Abstract
Purpose of Review
Trauma damage control has undergone a recent paradigm shift, broadening its focus from surgery to resuscitation. This review details central components of damage control resuscitation (DCR) across the phases of major injury care and the evidence behind its adoption.
Recent Findings
Permissive hypotension, minimization of crystalloid fluids, and early balanced blood product resuscitation have each been associated with improved outcomes in hemorrhaging patients. These tactics compliment current strategies of achieving hemorrhage control, including damage control surgery.
Summary
DCR is now integrated into care from the injury scene, through the resuscitation bay, the operating room, and into the intensive care unit. Its use limits the physiologic derangement experienced by the injured patient and minimizes preventable death from hemorrhage. It has become the accepted standard of modern trauma care and is shaping contemporary trauma systems and education. Future evidence-based advancements in trauma care will be scrutinized against this standard.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Trauma is a significant burden to modern society. Worldwide, it remains the leading cause of death for individuals <45 years of age and is the primary source of productive years of life lost [1]. Survivors of injury suffer considerable morbidity and long-term disability, and as a result, trauma remains associated with substantial direct and indirect health care costs. Despite this, civilian trauma secondary to motor vehicle collisions, falls, workplace accidents, and interpersonal violence remains common across all societies. Concerns regarding mass casualty incidents secondary to terrorism are prevalent worldwide. Military conflict also continues to result in trauma that is associated with high mortality and substantial direct and indirect economic costs to society. While traumatic brain injury leads the way as the single greatest source of post-traumatic death, hemorrhage is the primary cause of potentially preventable death after injury [2]. As a result, significant emphasis has been placed on improving the outcome of bleeding trauma patients [3•].
Advances in understanding the physiologic abnormalities of the injured patient have led to significant changes in the trauma care paradigm over the last 10 years. Trauma is generally considered a disease affecting young males, and the assumption previously made was that these patients have normal physiology. However, in recent years it has become clear that trauma patients often present with substantially deranged physiology. In many instances, the anatomic injury is actually of secondary importance to the metabolic abnormality. As a result, focus has shifted from the patient’s physical defect(s) to their physiologic insult as the priority. This concept of identifying and managing a patient’s metabolic derangements, as the earliest priority in care, is best described by the term damage control resuscitation (DCR). This relatively new notion grew out of the damage control surgery (DCS) concept to encompass the complete care of the trauma patient from the time of injury through to the normalization, or at least stabilization, of their physiology. Damage control itself is an idea borrowed from the US Navy, where it refers to the ability of a ship to sustain and absorb damage while still maintaining overall mission integrity [4]. When adapted to trauma, this means that definitive injury management is deferred in favor of ensuring patient survival [5].
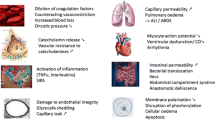
Key elements within DCR include permissive hypotension, minimization of crystalloid use, balanced blood product resuscitation ratios, and, most importantly, early control of hemorrhage (Fig. 1). Each of these techniques aims to limit metabolic derangements and normalize physiology as early as possible and, in doing so, minimize or prevent the lethal triad of trauma (acidosis, hypothermia, and coagulopathy). The use of these tools is no longer limited to one physical hospital location. Their use follows the patient from the site of injury through to the critical care bed, including every location in between. In this article, we review DCR in trauma. Specifically, we detail components of the lethal triad and how understanding of common derangements in patient physiology is key to the DCR strategy. We then discuss the principal components of DCR and consider how each plays a role across the prehospital, resuscitation bay, operating room (OR), interventional suite, and ICU settings (Fig. 2).
The Lethal Triad
An established concept within trauma care is that of the lethal triad. This self-perpetuating, cyclical, pathophysiologic process was named in order to emphasize that hypothermia, acidosis, and coagulopathy contribute to ongoing hemorrhage and patient demise unless interrupted [6, 7].
Hypothermia is common in trauma patients and is associated with decreased survival [8, 9]. Trauma patients with a temperature <33 °C are reported to have a 69% mortality with an inflection point of 35 °C having been described [10, 11]. Heat is lost at the scene of injury, through removal of clothing in the emergency department (ED), and administration of cold or room temperature fluids. Furthermore, patients in shock inappropriately regulate their core body temperature and have a lower tissue metabolism. This decreases the amount of heat that they produce. In the OR, heat losses are exacerbated by exposure of the peritoneum and Burch et al. have estimated that a patient undergoing laparotomy loses as much as 4.6 °C of core body heat per hour [12]. As part of the lethal triad, hypothermia worsens coagulopathy by increasing platelet sequestration and impacting key components of the clotting cascade [13,14,15]. A focus within DCR is therefore to decrease the severity of hypothermia by minimizing patient exposure, covering patients with warm sheets, rewarming patients with convection blankets, administering heated fluids, reducing operative times, and perhaps most importantly, curtailing the duration that a patient is in shock.
Acidosis is another common finding in injured patients. It can cause bradycardia, decreased cardiac output, dysrhythmias, and hypotension (i.e., the post-injury “declamping syndrome”) [7]. Obtaining an arterial blood gas upon arrival to the trauma center allows direct measurement of a patient’s pH and base deficit. The base deficit is a particularly useful means to assess cellular oxygenation, severity of acidosis, extent of injury, and transfusion requirements [16,17,18]. Up to 80% of trauma patients present with a base deficit. A base deficit of −8 mmol/L has been associated with a mortality of approximately 25% while Eastridge et al. describe a mortality threshold of −4 based on data from the National Trauma Data Bank [18, 19]. Low arterial pH after injury is often caused by both lactate release secondary to tissue injury and decreased tissue oxygenation, and may be worsened by concomitant hypothermia [9, 15]. Time to normalization of lactate has also been shown to prognosticate trauma patient survival [20]. Acidosis contributes to coagulopathy by decreasing the function of several clotting factors, including factor VIIa [21, 22]. DCR strategies used to minimize acidosis include the early and aggressive treatment of hypothermia and the avoidance of contributory resuscitative fluids, such as normal saline. Using plasma for resuscitation is now favored as not only does it prevent acidosis after major injury, but actively treats it. Plasma has a 25–50× better buffering ability than crystalloid [23].
The final, and arguably most important, component of the lethal triad is coagulopathy. Both hypothermia and acidosis worsen coagulopathy, which in turn leads to further hemorrhage and shock that continue to drive the vicious cycle. Previously it was felt that trauma patients developed coagulopathy primarily because of the dilutional effects of large volume crystalloid and unbalanced packed red blood cell (pRBC) resuscitation [13, 24]. However, current research suggests that as many as 30% of patients present to the trauma center with trauma-induced coagulopathy (TIC) or the “acute coagulopathy of trauma” [25, 26]. Shown to portend a poor survival, TIC likely develops from the release of inflammatory mediators from damaged tissue at the time of injury. Both direct tissue injury and secondary tissue hypoperfusion appear to play a role in this process [27, 28]. As the clotting cascade responds to injury by forming increasingly more clot, platelets and coagulation factors are depleted and an additional consumptive coagulopathy can develop (which may be further worsened by the administration of crystalloid fluids and pRBCs devoid of clotting factors) [29]. With increasing injury severity, the frequency of this coagulopathy rises [30]. Multiple factors, including direct tissue injury, acidosis, hypothermia, inflammation, dilution, and shock, increase the risk of TIC [31,32,33]. Possibly the most important goal of DCR is to prevent the progression of, and rapidly reverse, coagulopathy. However, standard tests of coagulation [e.g., prothrombin time (PT)/partial thromboplastin time (PTT) or the international normalized ratio (INR)] take time to obtain, and even rapid thromboelastography (rTEG) results are not immediately available. As a result, DCR relies on the assumption that a severely injured patient is coagulopathic on arrival and focuses on preventing further coagulopathy through use of massive transfusion protocols and balanced blood product ratios. Hirshberg et al. laid the foundation for this concept by demonstrating that 5 units of pRBCs transfused in isolation appears to contribute to a dilutional coagulopathy while the early addition of plasma and platelets in respective ratios of 2:3 and 8:10 to pRBCs likely prevents this [24]. As a result, the early treatment of an assumed coagulopathy, using balanced blood product ratios, has become a central tenant in the treatment of TIC and the prevention of dilutional coagulopathy.
Permissive Hypotension
For injured patients with signs of hemorrhagic shock, although the Advanced Trauma Life Support Manual recommends rapidly administering an initial bolus of 1–2 L of crystalloid fluid, many trauma experts question this practice [34]. While fluid resuscitation may help normalize vital signs after injury, it has unintended and often detrimental consequences [35]. For example, preclinical studies have reported that the administration of fluids to bleeding animals may worsen acidosis, dilute clotting factors, decrease blood viscosity, increase arterial and venous pressure, and disrupt formed or forming thrombus (i.e., “pop the clot”) [36,37,38,39]. In a meta-analysis of nine RCTs of bleeding animals, normotensive resuscitation led to a more than doubling of the risk of mortality when compared to hypotensive resuscitation [36].
Therefore, hypotensive resuscitation is a component of DCR in patients without significant brain injury. Termed permissive hypotension, the goal is to treat hypotension that results in signs of end-organ dysfunction, the earliest and most sensitive of these being the patients’ levels of consciousness, assessed via the Glasgow Coma Scale (GCS), or decreased pulse character. Other signs of end-organ perfusion, such as urine output, are also helpful, but may not be as useful on the minute-to-minute basis needed in the active management of the acutely hemorrhaging patient. The end point of resuscitation in this setting should be normalization of clinical end-organ perfusion, not a “normal” systolic blood pressure.
Evidence is mounting to support the use of hypotensive resuscitation in both the pre- and in-hospital settings. In 2009, the Eastern Association for the Surgery of Trauma (EAST) reviewed the literature and was unable to identify a benefit of prehospital fluid administration to injured patients [35]. In a controlled trial published in 1994, Bickell et al. reported that hypotensive patients with penetrating torso injuries had an 8% absolute improvement in survival and a reduced hospital length of stay when they received no fluid before reaching the OR versus immediate standard fluid resuscitation both before reaching hospital and once in-hospital [37]. Interestingly, however, the respective mean systolic blood pressure (58 vs. 59 mmHg) and intraoperative blood loss (3127 vs. 2555 mL) were similar between the immediate and delayed groups. In a subsequent RCT published in 2002, Dutton et al. randomized 110 injured patients with signs of hemorrhagic shock to a target systolic BP >100 or 70 mmHg and reported no difference in survival between the groups [38]. Finally, in a multicenter RCT published in 2015, the Resuscitation Outcomes Consortium Investigators allocated 192 patients to a target systolic BP of 110 versus 70 mmHg and reported an improved 24-h survival in the hypotensive resuscitation group among patients with a blunt mechanism of injury [40••]. Surprisingly, however, no improvement in survival was observed in the subgroup of patients with penetrating trauma [40••]. Taken together, there is little evidence to support aggressive resuscitation and permissive hypotension has become a central tenant to DCR.
Minimizing Crystalloid Resuscitation
Although crystalloid-based fluid resuscitation has long been considered standard in injury care, this approach should be abandoned. Administration of large volumes of normal saline produces hyperchloremic metabolic acidosis and can worsen pre-existing acidosis. It also appears to decrease renal perfusion and cardiac contractility [41, 42]. Further, infusion of cold or unheated crystalloids has been associated with hypothermia, dilutional coagulopathy, and multiorgan dysfunction syndrome (MODS) and, as a result, may influence the decision to perform damage control over definitive surgery after injury [10, 43, 44].
Use of large volumes of crystalloid in severely injured patients has also been associated with significant abdominal visceral edema, abdominal compartment syndrome (ACS), and an increased frequency of open abdominal management after laparotomy [45]. Post-injury abdominal visceral edema and ACS result from an ischemia-reperfusion injury of the bowel termed the “acute intestinal distress syndrome” [46,47,48]. In trauma patients receiving massive crystalloid fluid volumes, the abdominal viscera can sequester liters of fluid, and has been reported to increase in size to more than twice the volume of the abdominal cavity [49,50,51]. Thus, the volume of crystalloid fluids administered in the early post-injury period has been independently associated with an increased risk of ACS and a lower incidence of primary abdominal fascial closure [52,53,54]. It has also been linked with an increased risk of enteric fistulae among trauma patients with an open abdomen [55].
Even small volume crystalloid fluid resuscitation has been associated with poor outcomes in injured patients. In a propensity-adjusted, multicenter prospective cohort study of 1216 severely injured blunt trauma patients transported directly from the scene to a trauma center, Brown et al. reported that crystalloid resuscitation >500 versus ≤500 mL was associated with an increased odds of mortality and acute coagulopathy [56]. Interestingly, no association with survival was observed for those patients with evidence of prehospital hypotension, suggesting that the administration of crystalloid fluid may need to be based on the hemodynamics of the patient before arrival to the trauma center. Based on the previous evidence, and other data, limiting crystalloid fluids and instead focusing on the use of balanced blood products in bleeding trauma patients remains one of the key components of DC resuscitation.
Balanced Blood Product Ratios
In severely hemorrhaging patients, the restoration of circulating volume is often essential for survival and, as a result, many injured patients require a massive transfusion of blood products. Traditionally defined as the administration of more the 10 units of pRBCs within 24 h, contemporary measures such as resuscitation intensity (number of units transfused within 30 min of arrival) and the critical administration threshold (requiring 3 or more units of blood within any 1 h of the first 24 h) are more pragmatic measures of transfusion need [57, 58]. Modern resuscitation work has focused on the ideal ratio of blood products to be given when a massive transfusion is required. In the mid-2000s, Malone et al. and Ho et al. were among the first groups to aim for 1:1:1 ratios that approximated whole blood [13, 59]. A decade of extensive work in this area culminated in the publication of the PROPPR trial in 2015, which established that in trauma patients predicted to need a massive transfusion, those that received 1:1:1 product ratios, when compared to a 1:1:2 ratio, were more likely to achieve hemostasis and were less likely to die from hemorrhage [60••].
As a result, within DCR, the use of balanced ratios of pRBCs, plasma, and platelets in a 1:1:1 ratio that approximates the makeup of whole blood has become the accepted means of restoring lost intravascular volume during the early phase of resuscitation. Most trauma centers now standardize this 1:1:1 resuscitation through the use of a massive transfusion protocol (MTP) [61]. Like crystalloid, the infusion of these products restores circulating volume and improves end-organ perfusion. However, unlike crystalloids, the use of balanced product transfusion resolves acidosis, prevents endothelial damage, treats TIC, and begins to reverse, rather than contribute to, dilutional coagulopathy [62]. The transfusion of pRBCs provides a source of hemoglobin to carry oxygen to end organs. Plasma absorbs carbon dioxide for elimination, contains numerous clotting factors, is an excellent buffer, and acts to raise osmotic pressure, which in turn increases intravascular volume [23, 63]. It has also been shown to improve endothelial integrity and limit capillary permeability [62, 64]. Platelets act with clotting factors to clump together and form clot. As the use of balanced product ratios has become accepted as the modern standard, this concept has become an increasingly accepted practice throughout all phases of the initial care of the trauma patient from their prehospital transport through to the intensive care unit (ICU).
In the prehospital setting, it has now been shown that both the early administration of pRBCs and plasma is associated with improved acid-base status and early outcomes [65,66,67]. French combat data suggests that the administration of plasma in the field improves hemostasis prior to achievement of surgical hemorrhage control and the Israel Defense Forces Medical Corps uses plasma as its first-line field resuscitation fluid in many circumstances [68, 69]. Patients that receive product en route to hospital have lower rates of coagulopathy at little cost of product waste. As a result, the early implementation of product transfusion by medics using matured protocols on established aero-medical transport systems has become accepted and increasingly commonplace.
When the patient arrives in the resuscitation bay, two important components contribute to the early implementation of balanced blood product transfusion during DCR. First, prompt implementation of a MTP allows for the early protocolized transfusion of established 1:1:1 product ratios. Several methods of evaluating whether a MTP is likely to be required exist, including the trauma surgeon’s clinical gestalt and several clinical scoring systems [70]. The Assessment of Blood Consumption (ABC) score is an established, reliable, and straightforward scoring system for determining this need in which two positive variables out of a heart rate of 120 beats per minute or greater, blood pressure 90 mmHg or less, a positive focused assessment with sonography in trauma (FAST), and penetrating mechanism should initiate a MTP [71]. Early activation of a MTP in the resuscitation bay has been shown to be an independent predictor of both 24-h and 30-day survival in trauma patients [72]. Achieving balanced blood product ratios has also been shown to improve survival in trauma patients [73].
Second, the availability of pRBCs and plasma in the resuscitation bay directly impacts the outcome of trauma patients. Most trauma centers stock refrigerated pRBCs in the ED. However, having thawed or liquid plasma readily at hand in the resuscitation bay has been shown to be an independent predictor of improved mortality at 30 days in trauma patients while also decreasing their pRBC, plasma, and platelet transfusion requirements over the first 24 h [74]. Thawed AB plasma can be transfused in a balanced ratio with pRBCs until type-specific thawed plasma becomes available from the blood bank. This allows for the early infusion of an excellent buffer solution to treat acidosis, the rapid replenishment of key coagulation factors, stabilization of the endothelial wall, and an increase in circulating intravascular volume. In the 1:1:1 group in the PROPPR trial, patients received platelets as their first product transfused, suggesting that availability of platelets in the trauma bay may also be an appropriate future consideration [60••].
As a patient moves from the resuscitation bay to either the OR or the radiology suite for direct control of hemorrhage, a resuscitation strategy using a balanced ratio of blood products should be continued. While limiting crystalloid and permissive hypotension continue to play a role in these settings, a 1:1:1 ratio, as shown by the PROPPR trail, remains central [60••]. In patients requiring DCS, Cotton et al. found that the combination of all three of these strategies led to improved 30-day survival when compared to those that did not [75]. The balanced blood product resuscitation strategy should not change in the OR or interventional radiology (IR) until control of active hemorrhage is obtained [76].
After surgical or interventional bleeding control, many severely injured patients are transported to the ICU. By this time, the end points of resuscitation, usually considered to be operative or interventional hemorrhage control and stability or improvement in vital signs or measures of end-organ perfusion, have likely been achieved. Any ongoing blood loss is most commonly considered “medical” and secondary to residual coagulopathy. If there are doubts about surgical or interventional bleeding control in the post-operative period, a low threshold to return to the OR or angiography suite should be maintained. However, usually, a transition can be made from a ratio-driven MTP use to goal-directed therapy where laboratory values, either based on thromboelastography or traditional coagulation tests, are obtained in a timely fashion [76]. Of note, the PROMMTT study found that higher product ratios are not of benefit to trauma patients beyond 6 h, which is likely to reflect when these patients have moved into the ICU [77].
Early Control
One of the most critical components of DCR is to obtain early hemorrhage control and prevent additional physiologic derangement as a result of ongoing blood loss. In fact, the central aim of DCR is to rapidly prevent further blood loss (whether indirectly or directly). Historically, direct hemorrhage control has occurred primarily in the OR. While this remains the principal setting for hemorrhage control in the trauma patient, several strategies now provide a means to achieve temporary, and sometimes even definitive, means of direct hemorrhage control earlier in the care of the trauma patient (Table 1). The applicability of these strategies often depends on the anatomic location of injury, whether extremity, truncal, or junctional (at a connection between an extremity and the torso). In the field, direct pressure has long been taught as a basic tenant of first aid. The application of compressive dressings and hemostatic agents such as “combat gauze” has become an important tool for medics in both the civilian and military setting. These principles are emphasized in The Hartford Consensus and the “Stop the Bleed” campaign [78, 79].
In patients with significant hemorrhage secondary to pelvic fractures, the use of pelvic binders (a.k.a. pelvic orthotic devices, circumferential binders, and circumferential compression devices) has been shown to decrease intrapelvic volume, reduce transfusion requirements, and decrease lengths of stay [80,81,82]. Although a sheet can also accomplish this goal, commercial pelvic binders are readily available in most trauma centers and are used in many trauma systems. These proprietary devices may be more effective than wrapping a fractured pelvis with a simple sheet. Pelvic binders can be applied in the field by emergency personnel or by physicians in the resuscitation bay, thereby assisting with early hemorrhage control.
In extremity injuries, tourniquets are a means of obtaining early, temporary control of hemorrhage. Considered an effective and useful tool in the military setting, their use was often seen as controversial in the civilian setting because of concerns over potential complications [83,84,85,86,87]. However, the early application of tourniquets has now been shown to improve survival in the civilian prehospital and ED setting and is emphasized by both The Hartford Consensus and the Stop the Bleed campaign [78, 79, 88]. The use of tourniquets by incident bystanders, by prehospital emergency personnel, or by medical staff in the ED is often an effective means of obtaining temporary hemorrhage control.
Moving from the field to the resuscitation bay, a few further options for early control become available. The resuscitative thoracotomy and clamshell thoracotomy, used for trauma patients in extremis, both provide a potentially effective method for control of bleeding cardiac and great vessel wounds and for occlusion of the pulmonary hilum and descending thoracic aorta. In pulseless patients with signs of life after penetrating thoracic injury, survival to hospital discharge after resuscitative thoracotomy has been reported to be as high as 21% [89]. Resuscitative thoracotomy remains an invasive and heroic intervention that is not appropriate in all trauma patients in extremis. The 2015 EAST guidelines provide a set of reasonable and current indications as to when this intervention may be appropriate based on a systematic review of the literature [89].
A further tool available for hemorrhage control is Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA). With the balloon placed via the common femoral artery and inflated either in the descending thoracic aorta (zone I) or infra-renal aorta (zone III), REBOA provides a means of early, yet temporary, proximal hemorrhage control in patients with significant intraabdominal and pelvic bleeding, and may even play a role in control of hemorrhage in patients with lower extremity junctional injuries [90,91,92]. REBOA can be used for temporary hemorrhage control in many patients with non-compressible truncal hemorrhage. Once the balloon is in place and inflated, the patient can be transported to the OR or IR for definitive surgical or endovascular control of their bleeding. REBOA is becoming increasingly used and accepted as a means of temporary hemorrhage control and has a growing body of supporting evidence. The potential of REBOA extends into the prehospital setting as reports exist of its successful use in the field [93].
From the resuscitation bay, patients with active hemorrhage move on to either the IR suite or the OR, depending on their stability and hemorrhage location. IR has become a central tool in the early control of hemorrhage in the trauma patient. Injuries commonly treated by angioembolization include pelvic, splenic, and hepatic hemorrhage. However, endovascular therapy for trauma is hardly limited to these injuries. Embolization can be used for definitive control of bleeding from a wide range of injuries. The decision to take a patient to IR for possible embolization can be complex and requires an evaluation of the patient’s stability, overall injury burden, and the timeline of availability of the resource [94,95,96]. Overall, IR plays an important, and ever increasing, role in DCR for the trauma patient.
When a patient moves to the OR from the resuscitation bay, a plethora of tools, strategies, and techniques, too extensive to be detailed in this review, become available for further hemorrhage control. The OR is the primary location where decisive hemorrhage control is achieved. Tools for temporary control placed in the prehospital setting or the resuscitation bay are often exchanged for more definitive control means. Simultaneously, resuscitative efforts that minimize crystalloid use and focus on balanced product transfusion ratios are handed over to the anesthesia team, thereby delegating DCR between teams of experts. Frequent communication between these two groups of specialists is mandatory, to optimize the outcome of the patient. It is imperative that the surgeons focus on hemorrhage control and conduct of the operation, but also must remain aware of the overall trajectory of the patient, as modifications to the surgical procedure may be necessary. As discussed previously, the idea of DCR within the field of trauma evolved from the OR itself. Packing of truncal cavities, leaving bowel in discontinuity, placing temporary intravascular shunts, and temporary closure of the abdomen or chest once hemorrhage and contamination have been controlled allow the patient an opportunity to recover from their significant physiologic insult before moving toward the definitive repair of their remaining injuries.
In a series of recent studies, Roberts et al. compiled a list of specific DCS indications that had been reported in the literature and were assessed to be appropriate for use in practice by a panel of nine international trauma surgery experts and 201 surgeons practicing in the USA, Canada, Australia, and New Zealand [97,98,99,100]. The group at the Red Duke Trauma Institute, in Houston, TX, has begun to study DCS indications in detail and to date has published data on a quality improvement (QI) project that successfully decreased DCS rates from 39 to 23% without impacting morbidity or mortality [101]. From this QI project, they identified four indications for DCS felt to be acceptable including packing for hemorrhage control, expedited transfer to IR for hemorrhage control, hemodynamic instability defined by ongoing transfusion requirement or continuous vasopressor use, and ACS treatment or prophylaxis [101]. They identified four further indications where, based on a lack of high-quality data, the appropriateness of DCS remains unclear including hemodynamic instability defined by persistent acidosis without ongoing transfusion requirement or continuous vasopressor use, second-look laparotomy, expedited transfer to CT or ICU, and contamination [101]. In a descriptive study, they have since detailed the frequency with which each indication was used to pursue DCS and the indication-specific outcomes for patients undergoing DCS at their institution [102]. Finally, the group has begun a pilot randomized controlled trial comparing the morbidity of DCS with definitive laparotomy [101]. Some of the early results of the Houston group’s work on DCS indications are summarized in Table 2.
Stabilization and Transition to Definitive Repair
DCR strategies continue beyond DCS in the OR. As the patient is transferred to the ICU, the focus shifts away from direct hemorrhage control and back to treating ongoing physiologic derangement, including acidosis, hypothermia, and coagulopathy. Aggressive rewarming and further resuscitation with blood products are continued as a means to reverse remaining non-surgical hemorrhage arising from coagulopathy. The process continues in response to both physiologic parameters and laboratory investigations such as pH, base deficit, lactate, hemoglobin, hematocrit, platelet count, fibrinogen levels, and coagulation factors that may include the thromboelastography values of activated clotting time (ACT), maximum amplitude (MA), alpha-angle, and percent lysis, or more traditional values such as INR and PTT, depending on the measures used at a particular center. Close monitoring for failed temporary or definitive hemorrhage control strategies is vital so that ongoing surgical hemorrhage can be dealt with early via a rapid return to the OR or IR.
As the patient’s physiology normalizes in the ICU, focus finally turns from DCR to definitive management such as removal of intraabdominal packs, definitive vascular repair, re-establishment of bowel continuity, abdominal closure, and definitive orthopedic fixation as needed. The decision to transition from a DCR approach to a definitive repair strategy should be guided by the patient. Physiology and laboratory values all play into this assessment. A full catalogue of a patient’s injuries is important in this decision making process as prioritizing injury repair becomes important to the patient’s outcome and their overall length of ICU and hospital stay. Although a full discussion of this process falls outside the realm of DCR and is beyond the scope of this review, it is important to note that the approaches used vary among centers and practitioners, with most centers advocating for repair of injuries within 24 h of stopping DCR.
Conclusions
Emerging out of the concept of DCS, DCR has become the central management strategy for the primary care of the severely injured trauma patient. Its central tenants of permissive hypotension, minimization of crystalloid, balanced blood product transfusion ratios, and early control of hemorrhage have become so central to initial trauma care that they now follow the injured patient as they travel from the very scene of injury through to the ICU. They encompass all the key steps in between, including patient transport, initial treatment in the resuscitation bay, and early surgical or interventional management. The overarching strategy is to defer definitive, and sometimes anatomic, repair of injury, in favor of treating metabolic disturbance early. Treatment of derangements in physiology such as hypothermia, acidosis, and coagulopathy is emphasized in an effort to prevent hemorrhagic death. As injured patients with severe hemorrhage are seen as the largest group of potentially preventable deaths in trauma, DCR has become an effective and accepted strategy to maximize survival outcomes among this trauma population.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Cothren CC, Moore EE, Hedegaard HB, et al. Epidemiology of urban trauma deaths: a comprehensive reassessment 10 years later. World J Surg. 2007;31:1507–11. doi:10.1007/s00268-007-9087-2.
Centers for Disease Control and Prevention: Web-based injury statistics query and reporting system. Atlanta: US Department of Health and Human Services, CDC, National Center for Injury Prevention and Control, 2003.
• Oyeniyi BT, Fox EE, Scerbo M, et al. Trends in 1029 trauma deaths at a level 1 trauma center: impact of a bleeding control bundle of care. Injury. 2017;48:5–12. doi:10.1016/j.injury.2016.10.037. Evidence demonstrating that DCR, by decreasing hemorrhagic death, may decrease overall trauma mortality
Surface Ship Survivability. Naval War Publication 3–20.31. Washington, DC: Department of Defense; 1996.
Shapiro MB, Jenkins DH, Schwab CW, et al. Damage control: collective review. J Trauma. 2000;49:969–78.
Moore EE. Staged laparotomy for the hypothermia, acidosis, and coagulopathy syndrome. Am J Surg. 1996;172:405–10.
Mikhail J. The trauma triad of death: hypothermia, acidosis, and coagulopathy. AACN Clin Issues. 1999;10:85–94.
Luna GK, Maier RV, Pavlin EG, et al. Incidence and effect of hypothermia in seriously injured patients. J Trauma. 1987;27:1014–8.
Tsuei BJ, Kearney PA. Hypothermia in the trauma patient. Injury. 2004;35:7–15.
Jurkovich GJ, Greiser WB, Luterman A, et al. Hypothermia in trauma victims: an ominous predictor of survival. J Trauma. 1987;27:1019–24.
Wade CE, Salinas J, Eastridge BJ, et al. Admission hypo- or hyperthermia and survival after trauma in civilian and military environments. Int J Emerg Med. 2011;4:35. doi:10.1186/1865-1380-4-35.
Burch JM, Denton JR, Noble RD. Physiologic rationale for abbreviated laparotomy. Surg Clin North Am. 1997;77:779–82.
Ho AM, Karmakar MK, Dion PW. Are we giving enough coagulation factors during major trauma resuscitation? Am J Surg. 2005;190:479–84. doi:10.1016/j.amjsurg.2005.03.034.
Martini WZ, Pusateri AE, Uscilowicz JM, et al. Independent contributions of hypothermia and acidosis to coagulopathy in swine. J Trauma. 2005;58:1002–9.
Watts DD, Trask A, Soeken K, et al. Hypothermic coagulopathy in trauma: effect of varying levels of hypothermia on enzyme speed, platelet function, and fibrinolytic activity. J Trauma. 1998;44:846–54.
Bannon MP, O’Neill CM, Martin M, et al. Central venous oxygen saturation, arterial base deficit, and lactate concentration in trauma patients. American Surg. 1995;61:738–45.
Davis JW, Kaups KL, Parks SN. Base deficit is superior to pH in evaluating clearance of acidosis after traumatic shock. J Trauma. 1998;44:114–8.
Rutherford EJ, Morris JA Jr, Reed GW, et al. Base deficit stratifies mortality and determines therapy. J Trauma. 1992;33:417–23.
Eastridge BJ, Salinas J, McManus JG, et al. Hypotension begins at 110 mm Hg: redefining “hypotension” with data. J Trauma. 2007;63:291–7. doi:10.1097/TA.0b013e31809ed924.
Abramson D, Scalea TM, Hitchcock R, et al. Lactate clearance and survival following injury. J Trauma. 1993;35:584–8.
Meng ZH, Wolberg AS, Monroe DM 3rd, et al. The effect of temperature and pH on the activity of factor VIIa: implications for the efficacy of high-dose factor VIIa in hypothermic and acidotic patients. J Trauma. 2003;55:886–91. doi:10.1097/01.TA.0000066184.20808.A5.
Cosgriff N, Moore EE, Sauaia A, et al. Predicting life-threatening coagulopathy in the massively transfused trauma patient: hypothermia and acidoses revisited. J Trauma. 1997;42:857–61.
Traverso LW, Medina F, Bolin RB. The buffering capacity of crystalloid and colloid resuscitation solutions. Resuscitation. 1985;12:265–70.
Hirshberg A, Dugas M, Banez EI, et al. Minimizing dilutional coagulopathy in exsanguinating hemorrhage: a computer simulation. J Trauma. 2003;54:454–63. doi:10.1097/01.TA.0000053245.08642.1F.
Brohi K, Singh J, Heron M, et al. Acute traumatic coagulopathy. J Trauma. 2003;54:1127–30. doi:10.1097/01.TA.0000069184.82147.06.
MacLeod JB, Lynn M, McKenney MG, et al. Early coagulopathy predicts mortality in trauma. J Trauma. 2003;55:39–44. doi:10.1097/01.TA.0000075338.21177.EF.
Duchesne JC, McSwain NE Jr, Cotton BA, et al. Damage control resuscitation: the new face of damage control. J Trauma. 2010;69:976–90. doi:10.1097/TA.0b013e3181f2abc9.
Ostrowski SR, Henriksen HH, Stensballe J, et al. Sympathoadrenal activation and endotheliopathy are drivers of hypocoagulability and hyperfibrinolysis in trauma: a prospective observational study of 404 severely injured patients. J Trauma Acute Care Surg. 2017;82:293–301. doi:10.1097/TA.0000000000001304.
Spahn DR, Rossaint R. Coagulopathy and blood component transfusion in trauma. Br J Anaesth. 2005;95:130–9. doi:10.1093/bja/aei169.
May AK, Young JS, Butler K, et al. Coagulopathy in severe closed head injury: is empiric therapy warranted? Am Surg. 1997;63:233–6.
Hess JR, Brohi K, Dutton RP, et al. The coagulopathy of trauma: a review of mechanisms. J Trauma. 2008;65:748–54. doi:10.1097/TA.0b013e3181877a9c.
Cardenas JC, Wade CE, Holcomb JB. Mechanisms of trauma-induced coagulopathy. Curr Opin Hematol. 2014;21:404–9. doi:10.1097/MOH.0000000000000063.
Chang R, Cardenas JC, Wade CE, et al. Advances in the understanding of trauma-induced coagulopathy. Blood. 2016;128:1043–9. doi:10.1182/blood-2016-01-636423.
American College of Surgeons Committee on Trauma. ATLS, Advanced trauma life support student course manual. 9th ed. Chicago: American College of Surgeons; 2012.
Cotton BA, Jerome R, Collier BR, et al. Guidelines for prehospital fluid resuscitation in the injured patient. J Trauma. 2009;67:389–402. doi:10.1097/TA.0b013e3181a8b26f.
Mapstone J, Roberts I, Evans P. Fluid resuscitation strategies: a systematic review of animal trials. J Trauma. 2003;55:571–89. doi:10.1097/01.TA.0000062968.69867.6F.
Bickell WH, Wall MJ Jr, Pepe PE, et al. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. New Engl J Med. 1994;331:1105–9. doi:10.1056/NEJM199410273311701.
Dutton RP, Mackenzie CF, Scalea TM. Hypotensive resuscitation during active hemorrhage: impact on in-hospital mortality. J Trauma. 2002;52:1141–6.
Sondeen JL, Coppes VG, Holcomb JB. Blood pressure at which rebleeding occurs after resuscitation in swine with aortic injury. J Trauma. 2003;54:S110–7. doi:10.1097/01.TA.0000047220.81795.3D.
•• Schreiber MA, Meier EN, Tisherman SA, et al. A controlled resuscitation strategy is feasible and safe in hypotensive trauma patients: results of a prospective randomized pilot trial. J Trauma Acute Care Surg. 2015;78:687–95. doi:10.1097/TA.0000000000000600. A randomized trial demonstrating that permissive hypotension may lead to an early improvement in survival in trauma patients
Scheingraber S, Rehm M, Sehmisch C, et al. Rapid saline infusion produces hyperchloremic acidosis in patients undergoing gynecologic surgery. Anesthesiology. 1999;90:1265–70.
Williams EL, Hildebrand KL, McCormick SA, et al. The effect of intravenous lactated Ringer’s solution versus 0.9% sodium chloride solution on serum osmolality in human volunteers. Anesth Analg. 1999;88:999–1003.
Kirkpatrick AW, Chun R, Brown R, et al. Hypothermia and the trauma patient. Can J Surg. 1999;42:333–43.
Jensen SD, Cotton BA. Damage control laparotomy in trauma. Br J Surg. 2017; doi:10.1002/bjs.10519.
Roberts DJ, Ball CG, Feliciano DV, et al. History of the innovation of damage control for management of trauma patients: 1902-2016. Annals Surg. 2017;265:1034–44. doi:10.1097/SLA.0000000000001803.
Roberts DJ, Ball CG, Kirkpatrick AW. Increased pressure within the abdominal compartment: intra-abdominal hypertension and the abdominal compartment syndrome. Curr Opin Crit Care. 2016;22:174–85. doi:10.1097/MCC.0000000000000289.
Malbrain ML, De Laet I. It’s all in the gut: introducing the concept of acute bowel injury and acute intestinal distress syndrome…. Crit Care Med. 2009;37:365–6. doi:10.1097/CCM.0b013e3181935001.
Malbrain ML, De Laet I. AIDS is coming to your ICU: be prepared for acute bowel injury and acute intestinal distress syndrome. Intensive Care Med. 2008;34:1565–9. doi:10.1007/s00134-008-1135-3.
Roberts DJ, De Waele J, Kirkpatrick AW, et al. Intra-abdominal hypertension and the abdominal compartment syndrome. In: Gravlee GP, Davis RF, Hammon JW, Kussman BD, editors. Surgical Intensive Care Medicine. 3rd ed. Switzerland: Springer International Publishing; 2016.
Carr JA. Abdominal compartment syndrome: a decade of progress. J Am Coll Surg. 2013;216:135–46. doi:10.1016/j.jamcollsurg.2012.09.004.
Miller PR, Thompson JT, Faler BJ, et al. Late fascial closure in lieu of ventral hernia: the next step in open abdomen management. J Trauma. 2002;53:843–9. doi:10.1097/01.TA.0000027879.14969.C9.
Holodinsky JK, Roberts DJ, Ball CG, et al. Risk factors for intra-abdominal hypertension and abdominal compartment syndrome among adult intensive care unit patients: a systematic review and meta-analysis. Crit Care. 2013;17:R249. doi:10.1186/cc13075.
Pommerening MJ, DuBose JJ, Zielinski MD, et al. Time to first take-back operation predicts successful primary fascial closure in patients undergoing damage control laparotomy. Surgery. 2014;156:431–8. doi:10.1016/j.surg.2014.04.019.
Hatch QM, Osterhout LM, Ashraf A, et al. Current use of damage-control laparotomy, closure rates, and predictors of early fascial closure at the first take-back. J Trauma. 2011;70:1429–36. doi:10.1097/TA.0b013e31821b245a.
Bradley MJ, Dubose JJ, Scalea TM, et al. Independent predictors of enteric fistula and abdominal sepsis after damage control laparotomy: results from the prospective AAST Open Abdomen registry. JAMA Surg. 2013;148:947–54. doi:10.1001/jamasurg.2013.2514.
Brown JB, Cohen MJ, Minei JP, et al. Goal-directed resuscitation in the prehospital setting: a propensity-adjusted analysis. J Trauma Acute Care Surg. 2013;74:1207–12. doi:10.1097/TA.0b013e31828c44fd.
Rahbar E, Fox EE, del Junco DJ, et al. Early resuscitation intensity as a surrogate for bleeding severity and early mortality in the PROMMTT study. J Trauma Acute Care Surg. 2013;75:S16–23. doi:10.1097/TA.0b013e31828fa535.
Savage SA, Zarzaur BL, Croce MA, et al. Redefining massive transfusion when every second counts. J Trauma Acute Care Surg. 2013;74:396–400. doi:10.1097/TA.0b013e31827a3639.
Malone DL, Hess JR, Fingerhut A. Massive transfusion practices around the globe and a suggestion for a common massive transfusion protocol. J Trauma. 2006;60:S91–6. doi:10.1097/01.ta.0000199549.80731.e6.
•• Holcomb JB, Tilley BC, Baraniuk S, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313:471–82. doi:10.1001/jama.2015.12. An RCT that highlights that 1:1:1 resuscitation ratios achieves greater hemostasis and leads to fewer bleeding deaths by 24 hours compared to a 1:1:2 ratio
Cannon JW, Khan MA, Raja AS, et al. Damage control resuscitation in patients with severe traumatic hemorrhage: a practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg. 2017;82:605–17. doi:10.1097/TA.0000000000001333.
Pati S, Matijevic N, Doursout MF, et al. Protective effects of fresh frozen plasma on vascular endothelial permeability, coagulation, and resuscitation after hemorrhagic shock are time dependent and diminish between days 0 and 5 after thaw. J Trauma. 2010;69:S55–63. doi:10.1097/TA.0b013e3181e453d4.
Cardenas JC, Cap AP, Swartz MD, et al. Plasma resuscitation promotes coagulation homeostasis following shock-induced hypercoagulability. Shock. 2016;45:166–73. doi:10.1097/SHK.0000000000000504.
Ketchum L, Hess JR, Hiippala S. Indications for early fresh frozen plasma, cryoprecipitate, and platelet transfusion in trauma. J Trauma. 2006;60:S51–8. doi:10.1097/01.ta.0000199432.88847.0c.
Holcomb JB, Donathan DP, Cotton BA, et al. Prehospital transfusion of plasma and red blood cells in trauma patients. Prehosp Emerg Care. 2015;19:1–9. doi:10.3109/10903127.2014.923077.
Brown JB, Sperry JL, Fombona A, et al. Pre-trauma center red blood cell transfusion is associated with improved early outcomes in air medical trauma patients. J Am Coll Surg. 2015;220:797–808. doi:10.1016/j.jamcollsurg.2015.01.006.
Henriksen HH, Rahbar E, Baer LA, et al. Pre-hospital transfusion of plasma in hemorrhaging trauma patients independently improves hemostatic competence and acidosis. Scand J Trauma Resusc Emerg Med. 2016;24:145. doi:10.1186/s13049-016-0327-z.
Martinaud C, Ausset S, Deshayes AV, et al. Use of freeze-dried plasma in French intensive care unit in Afghanistan. J Trauma. 2011;71:1761–4. doi:10.1097/TA.0b013e31822f1285.
Glassberg E, Nadler R, Gendler S, et al. Freeze-dried plasma at the point of injury: from concept to doctrine. Shock. 2013;40:444–50. doi:10.1097/SHK.0000000000000047.
Cantle PM, Cotton BA. Prediction of massive transfusion in trauma. Crit Care Clin. 2017;33:71–84. doi:10.1016/j.ccc.2016.08.002.
Nunez TC, Voskresensky IV, Dossett LA, et al. Early prediction of massive transfusion in trauma: simple as ABC (assessment of blood consumption)? J Trauma. 2009;66:346–52. doi:10.1097/TA.0b013e3181961c35.
Cotton BA, Dossett LA, Au BK, et al. Room for (performance) improvement: provider-related factors associated with poor outcomes in massive transfusion. J Trauma. 2009;67:1004–12. doi:10.1097/TA.0b013e3181bcb2a8.
Gunter OL Jr, Au BK, Isbell JM, et al. Optimizing outcomes in damage control resuscitation: identifying blood product ratios associated with improved survival. J Trauma. 2008;65:527–34. doi:10.1097/TA.0b013e3181826ddf.
Radwan ZA, Bai Y, Matijevic N, et al. An emergency department thawed plasma protocol for severely injured patients. JAMA Surgery. 2013;148:170–5. doi:10.1001/jamasurgery.2013.414.
Cotton BA, Reddy N, Hatch QM, et al. Damage control resuscitation is associated with a reduction in resuscitation volumes and improvement in survival in 390 damage control laparotomy patients. Ann Surg. 2011;254:598–605. doi:10.1097/SLA.0b013e318230089e.
Johansson PI, Stensballe J, Oliveri R, et al. How I treat patients with massive hemorrhage. Blood. 2014;124:3052–8. doi:10.1182/blood-2014-05-575340.
Holcomb JB, del Junco DJ, Fox EE, PROMMTT Study Group, et al. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surgery. 2013;148:127–36. doi:10.1001/2013.jamasurg.387.
Joint Committee to Create a National Policy to Enhance Survivability From Intentional Mass Casualty Shooting Events. Improving survival from active shooter events: The Hartford Consensus. American College of Surgeons. 2013.
Jacobs, LM and Joint Committee to Create a National Policy to Enhance Survivability From Intentional Mass Casualty Shooting Events. The Hartford Consensus III: implementation of bleeding control. American College of Surgeons. 2015.
Spanjersberg WR, Knops SP, Schep NWL, et al. Effectiveness and complications of pelvic circumferential compression devices in patients with unstable pelvic fractures: a systematic review of literature. Injury. 2009;40:1031–5. doi:10.1016/j.injury.2009.06.164.
Croce MA, Magnotti LJ, Savage SA, et al. Emergent pelvic fixation in patients with exsanguinating pelvic fractures. J Am Coll Surg. 2007;204:935–9. doi:10.1016/j.jamcollsurg.2007.01.059.
Krieg JC, Mohr M, Ellis TJ, et al. Emergent stabilization of pelvic ring injuries by controlled circumferential compression: a clinical trial. J Trauma. 2005;59:659–64.
Eastridge BJ, Mabry RL, Seguin P, et al. Death on the battlefield (2001–2011): implications for the future of combat casualty care. J Trauma Acute Care Surg. 2012;73:S431–7. doi:10.1097/TA.0b013e3182755dcc.
Kragh JF Jr, Walters TJ, Baer DG, et al. Survival with emergency tourniquet use to stop bleeding in major limb trauma. Ann Surg. 2009;249:1–7. doi:10.1097/SLA.0b013e31818842ba.
Kragh JF Jr, Walters TJ, Baer DG, et al. Practical use of emergency tourniquets to stop bleeding in major limb trauma. J Trauma. 2008;64:S38–50. doi:10.1097/TA.0b013e31816086b1.
Lee C, Porter KM, Hodgetts TJ. Tourniquet use in the civilian prehospital setting. Emerg Med J. 2007;24:584–7. doi:10.1136/emj.2007.046359.
Doyle GS, Taillac PP. Tourniquets: a review of current use with proposals for expanded prehospital use. Prehosp Emerg Care. 2008;12:241–56. doi:10.1080/10903120801907570.
Scerbo MH, Mumm JP, Gates K, et al. Safety and appropriateness of tourniquets in 105 civilians. Prehosp Emerg Care. 2016;20:712–22. doi:10.1080/10903127.2016.1182606.
Seamon MJ, Haut ER, Van Arendonk K, et al. An evidence-based approach to patient selection for emergency department thoracotomy: a practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg. 2015;79:159–73. doi:10.1097/TA.0000000000000648.
Stannard A, Eliason JL, Rasmussen TE. Resuscitative endovascular balloon occlusion of the aorta (REBOA) as an adjunct for hemorrhagic shock. J Trauma. 2011;71:1869–72. doi:10.1097/TA.0b013e31823fe90c.
Brenner ML, Moore LJ, DuBose JJ, et al. A clinical series of resuscitative endovascular balloon occlusion of the aorta for hemorrhage control and resuscitation. J Trauma Acute Care Surg. 2013;75:506–11. doi:10.1097/TA.0b013e31829e5416.
Moore LJ, Brenner M, Kozar RA, et al. Implementation of resuscitative endovascular balloon occlusion of the aorta as an alternative to resuscitative thoracotomy for noncompressible truncal hemorrhage. J Trauma Acute Care Surg. 2015;79:523–30. doi:10.1097/TA.0000000000000809.
Sadek S, Lockey DJ, Lendrum RA, et al. Resuscitative endovascular balloon occlusion of the aorta (REBOA) in the pre-hospital setting: an additional resuscitation option for uncontrolled catastrophic haemorrhage. Resuscitation. 2016;107:135–8. doi:10.1016/j.resuscitation.2016.06.029.
Smith A, Ouellet JF, Niven D, et al. Timeliness in obtaining emergent percutaneous procedures in severely injured patients: how long is too long and should we create quality assurance guidelines? Can J Surg. 2013;56:E154–7.
Schwartz DA, Medina M, Cotton BA, et al. Are we delivering two standards of care for pelvic trauma? Availability of angioembolization after hours and on weekends increases time to therapeutic intervention. J Trauma Acute Care Surg. 2014;76:134–9. doi:10.1097/TA.0b013e3182ab0cfc.
Holcomb JB, Fox EE, Scalea TM, et al. Current opinion on catheter-based hemorrhage control in trauma patients. J Trauma Acute Care Surg. 2014;76:888–93. doi:10.1097/TA.0000000000000133.
Roberts DJ, Bobrovitz N, Zygun DA, et al. Indications for use of damage control surgery and damage control interventions in civilian trauma patients: a scoping review. J Trauma Acute Care Surg. 2015;78:1187–96. doi:10.1097/TA.0000000000000647.
Roberts DJ, Bobrovitz N, Zygun DA, et al. Indications for use of thoracic, abdominal, pelvic, and vascular damage control interventions in trauma patients: a content analysis and expert appropriateness rating study. J Trauma Acute Care Surg. 2015;79:568–79. doi:10.1097/TA.0000000000000821.
Roberts DJ, Bobrovitz N, Zygun DA, et al. Indications for use of damage control surgery in civilian trauma patients: a content analysis and expert appropriateness rating study. Ann Surg. 2016;263:1018–27. doi:10.1097/SLA.0000000000001347.
Roberts DJ, Zygun DA, Faris PD, et al. Opinions of practicing surgeons on the appropriateness of published indications for use of damage control surgery in trauma patients: an international cross-sectional survey. J Am Coll Surg. 2016;223:515–29. doi:10.1016/j.jamcollsurg.2016.06.002.
Harvin JA, Kao LS, Liang MK, et al. Decreasing the use of damage control laparotomy in trauma: a quality improvement project. J Am Coll Surg 2017. Accepted Manuscript. Doi: 10.1016/j.jamcollsurg.2017.04.010.
Taylor JR, Adams SD, McNutt MK, et al. Indication-specific outcomes for damage control laparotomy: a descriptive study. Am J Surg 2017. Submitted.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Holcomb is the chief medical officer of Prytime Medical Devices (The REBOA Company™), a private company that manufactures a proprietary REBOA balloon catheter. Drs. Cantle and Roberts declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Human and Animal Rights
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki Declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
This article is part of the Topical Collection on Modern Trauma Resuscitation
Rights and permissions
About this article
Cite this article
Cantle, P.M., Roberts, D.J. & Holcomb, J.B. Damage Control Resuscitation Across the Phases of Major Injury Care. Curr Trauma Rep 3, 238–248 (2017). https://doi.org/10.1007/s40719-017-0096-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40719-017-0096-9