberle B, Nagler M. Next generation viscoelasticity assays in cardiothoracic surgery: feasibility of the TEG6s system. PLoS One. 2018;13(14):e0209360.
Roullet S, de Maistre E, Ickx B, Blais N, Susen S, Faraoni D, et al. Position of the French Working Group on Perioperative Hemostasis (GIHP) on viscoelastic tests: what role for which indication in bleeding situations? Anaesth Crit Care Pain Med. 2019;38(5):539–48.
Alamo JM, Leon A, Mellado P, Bernal C, Marin LM, Cepeda C, et al. Is “intra-operating room” thromboelastometry useful in liver transplantation? A case-control study in 303 patients. Transplant Proc. 2013;45(12):3637–9.
Wang YX, Griffith JF, Deng M, Heather TM, Zhang YF, Yan SX, et al. Compromised perfusion in femoral head in normal rats: distinctive perfusion MRI evidence of contrast washout delay. Br J Radiol. 2012;85(1016):e436–41.
Leon-Justel A, Noval-Padillo JA, Alvarez-Rios AI, Mellado P, Gomez-Bravo MA, Alamo JM, et al. Point-of-care hemostasis monitoring during liver transplantation reduces transfusion requirements and improves patient outcome. Clin Chim Acta. 2015;446:277–83.
Gurusamy KS, Pissanou T, Pikhart H, Vaughan J, Burroughs AK, Davidson BR. Methods to decrease blood loss and transfusion requirements for liver transplantation. Cochrane Database Syst Rev. 2011;(14):CD009052.
McCrossin KE, Bramley DE, Hessian E, Hutcheon E, Imberger G. Viscoelastic testing for hepatic surgery: a systematic review with meta-analysis-a protocol. Syst Rev. 2016;5(1):151.
Spinella PC, Pidcoke HF, Strandenes G, Hervig T, Fisher A, Jenkins D, et al. Whole blood for hemostatic resuscitation of major bleeding. Transfusion. 2016;56(Suppl 2):S190–202.
Spinella PC, Cap AP. Whole blood: back to the future. Curr Opin Hematol. 2016;23(6):536–42.
Yazer MH, Jackson B, Sperry JL, Alarcon L, Triulzi DJ, Murdock AD. Initial safety and feasibility of cold-stored uncrossmatched whole blood transfusion in civilian trauma patients. J Trauma Acute Care Surg. 2016;81(1):21–6.
Neal MD, Moore HB, Moore EE, Freeman K, Cohen MJ, Sperry JL, et al. Clinical assessment of trauma-induced coagulopathy and its contribution to postinjury mortality: a TACTIC proposal. J Trauma Acute Care Surg. 2015;79(3):490–2.
Geerts WH, Code KI, Jay RM, Chen E, Szalai JP. A prospective study of venous thromboembolism after major trauma. N Engl J Med. 1994;331(26):1601–6.
Park MS, Perkins SE, Spears GM, Ashrani AA, Leibson CL, Boos CM, et al. Risk factors for venous thromboembolism after acute trauma: a population-based case-cohort study. Thromb Res. 2016;144:40–5.
Frohlich M, Lefering R, Probst C, Paffrath T, Schneider MM, Maegele M, et al. Epidemiology and risk factors of multiple-organ failure after multiple trauma: an analysis of 31,154 patients from the Trauma Register DGU. J Trauma Acute Care Surg. 2014;76(4):921–7 discussion 7–8.
Kuo SCH, Kuo PJ, Chen YC, Chien PC, Hsieh HY, Hsieh CH. Comparison of the new Exponential Injury Severity Score with the Injury Severity Score and the New Injury Severity Score in trauma patients: a cross-sectional study. PLoS One. 2017;12(13):e0187871.
Blasi A, Molina V, Sanchez-Cabus S, Balust J, Garcia-Valdecasas JC, Taura P. Prediction of thromboembolic complications after liver resection for cholangiocarcinoma: is there a place for thromboelastometry? Blood Coagul Fibrinolysis. 2018;29(1):61–6.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
Dr. H. Moore reports grants from the National Institutes of Health during the conduct of the study, another from Thrombo Therapeutics, Inc. outside the submitted work. In addition, Dr. Moore has a patent pending A Modified Coagulation Assay That Rapidly Unmasks Pathological Fibrinolysis Phenotypes in a Wide Spectrum of Human Diseases. In addition, Dr. Moore has a patent Fibrinolysis Detection issued.
Dr. Ernest Moore reports non-financial, research support from Haemonetics, non-financial support from Instrumentation Laboratory, and non-financial support from Stago outside the submitted work. In addition, Dr. E. Moore has a patent Fibrinolysis Detection issued and is Co-Founder of ThromboTherapeutics.
Dr. Barrett reports grants from National Institutes of Health during the conduct of the study and another from Thrombo Therapeutics, Inc. outside the submitted work. In addition, Dr. Barrett has a patent pending A Modified Coagulation Assay That Rapidly Unmasks Pathological Fibrinolysis Phenotypes in a Wide Spectrum of Human Diseases.
Dr. Yaffe reports grants from National Institutes of Health during the conduct of the study and another from Thrombo Therapeutics, Inc. outside the submitted work. In addition, Dr. Barrett has a patent pending A Modified Coagulation Assay That Rapidly Unmasks Pathological Fibrinolysis Phenotypes in a Wide Spectrum of Human Diseases.
Mr. Dhara has nothing to disclose.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors were performed in accordance with all applicable ethical standards including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Hemostasis after Trauma
Rights and permissions
About this article
Cite this article
Dhara, S., Moore, E.E., Yaffe, M.B. et al. Modern Management of Bleeding, Clotting, and Coagulopathy in Trauma Patients: What Is the Role of Viscoelastic Assays?. Curr Trauma Rep 6, 69–81 (2020). https://doi.org/10.1007/s40719-020-00183-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40719-020-00183-w