Abstract
Purpose of review
This review focuses on the cutting edge of surgery and technologies following upper extremity loss. A range of technologies will be reviewed with the common theme of bionics.
Recent findings
Traditional surgical amputation techniques are antiquated, especially with commercially advanced prosthetic technologies available following Targeted Muscle Reinnervation (TMR) surgery for the upper extremity. TMR should be standard of care not only for its benefits for advanced prosthetic control but also for its improvement in neuroma pain and prevention of neuroma formation. Current investigations of different technologies hold a bionic solution to arm restoration.
Summary
Many technologies currently exist in different phases of clinical research while others are immediately available and should be standard of care following upper extremity amputation. It is important that the healthcare community has understanding of the developing and available technology in the area of bionics and its application to upper extremity prosthetics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
War often inspires advancements in medicine, surgery, technology, and innovation. When looking at the history of combat and medicine, the American Civil War represents an evolutionary turning point triggering technological advancements in surgical tools and techniques, the development of artificial limbs, and new systems of evacuation and hospital care. [1] The Civil war also gave the emergence of distinct subspecialties of plastic reconstruction surgery forcing surgeons to be creative, reframe possibility, and break from tradition and rigid adherence to tradition. The most common surgery performed was amputation. Recognizing the alarming number of veterans who lost limbs during combat, the US Government issued what became the known as the “Great Civil War Benefaction”: an unconditional guarantee to provide prostheses to all veterans who lost a limb during war. The civil war marked the end of an era of wooden peg legs and simple hooks and paved the way for the prosthetics industry and has led to the bionic limbs of today.
We are again faced with the same challenges with the most recent conflicts in Iraq and Afghanistan having 82% of casualties resulting in extremity injuries [2]. This is a multifactorial result of protective body amour coupled with the widespread use of improvised explosive devices as the weapon of choice of the enemy resulting in the loss of exposed extremities in the battlefield. In accordance with military regulations, US military personnel are considered unfit for military service if they have a major limb amputation. However, if an amputee wishes to remain on active duty one must demonstrate a higher level of function and have the recommendation of two medical officers [3]. If we compare current return to duty rates to the 1980s, the number of amputees returning to active duty has significantly increased from 2.3 to 16.5% due to advancements in combat casualty care and the establishment of centralized amputee centers [2]. However, when examining the distribution of those who are able to return to active duty, the majority of those service men and women have suffered a loss of a lower extremity. Despite the promising improvements in upper extremity prosthesis, the technology advancement and functionality in lower extremity prosthesis have far surpassed its upper extremity counterpart. The loss of an arm often means the end of one’s military career and significant impact on their daily lives. Upper extremity amputees are not only more likely to be permanently retired but also suffer increased disability from PTSD. [4]
In recognition of the huge technology gap between upper and lower prosthetics, the Defense Advanced Research Projects Agency funded the Revolutionizing Prosthetics program, which has the goal of developing a neurally controlled upper limb prosthesis to provide the wounded warrior pre-injury levels of function including size, strength, dexterity, and sensorization. The Revolutionizing Prosthetics program resulted in the development of two advanced limb systems: the LUKE arm developed by DEKA Integrated Solutions, and the Modular Prosthetic Limb (MPL) developed by the Johns Hopkins University Applied Physics Laboratory (Fig. 1). In parallel, a new surgical technique, Targeted Muscle Reinnervation (TMR), developed by Dr. Todd Kuiken and Dr. Greg Dumanian, allowed for intuitive control for advanced prosthetics for the upper extremity and has revolutionized bionics. These technologies and surgical techniques continue to evolve and expand to include features such as sensory reinnervation in parallel with TMR and the use of osseointegrated implants for robust attachment of the prosthetic device. We are currently at a crossroads of breakthrough technology and surgery, with the muse of advancement accelerated by the recent conflicts with limb loss at the forefront. This review focuses on updates of the current status of targeted muscle reinnervation, the LUKE Arm, and Modular Prosthetic Limb as well as new technologies being investigated in the field of bionics for the upper extremity.
Traditional Control
Prior to the invention of TMR, the most sophisticated devices available were myoelectric prosthetic devices which deployed non-intuitive control strategies activated through muscle contractions or electromyogram (EMG) signals of residual muscles groups. For the transhumeral amputee, elbow and flexion would be triggered through biceps and triceps contractions; however, in order to control wrist rotation, the user must trigger a “mode switch” that would sequentially move control to the wrist or hand; however, motions would still require biceps and triceps contractions. Using this type of control, many upper extremity amputees are dissatisfied and often reject using a prosthesis altogether with rejection rates higher with more proximal levels of limb loss. Transhumeral users have rejection rates as high as 57% with shoulder disarticulations rejection rates at 60% [5•]. The most frequent reason for abandoning prosthetic devices was “too much fuss” and other reasons which included poor functionality, pain, comfort, mechanical failure, and lack of tactile sensation. [6, 7]
Targeted Muscle Reinnervation
Targeted muscle reinnervation is a surgical technique which can improve control of myoelectric prostheses [8, 9••, 10]. With TMR, remaining nerves are transferred to residual chest or upper arm muscles that are no longer biomechanically functional. Once reinnervated, these muscles serve as biological amplifiers of motor commands from the transferred arm nerves and provide physiologically appropriate electromyography (EMG) signals for control of the elbow, wrist, and hand. The TMR technique has been successfully performed in patients with transhumeral and shoulder disarticulation amputations and has markedly improved their functional use of prostheses [8, 9••, 10].
Using a simple “direct” control method based only on the amplitude of EMG signals from reinnervated muscles, patients who have undergone TMR surgery can intuitively and simultaneously control opening and closing of the hand as well as extension and flexion of the elbow intuitively. This type of intuitive direct control has shown to outperform and traditional control with basic prosthetic measurements. Using the Box and Blocks as well as Clothespins Relocation Test, performance times for individuals who have undergone TMR at the transhumeral and shoulder disarticulation levels have performance times increased up to six times [11]. Using commercially available devices, subjects are able to simultaneously operate the hand and elbow without using switch controls and allows for seamless positioning and operation of the hand. Other tasks which were also reported to be easier are activities of daily living such as cooking, cleaning, housework, yard work, and home maintenance. Due to its intuitive nature, less training is required and control optimization can often be effectively reinforced at home.
Pattern Recognition
Further investigation has shown that TMR provides a rich source of motor control information. By using multiple electrode array recordings of EMG signals in patients who had undergone TMR surgery, many more motions involving the elbow, wrist, thumb, and fingers can be detected. The patterns produced by the combined EMG signals during the performance of different movements can be used by a computer to create a machine learning based “classifier.” The classifier can then decipher which motions are being performed based on the current pattern of EMG signals. By simultaneously recording EMG activity from multiple channels and identifying key features within each channel, hundreds of thousands of classifications are possible. Consider a movement as a signature symphony of muscle activity that is reliable and repeatable which can be mapped to a classifier. In January 2015, the first device based on pattern recognition and surface electrodes (Coapt, LLC) was commercialized. In a recent randomized clinical trial examining direct control verses pattern recognition in transhumeral amputees after TMR, pattern recognition outperformed direct control in the Southampton Hand Assessment Procedure and Clothes Pin Relocation Test. No statistical significance was found with the Box and Blocks and Assessment for Capacity Myoelectric control (ACMC) tests [12•]. Seven of the eight subjects indicated that they preferred pattern recognition control over direct control.
Virtual Rehabilitation
The key to success of any prosthetic fitting is a cohesive team of surgeons, physiatrists, prosthetists and occupational therapists. This is particularly true for pattern recognition and developing consistent and distinguishable muscle patterns post TMR surgery. It is our practice to implement a Virtual Integration Environment (VIE) [13,14,15,16,17] that allows real-time feedback as well as performance measures as the patient continues to strengthen the neural connections postoperatively. Virtual computer-based training systems have been studied which allow real-time signal processing and calibration of pattern recognition algorithms by animating a virtual prosthesis on a computer screen or head-mounted virtual reality display. This virtual prosthesis can be programmed to reflect any natural configuration of the upper limb and approximates the rate of movement observed in commercially available prostheses. The developed computer-based training system provides a platform for the amputee to learn myoelectric control while exploring different prosthesis options or while awaiting a final prosthesis fitting [18•, 19].
In the VIE pattern recognition training system, the user follows visual cues presented on a computer screen and executes the same movement with his or her phantom limb. “Consistency” and “distinguishability” are the two key words to repeat throughout training as these are the foundation of successful pattern recognition. Practice and repetition are necessary to build consistency in any motor movement. We typically start with a basic set of movements (such as hand open/close and elbow up/down), and as the subject continues to be successful with basic movement sets, we continue adding desirable movements (such as wrist control and multiple grasp patterns) until there is some difficulty achieving all the desired movements. Virtual rehabilitation sessions typically consist of 1-h training sessions which are performed throughout the week at 1, 3, and 6 months intervals. A subject repeatedly provides training data to the movement classification algorithm and over time learns how best to train the system. Results are saved and tracked for each evaluation to quantify any improvement in performance over multiple sessions. To date, all patients who have entered our virtual rehabilitation program after TMR for prosthesis training have exceeded the number pattern recognition classification which is commercially available. The true potential and limitation of pattern recognition has yet to be realized. In the research setting, up to 15 distinct classifications have been demonstrated with the ability for individual finger movements, allowing for fine motor activities such as playing the piano. The key to success has been the ability for continued virtual training and rehabilitation.
Standard of Care
It is the opinion of the authors that TMR should be considered standard of care for all upper extremity amputation. Goals should be preservation of muscles and nerve length with aims of advanced prosthetic control and future planning of prosthetic fitting. Specific transfers of nerves location should be guided by existing residual musculature and nerve length with primary focus of a tension-free coaptation rather than skeletal anatomy. This is a significant departure from consideration of the bones and joints as the prime determinants of operative procedure. [20] TMR typically involves the transfer of the median, radial, and ulnar nerves for the transhumeral amputee and the additional transfer of the musculocutaneous nerve for the shoulder disarticulation case. Whereas TMR in the transhumeral patient is straightforward and predictable, TMR at the shoulder disarticulation level requires intraoperative flexibility. [20] Original descriptions of TMR for the shoulder disarticulations used the segments of the pectoralis major and pectoralis minor as muscle targets. Dumanian et al., who have the largest experience and expertise with the procedure have also used the serratus and latissimus as targets. They have also described dividing a single nerve lengthwise along its inner epineurium and coapting split nerve endings to separate muscle targets in order to improve prosthetic control. This variability emphasizes the need for flexibility and surgical expertise when considering TMR as well as highlights future directions of TMR procedure itself.
In addition to advanced prosthetic control, TMR also represents one of the more promising treatments for post-amputation neuroma pain. Pain remains a rate limiting step for successful prosthetic fitting resulting from neuromas. In addition to neuroma pain, patients also experience phantom limb pain (PLP), which was coined by Civil War Surgeon, Silas Weir Mitchell, once believed to be a “haunting” of the missing limb. Although the exact mechanism of PLP is poorly understood, it is surmised that spontaneous and abnormal peripheral nerve signals along with central changes including cortical reorganization and gray matter changes may play a role. [21] As TMR has become more mainstream, anecdotal experience from clinicians and prosthetists reported the resolution of neuroma pain. This is supported by early TMR experiences by Souza et al., who reported the relief of neuroma pain 14/15 patients who experience pre-operative pain and no new neuroma pain in patients who underwent TMR. The coaptation of the amputated nerve stumps to recipient motor nerve branches encourages organized nerve regeneration into the denervated target muscles, thus preventing the chaotic and misdirected nerve growth that leads to neuroma formation [21]. Current studies are underway that suggest surgical treatment of neuroma pain with TMR is far superior to alternative modalities. It is the opinion of the authors that TMR should be considered for all upper extremity amputations at the time of the primary surgery as well as offered TMR for treatment of neuroma pain alone.
Advanced Limb Systems
The Luke Arm
The LUKE arm, short for Life Under Kinetic Evolution, but also in homage to the fictional character Luke Skywalker, was the first advanced limb system from the DARPA Revolutionizing Prosthetic program to receive FDA approval, which occurred in 2014. The DEKA Arm is unique compared to prior commercially available arms in its wrist and finger design, and that it can carry out multiple, simultaneous, and coordinated powered movements using 10 independent joint actuators. It can adjust its positions to perform six different user-selectable grips such as power, chuck, tool, lateral pinch, fine pinch open, and fine pinch closed. In addition, force sensors let the robotic hand precisely control its grasp ranging from delicately picking a grape as well as being rugged enough to handle a power drill. Modular in design, it can be adapted to limb loss at every level of major amputation from transradial, transhumeral, and shoulder disarticulation. The key innovation is the use of foot-mounted inertial measurement units (IMU) that wirelessly communicates to the arm and offers natural endpoint control. Traditional EMG control has been applied as well with pattern recognition and offers custom hybrid types of control available to the user. Currently manufactured by Mobius Bionics, 10 LUKE Arms have been delivered worldwide, with the first LUKE Arm paid for by private insurance recently in 2018. Summary of the breath of literature for the LUKE arm is challenging. Little negative findings can be reported, which include device weight and complaints of shoulder appearance and harnessing. Initial reports of slower performance and activity speed, which can be expected, were equivalent to conventional prostheses with more user time and home use. Advantages appear to far outweigh the minor complaints in comparison to offering the only commercially available powered shoulder on the market. Its unique IMU control offering end point control is a potential prosthetic option for individuals with brachial plexus injuries and non-functional limbs; however, users with concomitant lower leg amputations would be restricted from using the foot-based control methodology. Rather than individual component device assembly for a prosthetic system, having a single manufacturer offers seamless device integration and offers a one stop shop for all prosthetic needs. In a recent comparison of outcomes of the LUKE arm to conventional prostheses, Resnik et al. concluded in comparison with conventional prostheses, the LUKE arm surpassed all conventional prosthesis scores, participants reported being able to perform more activities and perceived less disability and less difficulty in activities [22]. The LUKE arm is currently the most advanced limb system commercially available to date.
Modular Prosthetic Limb
The Modular prosthetic limb (MPL) [23] developed by the Johns Hopkins Applied Physics Laboratory is a novel electromechanical upper limb prosthesis that aims to fully replicate the functionality of a normal human hand and arm in terms of dexterity, flexibility, speed, strength, size, and weight (Table 1). It is designed as a modular system that can be assembled by a prosthetist to accommodate amputation injuries ranging from wrist to shoulder. The MPL’s upper arm and hand represent significant engineering achievements demonstrating human-like actuation, allowing infinite customized grasp configurations to mimic the performance of the natural limb. To this end, the MPL has 26 different joint degrees of freedom (DOF), controlled by 17 independent motors in the upper arm and hand, essentially anything the human arm can perform the MPL can emulate. Not only is the MPL motor capable, but sensory capable as well. There are over 200 sensors that continuously give information on joint angle, joint torque, and joint velocity. Tactile sensors on the fingertips measure contact, temperature, vibration, and force. Absolute position information is measured at the joint level using potentiometers which could feedback even proprioception.
Several MPL investigations in addition to its application to amputees are worth mentioning. With the aim of assistive technology towards helping those with tetraplegia, intracortical implants using a 96-channel array in the motor cortex were used to successfully demonstrate full control of the MPL in three-dimensional space by an individual with tetraplegia Subjects, using only thoughts, were capable of controlling the limb in a three-dimensional space and performed coordinated reaching and grasping tasks [24]. In efforts to bring noninvasive solutions to this same population, a semi-autonomous control system that implements computer vision, prosthesis feedback, smooth movement trajectories, and appropriate hand conformations to interact with objects of interest called the Hybrid Augmented Reality Multimodal Operation Neural Integration Environment (HARMONIE) is under development. Users can direct a prosthetic limb through an intuitive graphical user interface to complete multi-stage tasks using patient appropriate combinations of control inputs such as eye tracking, conventional controls (joystick/switch), surface electromyography (sEMG) signals, and or neural interfaces. [25] The HARMONIE system through eye gaze is capable of directing the prosthetic to specific locations, grasp objects by automatically modulating hand conformation, and perform actions on objects such as self-feeding. This flexible and intuitive control system lowers the cognitive load on the user and could potentially serve patient populations ranging from wheelchair-bound quadriplegics to upper-limb amputees.
For amputees, case studies of the MPL have shown feasibility of bilateral simultaneous control with multiple degrees of freedom of control of the shoulder, elbow, wrist, and hand for a bilateral TMR shoulder disarticulation (Fig. 2).
Sensory studies have been conducted integrating the MPL haptic feedback using vibrotactile sensors to successfully discriminate between hard and soft object and finger discrimination. Longitudinal studies have shown that over time, additional classification can be added and improve the number of tasks that can be performed. A recent study reports, a transradial subject achieved performance of all 13 attempted powered motions: hand open, wrist flexion/extension, cylindrical/spherical/fine pinch/pointer/lateral grasps, and articulation of five digits, in comparison to conventional prosthesis having only four discrete motions (hand open/close, wrist pronation/ supination) [26]. The first year-long home study of the MPL began in December 2017. The current take home system is completely self-contained EMG wireless system which uses a Bluetooth web-based smart phone app that allows for classifications and status updates of the MPL system such as battery life and frequency of data streaming as well as customized modes for specific desired activities of daily living.
Osseointegration
The stump–socket interface is of utmost importance for prosthetic function. Uncomfortable and unstable socket constructions together with clumsy control mechanisms are responsible for unacceptably high rejection rates [27]. In the era of TMR and advanced prosthetics, efforts have been focused on the man–machine interface, specifically osseointegration (OI). Osseointegration is also defined as the formation of a direct interface between an implant and bone, without intervening soft tissue. These are intramedullary implants containing pores into which osteoblasts and connective tissue integrates and is then externalized and acts as a direct skeletal attachment for prosthetic devices. Osseointegration was pioneered by Per-Ingvar Brånemark, who in the early 1960s discovered that bone can integrate with titanium components in the field of dental implants. His son, Rickard Brånemark, an orthopedic surgeon, applied this OI technology to amputee rehabilitation in the 1990s and developed the Osseoanchored Prostheses for the Rehabilitation of Amputees (OPRA) system in Sweden. Following the success of the OPRA system, several other implant systems with conceptually unique designs and operative approaches developed. To date, only three systems are commercially available internationally, the OPL (Osseointegrated Prosthetic Limb) from Australia, ILP (Integral Leg Prosthesis) from Germany, and OPRA. Currently there exist two separate design concepts for OI skeletal stabilization: anchored screw fixation verses compressive designs. Compressive designs provide immediate, stable anchorage and helps to avoid the long-term complication of aseptic loosening secondary to stress shielding and particle-induced osteolysis [28]. Surgical approaches also differ with investigations of one stage versus two-stage procedures. Currently in the USA, osseointegration has been given humanitarian approval by the FDA and current clinical trials are underway for both lower and upper extremity amputees. Approximately 200 individuals in the USA have had OI performed. Among those, it is estimated half of those individuals have sought out the OI technology outside of the USA and acquired their implants through medical tourism internationally. Thus far, the OI experience has been largely studied internationally and has gained wide acceptance particularly for its application for the lower extremity; however, it has recently extended to the upper extremity as well. The advantages of a bone-anchored prosthesis are improved control, stability, fixation, increased range of motion, quick donning and doffing, better body perception, improved functional capacity, and an overall increase in quality of life [29].
Osseointegration primarily provides mechanical stability for attaching an external prosthesis; however, these implants also have the potential to act as a signal interface to the body (discussed below) when combined with sub-dermal motor/sensory implants interfacing with muscles or peripheral nerves [30].
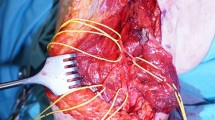
The first upper extremity OI was performed in the USA in March 2015 by Dr. Richard McGough from the University of Pittsburg Medical Center using the Zimmer Biomet COMPRESS Device in a transhumeral patient with previous TMR surgery. This individual, who has often been referred to as “The Real Bionic Man” is also the first individual to participate in a long-term study of the Modular Prosthetic Limb currently through Walter Reed National Military Medical Center. Current clinical investigations are underway which hopefully will answer differences between device designs, surgical approaches, develop rehabilitation protocols and address potential long-term infection risks. This technology truly represents advancements in bionics and the evolution of man and machine (Fig. 3).
Sensor Technology
Implanted Myoelectric Sensors
Modern prosthetic devices are controlled by surface electrodes on the skin that recognize underlying EMG signals from residual limb muscles. This modality has a limited number of surface electrode site possibilities that restricts the number of degrees of freedom using intuitive control below the capabilities of currently available prosthetic devices. Furthermore, surface electrodes also have problems reliably recording EMG signals due to poor skin contact, sweat production, socket rotation, and obtaining signals from deeper musculature [31]. These issues lead to an inability for a patient to operate a sophisticated prosthetic device at its maximal capacity.
As opposed to surface electrodes, Implantable Myoelectric Sensors (IMES®) are small electrodes intended to detect and wirelessly transmit EMG signals to an electromechanical prosthetic hand via an electromagnetic coil within the prosthetic socket. This system is designed to simultaneously capture EMG signals from multiple residual limb muscles, allowing for simultaneous natural control and multiple degrees of freedom of the prosthesis. Each of the implanted electrodes transmits individual EMG signals that are processed by the prosthetic device [32]. The increased number of electrodes allows for training a greater number of movement combinations while implantation into multiple residual muscle bodies permits intuitive control that does not require sequential control or special switch signals to transition between degrees of freedom [33].
Recent advancements in the IMES technology allows for a low-power mode during times of inactivity to preserve battery life and allow for reduction of the overall weight of the prosthetic device [34]. As experience with this technology increases, prosthetic devices are now able to process multiple degrees of freedom simultaneously for more natural movements of the limb [31]. Clinical trials with human patients have commenced to evaluate objective and subjective measures of prosthetic control using the IMES technology with promising early results of increased patient satisfaction, capabilities, and reliability compared to previous prosthetic devices.
Osseointegration with Embedded Digital Controller
Many of the recent advancements in prosthetics over the last few decades allowed for enormous leaps forward in specific, individualized areas of prosthetic technology. While these advancements have been extraordinary, they have somewhat occurred within silos with few utilizing multiple aspects of these technologies. A recent case report by Mastinu et al. has detailed their team’s first experience with osseointegration along with implanted neurostimulator [35]. This technology combines the mechanical stability advantage of osseointegration with implanted neuromuscular electrodes to provide sensory feedback with motor function. These sensory and motor functions traverse through the same core processor, the Artificial Limb Controller (ALC) that is a wearable device which decodes and integrates the neural input from the implanted electrodes. The ALC contains three components, the Mixed Signal Processing Unit (MSPU), Prosthetic control and communication Unit (PCCU), and Neurostimulator (NS). The MSPU is the core unit of the ALC which processes digital signals, regulates power consumption, and has computation capabilities that allows for memory of particular neural signals and patterns. The PCCU is the portion of the ALC that carries out the motor control signals from the MSPU. The NS connects with the MSPU to process and deliver sensory information. The ALC also has the capability to wirelessly communicate via Bluetooth technology to a computer or mobile device for prosthetic fitting, training, monitoring, and data management for research capabilities [35].
Sensory Cuff
Advancements in the fields of prosthetics have often been focused on regaining the motor function of the limb, but improvements in the sensory component have gained traction in recent years. With traditional prosthetics, patients had to rely on other senses such as visual or auditory signals for feedback on where their prosthetic was positioned in space. Current technology can provide both tactile and proprioceptive feedback from the prosthesis to the patient’s residual nerves to allow the patient to have more innate control of their prosthetic. To produce this sensory feedback, small sensory cuff electrodes are surgically placed around the median, radial, and ulnar nerves and then attached via percutaneous leads to a programmable stimulator. [36] The stimulator then produces waveforms of a predetermined pulse width and amplitude that produce a sensation for the patient which can be correlated with a specific sensation in the prosthetic limb, such as pinpoint discrimination of the entire hand and the detection of surface textures. These neural inputs and stimuli can be trained overtime, much like the motor functions of prosthetic limb control. Although there is limited data on this technology to date, the initial case series improved control and confidence in using the prosthetic limb without any reported adverse events related to the percutaneous lead placement for 2–3 years. [36, 37] These results also appear to be improving over time with increased experience that the patients have with the tactile and proprioceptive feedback.
Conclusions
The term bionics is a linguistic blend of biology and electronics and often draws to mind science fiction imagery of man and machine. State-of-the-art technology in development is quickly advancing that can only be described as the evolution of bionics. Rates of prosthetic rejection continue to be high among upper limb amputees. However, with the availability of advanced prosthetic technologies and with the parallel advancement of surgical procedures for motor control for amputee, these rates may be reduced with sufficient training by a highly specialized, multidisciplinary team of surgeons, physiatrists, occupational therapists, prosthetists, and engineers. The next frontier of the Revolutionizing Prosthetic program is aimed at the restoration of sensation, connecting sensors of the advanced limb systems and returning haptic feedback directly to the patient through either peripheral nerve or direct cortical stimulation. As we have seen from the past, war advances technology and surgery. Our present has developed new innovative surgical techniques such as TMR which is changing perceptions of standard of care for upper extremity amputees and has developed the world’s most sophisticated robotic limb system, the LUKE Arm that is commercially available. As bionic innovation continues, breakthrough technologies offer an engineering solution to motor control and sensory feedback where even more advanced prosthetic systems like the MPL represent the future of arm restoration and science fiction becomes reality.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major Importance
Reznick JS, Koyle KM. Combat and the medical mindset--the enduring effect of civil war medical innovation. N Engl J Med. 2015;372(25):2377–80.
Owens BD, Kragh JF Jr, Macaitis J, Svoboda SJ, Wenke JC. Characterization of extremity wounds in operation Iraqi freedom and operation enduring freedom. J Orthop Trauma. 2007;21(4):254–7.
Stinner DJ, Burns TC, Kirk KL, Ficke JR. Return to duty rate in current conflicts in Afghanistan and Irag. J Trauma 2010;68(6):1476–9.
Tennent DJ, Wenke JC, Rivera JC, Krueger CA. Characterisation and outcomes of upper extremity amputations. Injury. 2014;45(6):965–9.
• Resnik L, et al. Advanced upper limb prosthetic devices: implications for upper limb prosthetic rehabilitation. Arch Phys Med Rehabil. 2012;93(4):710–7. A special communication that highlights the the need for specialized teams and importance of rehabiliatation with advanced prosthetics.
McFarland LV, Winkler SLH, Heinemann AW, Jones M, Esquenazi A. Unilateral upper-limb loss: satisfaction and prosthetic-device use in veterans and servicemembers from Vietnam and OIF/OEF conflicts. J Rehabil Res Dev. 2010;47(4):299–316.
Resnik L, et al. Function, quality of life, and community integration of DEKA Arm users after discharge from prosthetic training: Impact of home use experience. Prosthetics Orthot Int. 2018:309364618774054.
Kuiken TA, et al. The use of targeted muscle reinnervation for improved myoelectric prosthesis control in a bilateral shoulder disarticulation amputee. Prosthetics Orthot Int. 2004;28(3):245–53.
•• Kuiken TA, et al. Targeted muscle reinnervation for real-time myoelectric control of multifunction artificial arms. JAMA. 2009;301(6):619–28. The above reference describes the first experiences of TMR for transhumeral and shoulder diarticulation amputees using direct control.
Hijjawi JB, Kuiken TA, Lipschutz RD, Miller LA, Stubblefield KA, Dumanian GA. Improved myoelectric prosthesis control accomplished using multiple nerve transfers. Plast Reconstr Surg. 2006;118(7):1573–8.
Miller LA, Stubblefield KA, Lipschutz RD, Lock BA, Kuiken TA. Improved myoelectric prosthesis control using targeted reinnervation surgery: a case series. IEEE Trans Neural Syst Rehabil Eng. 2008;16(1):46–50.
• Hargrove LJ, et al. Myoelectric pattern recognition outperforms direct control for transhumeral amputees with targeted muscle reinnervation: a randomized clinical trial. Sci Rep. 2017;7(1):13840. Recent clincal trial showing the improved outcomes of pattern recognition over direct control.
Zeher MJ, Armiger RS, Burck JM, Moran C, Kiely JB, Weeks SR, et al. Using a virtual integration environment in treating phantom limb pain. Stud Health Technol Inform. 2011;163:730–6.
Alphonso AL, Monson BT, Zeher MJ, Armiger RS, Weeks SR, Burck JM, et al. Use of a virtual integrated environment in prosthetic limb development and phantom limb pain. Stud Health Technol Inform. 2012;181:305–9.
Bishop W, et al. A real-time virtual integration environment for the design and development of neural prosthetic systems. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:615–9.
Armiger RS, Tenore FV, Bishop WE, Beaty JD, Bridges MM, Burck JM, Vogelstein RJ, Harshbarger SD. An overview of the developmental process for the modular prosthetic limb. Johns Hopkins APL Tech Dig. 2011;30(3):198–205.
Hotson G, McMullen DP, Fifer MS, Johannes MS, Katyal KD, Para MP, et al. Individual finger control of a modular prosthetic limb using high-density electrocorticography in a human subject. J Neural Eng. 2016;13(2):026017–26017.
• Powell MA, Thakor NV. A Training strategy for learning pattern recognition control for myoelectric prostheses. J Prosthet Orthot. 2013;25(1):30–41. The referenced article describes the use of virtual training and its application to pattern recognition and prostethic control.
Chau B, Phelan I, Ta P, Humbert S, Hata J, Tran D. Immersive virtual reality therapy with myoelectric control for treatment-resistant phantom limb pain: case report. Innov Clin Neurosci. 2017;14(7–8):3–7.
Gart MS, Souza JM, Dumanian GA. Targeted muscle reinnervation in the upper extremity amputee: a technical roadmap. J Hand Surg Am. 2015;40(9):1877–88.
Bowen JB, Wee CE, Kalik J, Valerio IL. Targeted muscle reinnervation to improve pain, prosthetic tolerance, and bioprosthetic outcomes in the amputee. Adv Wound Care (New Rochelle). 2017;6(8):261–7.
Resnik LJ, Borgia ML, Acluche F, Cancio JM, Latlief G, Sasson N. How do the outcomes of the DEKA Arm compare to conventional prostheses? PLoS One. 2018;13(1):e0191326.
Johannes MS, Bigelow JD, Burck JM, Harshbarger SD, Kozlowski MV, Van Doren T. An overview of the developmental process for the modular prosthetic limb. Johns Hopkins APL Tech Dig. 2011;30:207–16.
Collinger JL, Wodlinger B, Downey JE, Wang W, Tyler-Kabara EC, Weber DJ, et al. High-performance neuroprosthetic control by an individual with tetraplegia. Lancet. 2013;381(9866):557–64.
Katyal K, Johannes MS, McGee TG, Harris AJ, Armiger RS, Firpi AH, et al. HARMONIE: a multimodal control framework for human assistive robotics, in 6th International IEEE/EMBS Conference on Neural Engineering (NER). 2013.
Perry BN, Moran CW, Armiger RS, Pasquina PF, Vandersea JW, Tsao JW. Initial clinical evaluation of the modular prosthetic limb. Front Neurol. 2018;9:153.
Salminger S, Gradischar A, Skiera R, Roche AD, Sturma A, Hofer C, et al. Attachment of upper arm prostheses with a subcutaneous osseointegrated implant in transhumeral amputees. Prosthetics Orthot Int. 2018;42(1):93–100.
Kramer MJ, Tanner BJ, Horvai AE, O’Donnell RJ. Compressive osseointegration promotes viable bone at the endoprosthetic interface: retrieval study of Compress implants. Int Orthop. 2008;32(5):567–71.
Van de Meent H, Hopman MT, Frolke JP. Walking ability and quality of life in subjects with transfemoral amputation: a comparison of osseointegration with socket prostheses. Arch Phys Med Rehabil. 2013;94(11):2174–8.
Ortiz-Catalan M, Hakansson B, Branemark R. An osseointegrated human-machine gateway for long-term sensory feedback and motor control of artificial limbs. Sci Transl Med. 2014;6(257):257re6.
Pasquina PF, Evangelista M, Carvalho AJ, Lockhart J, Griffin S, Nanos G, et al. First-in-man demonstration of a fully implanted myoelectric sensors system to control an advanced electromechanical prosthetic hand. J Neurosci Methods. 2015;244:85–93.
Merrill DR, Lockhart J, Troyk PR, Weir RF, Hankin DL. Development of an implantable myoelectric sensor for advanced prosthesis control. Artif Organs. 2011;35(3):249–52.
Weir R, et al. Technical details of the implantable myoelectric sensor (IMES) system for multifunction prosthesis control. Conf Proc IEEE Eng Med Biol Soc. 2005;7:7337–40.
DeMichele GA, et al. Low-power polling mode of the next-generation IMES2 implantable wireless EMG sensor. Conf Proc IEEE Eng Med Biol Soc. 2014;2014:3081–4.
Mastinu E, Doguet P, Botquin Y, Hakansson B, Ortiz-Catalan M. Embedded system for prosthetic control using implanted neuromuscular interfaces accessed via an osseointegrated implant. IEEE Trans Biomed Circuits Syst. 2017;11(4):867–77.
Schiefer M, Tan D, Sidek SM, Tyler DJ. Sensory feedback by peripheral nerve stimulation improves task performance in individuals with upper limb loss using a myoelectric prosthesis. J Neural Eng. 2016;13(1):016001.
Tan DW, Schiefer MA, Keith MW, Anderson JR, Tyler DJ. Stability and selectivity of a chronic, multi-contact cuff electrode for sensory stimulation in human amputees. J Neural Eng. 2015;12(2):026002.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on The Military Perspective
Rights and permissions
About this article
Cite this article
Chi, A., Smith, S., Womack, I. et al. The Evolution of Man and Machine—a Review of Current Surgical Techniques and Cutting Technologies After Upper Extremity Amputation. Curr Trauma Rep 4, 339–347 (2018). https://doi.org/10.1007/s40719-018-0142-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40719-018-0142-2