Abstract
Purpose of review
Gout is more prevalent than ever and is associated with multiple chronic comorbidities, including Chronic Kidney Disease (CKD). While goals of treatment are the same as in those without renal impairment, co-morbid CKD poses a challenge in treatment selection and requires a solid understanding of potential drug-drug interaction and drug-related toxicity.
Recent findings
In acute gout complicated by CKD, NSAIDs should be avoided, and colchicine used with caution. Systemic corticosteroids are effective but may be replaced by anakinra, in particular in inpatients with additional comorbidities that may make corticosteroids less desirable. Allopurinol remains the first line urate lowering therapy (ULT), starting at a low dose followed by careful goal driven up-titration. Febuxostat is a reasonable alternative, though second line in light of recent cardiovascular data. Uricosuric drugs are generally less effective, while pegloticase is reserved for refractory cases of polyarticular tophaceous disease.
Summary
Gout treatment must be guided by renal function but in spite of renal disease can be successfully managed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of gout has doubled in the past 20 years, as has the prevalence of comorbid conditions in those with gout, including CKD [1]. Gout and chronic kidney disease (CKD) are tightly linked [2] as renal function greatly impacts serum urate (sUA) concentration. CKD results in reduced fractional excretion of serum urate, leading to chronic hyperuricemia, the key risk factor for the development of gout. Additionally, patients with gout are more likely to have metabolic syndrome [3], which has been linked to hyperuricemia and further renal impairment through a wide range of mechanisms. Evidence pointing to a possible association between hyperuricemia and renal disease progression has resulted in a large body of active research evaluating the effect of urate lowering medications on renal impairment. This narrative review explores the data guiding the challenging management of gout in patients with CKD and focuses on practical aspects of gout treatment in the acute and chronic phases. Patients with gout and CKD are likely to have multiple co-morbidities including diabetes mellitus, hypertension, and congestive heart failure, which will impact appropriate medication choices and may pose therapeutic dilemma. In this manuscript, we work to summarize the major pearls and pitfalls of gout management in this population (Table 1).
Acute Gout Management in CKD
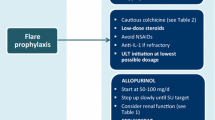
Commonly used drugs to treat an acute gout flare include oral colchicine, NSAIDs, corticosteroids either orally, intramuscularly, or by direct joint injection. Adrenocorticotropic hormone or IL-1 blocking medications are alternate effective options when others are contraindicated by comorbid illnesses. These treatment options should be guided by the presence or absence of renal impairment as reviewed below (Table 2).
Colchicine: Clearance Reduction in CKD
Colchicine may be used as a first-line acute gout treatment in those with CKD though with very careful dose adjustments [4]. Colchicine is rapidly absorbed, peaking in 1–2 h in fasting healthy volunteers, with bioavailability of about 45% [5]. The liver metabolizes colchicine into two primary metabolites, which then undergo excretion through renal, enterohepatic, and biliary sites [5]. Given the concern for colchicine toxicity in the setting of reduced renal elimination in CKD patients, colchicine to treat gout flares is generally not recommended in patients with renal impairment already receiving prophylactic colchicine. The use of low-dose colchicine for treating acute gout was investigated in a randomized, double-blind, placebo-controlled, parallel-group study in patients with CrCl ≥ 60, where low dose was defined as 1.2 mg followed by 0.6 mg 1 h later [6]. In this study, the low dose was well tolerated but only 37.8% of patients had reached the primary end point of a 50% reduction in pain at 24 h. This dosing, though likely inadequate for a substantial portion of patients, is the dose now approved for colchicine use for acute gout management. In those with mild (CrCl 50–80 mL/min) or moderate (CrCl 30–50 mL/min) renal impairment, close monitoring is advised but the dose of 1.2 mg initially and 0.6 mg 1 h later is not adjusted [5]. In patients with severe renal impairment (CrCl < 30 mL/min), dose adjustment is not required, but it is advised the dosing not be repeated more than every 2 weeks [5]. Patients requiring dialysis are advised to limit colchicine to 0.6 mg every 2 weeks. Of note, colchicine is not removed by hemodialysis [5]. Colchicine may interact with medications that are strong CYP3A4 inhibitors (clarithromycin, certain protease inhibitors), moderate CYP3A4 inhibitors (grapefruit juice, verapamil), and P-glycoprotein inhibitors such as cyclosporine [5] so that further dose reduction may be advised in patients concomitantly receiving these medications.
NSAIDS: Limit Use in CKD
NSAIDs are a frequently used option for acute gout in those with normal renal function but are not desirable in those with impaired renal function. Though the FDA has approved naproxen, indomethacin, and sulindac for the treatment of acute gout [4], all NSAIDs are generally considered equivalent when used at full dose. Patients with CKD are advised to limit or completely avoid NSAID intake due to risks of acute renal injury, hypertension, and electrolyte abnormalities [7]. Physicians are well aware of this risk so that the rate of NSAID prescriptions in patients with CKD is lower than that of opiates and other pain medications [8]. Despite this, in one study, 5% of Americans with moderate to severe CKD reported daily use of NSAIDs [9], emphasizing the need to educate patients with gout and CKD about the risk of NSAIDs and help them find alternative treatments.
Corticosteroids: Often Used
Corticosteroids represent a very useful alternative to NSAIDs and colchicine when treating acute gout in CKD. Intra-articular injections of corticosteroids are best used when a limited number of joints are affected. When multiple joints are involved or the affected joint is not amenable to injection, oral or intramuscular (IM) corticosteroids may be used effectively. Combination therapy of intra-articular injections in larger joints with oral colchicine for polyarticular symptoms for example may additionally be useful. Under-dosing of systemic corticosteroids may lead to incomplete gout flare resolution and relapse. The advised dose is at least 0.5 mg/kg/day for 5–10 days, versus 2–5 days at the full dose of 0.5 mg/kg/day followed by a taper lasting 7–10 days [4]. If patients prefer or require IM dosing of corticosteroid, a single dose of 60 mg triamcinolone acetonide, followed by oral prednisone or prednisolone, is recommended [4].
Corticotropin (ACTH): Costly
Corticotropin has the same indications as systemic corticosteroids and is available for subcutaneous or intramuscular injection. Doses of 40–80 IU have been reported to terminate acute gout attacks, with mild side effects that have included hypokalemia, hyperglycemia, and fluid retention [10]. Sodium and water retention and increased blood pressure are seen due to the mechanism of action of ACTH [11], which may be problematic in those with CKD. Corticotropin is reported to be quite effective, with response rates as high as 78–100% of patients responding [12]. The main barrier to use is cost, as one vial containing 5 mL at 80 units/mL and the equivalent of 5–10 doses costs $43,658 (www.goodrx.com).
IL-1 Antagonism: Newer Option
The use of recombinant or monoclonal interleukin-1 (IL-1) inhibitors has gained interest given the central role IL-1 plays in the inflammatory cascade triggered by monosodium urate crystals. Though expensive, IL-1 inhibitors may be justified to shorten the length of stay of a hospitalized patient with acute gout and comorbidities precluding the use of corticosteroids, such as heart failure, diabetes mellitus, active infection, or perioperative care. Anakinra , a recombinant IL-1 receptor blocker, is approved for daily use in the treatment of rheumatoid arthritis. Case reports and series have reported on the efficacy of anakinra in treating acute gout flares. Anakinra is typically used at 100 mg SQ daily (or every other day if CrCl < 30 mL/min) for 3–5 doses [13,14,15,16,17,18]. Anakinra was found to be safe in treating acute gout in a population with CKD stage IV or V, or renal transplant patients [19]. Canakinumab 150 mg SQ, a human monoclonal antibody directed against IL-1 beta, has also been used and is labeled for use in the European Union in patients with gout in whom treatment with other agents is not acceptable [20, 21].
Chronic Gout Management
In managing chronic gout, certain principles are good practice, regardless of the presence of CKD. These include the following:
Making adjustments in diet and lifestyle to help maintain a low sUA
Lowering the serum urate by initiating ULT treating to goal sUA of 6 mg/dL (or 5 mg/dL in those with tophi) while monitoring laboratory tests to ensure safe use
When starting or adjusting the dose of ULT, ensuring anti-inflammatory prophylaxis is concomitantly used to prevent acute gout attacks
Maintaining prophylactic therapy for at least 3–6 months after serum urate goal is achieved (or longer in those with large tophi burden), though this is based on grade B and C level of evidence and expert opinion [4]
Treating acute flares as they occur while not interrupting ULT
Prophylaxis: Limited Options in Those with CKD
Agents used in treating acute gout may also be used as anti-inflammatory prophylaxis as ULT is started or the dose is being adjusted (Table 3). Guidelines recommend the use of anti-inflammatory prophylaxis as long as there is evidence of clinically active gout or until the serum urate goal has been achieved for at least 3 months (in those without tophi) or 6 months (in those with tophi) [4].
Colchicine can be used as prophylaxis in those with CKD, though with caution and monitoring laboratory data. Colchicine long-term use requires a dose adjustment in those with CKD given lower elimination and a higher risk of toxicity including neuropathy, neutropenia, and myopathy [22]. With intact renal function, the dose is 0.6 mg once or twice daily. Patients with a GFR > 30 mL/min may take up to 0.6 mg daily, though this dose may be further reduced in the setting of concomitant treatment with CYP3A4 and glycoprotein P inhibitors as well as statins [23]. Patients with a creatinine clearance of 10–30 mL/min should not take more than 0.3 mg daily. The Colcrys package insert specifies that colchicine may be dosed as 0.3 mg orally twice weekly in patients receiving hemodialysis without guidance on timing of its administration relative to dialysis [5]. Following a single dose administration of colchicine, colchicine exposure was similar in patients with mild-moderate CKD and healthy controls, while in severe CKD, the exposure was doubled. In patients undergoing dialysis, there was no increased exposure to colchicine though only 5% was removed in the dialysate [24]. A case-control study reported on 22 patients on hemodialysis taking at least 0.5 mg daily of colchicine for an average of 8 years and found no difference in cell count abnormalities or neuromuscular symptoms or signs, aside from an increased WBC count in those patients compared to hemodialysis controls [25]. Nonetheless, caution should be used in those with severe CKD and ESRD on colchicine due to potential toxicity. Colchicine has a narrow therapeutic window and can be associated with a high mortality rate particularly with acute poisoning [26].
Gout prophylaxis with NSAIDs even in those with mild renal impairment is best avoided due to known long-term renal toxicity. Low-dose daily oral corticosteroids may be necessary when other options are not possible. However, patients need to be warned about the long-term risk of chronic corticosteroids including osteoporosis, osteonecrosis, iatrogenic Cushing’s syndrome, hyperglycemia, hypertension, and mood disorders. The daily recommended dose is 10 mg prednisone equivalents or less [4]. Dose and duration of use should be minimized if possible.
IL-1 antagonists for gout prophylaxis have been evaluated. Daily use of anakinra to prevent gout flares seems inconvenient and unnecessary and has not been studied. Canakinumab monthly injection was found to be superior to colchicine in preventing acute gout flares after initiating allopurinol, though no mention of renal function was made in this study [27]. Rilonacept, a fusion protein long acting IL-1 inhibitor, likewise has data to support its use in gout prophylaxis in those initiating allopurinol, with 14% of study participants having “renal and other urinary disorders” [28]. Neither canakinumab nor rilonacept are approved for gout flare prevention.
Allopurinol: Tried and True, Start Low and Go Slow
Allopurinol competitively inhibits xanthine oxidase and is the first line ULT (Table 4). Allopurinol is metabolized into oxypurinol (80%), allopurinol 1′riboside (10%), while 10% is not metabolized but undergoes renal excretion. Oxypurinol is also excreted almost unchanged through the kidneys and has an extended half-life of up to 1 week in those with severe renal impairment [29].When the CrCl is < 10 mL/min, oxypurinol is not excreted by the kidneys at all [30].
Allopurinol hypersensitivity syndrome (AHS) is of concern given its high mortality rate [31]. A history of CKD increases the risk of allopurinol hypersensitivity syndrome significantly [32]. Other risks include recently starting allopurinol in the past 6–8 weeks, a positive HLA-B5801 status, diuretic use, and a high allopurinol starting dose [33]. The latter appears to be more important than the maintenance dose of allopurinol in inducing AHS, so that a starting dose of 1.5 mg per unit of estimated GFR has been proposed [34]. AHS was not found to occur more frequently in those with reduced CrCl who are requiring higher than CrCl-based maintenance allopurinol dosing [35]. Because of this, close monitoring especially when starting and adjusting allopurinol dose is necessary. Nonetheless, maintenance doses of allopurinol above 300 mg daily may be used if the dose is titrated correctly.
The starting dose of allopurinol in those with normal renal function is 100 mg/day. This dose is gradually increased by 100 mg/day every 2–4 weeks until the sUA is at goal of 6 mg/dL (or 5 mg/dL in those with tophi). In those with stage IV CKD or worse, the starting dose is 50 mg/day with a gradual dose increase of 50 mg/day every 2–4 weeks until the target sUA has been achieved or the maximum dose of 800 mg/day has been reached. Patient education about hypersensitivity reactions, including rash, pruritus, and regular monitoring of laboratory tests looking for hepatic transaminase elevation and eosinophilia [36], is particularly important in this patient population. For patients who require hemodialysis, the dose is given after dialysis sessions, as oxypurinol concentrations were found to be decreased by 50% with dialysis [37]. For those undergoing daily hemodialysis, a dose of allopurinol up to 50% higher may be required [38].
Though allopurinol is an effective treatment for gout, adherence remains an issue as reported in a retrospective analysis, where patients were adherent to allopurinol therapy only 56% of the treatment period [39].
Whether ULT with allopurinol improves renal function is an active area of research. A recent meta-analysis of this topic found that allopurinol may slow the progression of CKD but had no effect on proteinuria and blood pressure [40]. Many of the trials analyzed had different levels of follow-up and baseline CKD, as well as cause of CKD, suggesting that more randomized controlled trials with adequate power are needed to answer this question. Studies since then have suggested allopurinol may improve CKD and decrease cardiovascular risk [41], perhaps through its effect on endothelial cell function [42, 43]. Allopurinol was found in another study to reduce risk of cardiovascular events in patients who had both gout and diabetes [44]. The recent CARES trial highlighted an increased risk of cardiovascular death and all-cause mortality in febuxostat vs. allopurinol-treated gout patients with a high burden of cardiovascular risk factors. Whether this difference reflects a protective effect of allopurinol or a cardiovascular risk inherent to febuxostat is unknown as a control group of gout patients not on ULT was not included [45].
Febuxostat: Second-Line ULT
Febuxostat is a non-purine selective inhibitor of xanthine oxidase, and like allopurinol, interferes with serum urate production. Febuxostat is metabolized primarily through glucuronidation by the liver, with only up to 6% of the drug eliminated unchanged through renal excretion [46]. A reduced creatinine clearance might therefore be anticipated to have little impact on the pharmacokinetic profile of febuxostat [47] and is an appealing alternative to allopurinol in patients with CKD who have failed an adequate trial or are intolerant of allopurinol. To evaluate the safety, pharmacokinetics, and pharmacodynamics of febuxostat in subjects with reduced renal function, one study administered febuxostat 80 mg per day for 7 days, to participants with mild (n = 6, CrCl 50–80), moderate (n = 7, CrCl 30–49), or severe kidney disease (n = 7, CrCl 10–29). The drug was well tolerated. Febuxostat time to maximum concentration and maximum concentrations were the same. Area under the curve, however, indicated a statistically significant linear relationship with creatinine clearance. Regardless of clearance, sUA decreased by 55–64% by day 7 [48]. One study indicated that febuxostat acyl glucuronide may undergo “futile cycling” regenerating the parent drug because of renal impairment. Whether this translates in more effective urate lowering in patients with reduced clearance deserves further study [49].
Phase 3 trials evaluating the use of febuxostat compared to allopurinol included patients with mild to moderate CKD but excluded those with severe renal impairment defined as eGFR of ≥ 15 to < 30 mL/min. The FACT trial, a 52-week double-blind randomized controlled trial, evaluated the safety of febuxostat 80 mg or 120 mg compared to allopurinol 300 mg in 762 patients with eGFR > 50 mL/min. Thirty-five percent of participants had an eGFR 50–79 mL/min. More patients receiving febuxostat achieved the primary outcome of a sUA < 6 mg/dL at the last 3-month visit. However, data according to eGFR was not reported. No differences were noted in terms of number of gout flares or tophus size reduction [50]. The CONFIRMS trial [51], the largest study evaluating the efficacy and safety of febuxostat to allopurinol in roughly 2200 patients, included patients with mild (eGFR 60–89 mL/min) to moderate (eGFR 30–59 mL/min) but excluded those with eGFR < 30 mL/min. Subjects were randomized to receive febuxostat 40 mg, 80 mg, or allopurinol 300 mg (reduced to 200 mg in those with moderate renal impairment). Overall, 18% of patients had moderate CKD, whereas 48% had mild CKD. Febuxostat 80 mg was significantly better than febuxostat 40 mg or allopurinol 300/200 mg in all groups in lowering sUA to below 6 mg/dl. In those with mild to moderate CKD, febuxostat 40 mg was superior to allopurinol 300/200 mg in reaching target sUA. The nature and rates of adverse events in those with mild to moderate renal impairment were similar to the overall treatment groups [51].
In a multicenter, double-blind placebo controlled trial, the efficacy and safety of febuxostat in 96 patients with gout and moderate to severe CKD were evaluated. Patients were randomized to receive febuxostat 30 mg twice daily, 40–80 mg febuxostat once daily, or placebo. Overall, 37.5% of patients had severe renal impairment (eGFR 15–29 mL/min) and 62.5% had moderate impairment (eGFR 30–50 mL/min). No change in creatinine or eGFR from baseline was noted among the groups. However, the proportion of patients with a sUA of < 6 mg/dl was significantly greater in both febuxostat groups compared to placebo. About 2/3 of patients assigned to the 40–80 mg group, had a dose titration to 80 mg once daily at 1 month. No safety concerns were reported [52].
Based on these data, febuxostat is currently approved in the USA at a dose of 40–80 mg for patients with eGFR 30 mL/min and above. Those with eGFR 10–29 mL/min can receive a dose up to 40 mg per day. Febuxostat is not approved for use in CKD stage 5 or in ESRD [53] though a case series of 17 ESRD patients reported on its use starting at 10 mg daily [54].
Uricosurics: Not Effective in Moderate to Severe CKD
Probenecid, benzbromarone, and lesinurad inhibit urate anion transporters located in the renal proximal tubule, and as such increase serum urate excretion by preventing tubular resorption. These may not be used in patients with high uric acid excretion, or those with nephrolithiasis given the risk of further precipitating stones, and must be taken with large amounts of fluids. Benzbromarone is not available in the USA and will not be discussed further in this review but may be used in patients with eGFR > 30 mL/min. Very little information is available on the use of probenecid in patients with CKD and gout. The ACR 2012 Gout Guidelines do not recommend its use in patients with eGFR < 50 mL/min [36]. Combining it with allopurinol may provide additional urate lowering effects in patients with CKD. A study of 20 patients with gout on a stable dose of allopurinol (100–400 mg/day) not at target sUA, assessed the effect of adding probenecid in incremental dosing, and included five subjects with an eGFR < 50 mL/min. All patients with renal impairment had an observed reduction in their sUA, and all but one patient achieved target sUA with combination therapy [55].
Lesinurad inhibits URAT-1 and OAT-4, major apical transporters for uric acid [56]. In pharmacologic studies, mild, moderate, and severe renal impairment increased lesinurad area under the curve plasma levels. Lower renal clearance and urinary excretion of lesinurad were related to the degree of renal impairment [57]. Lesinurad must be given in combination therapy with allopurinol, as monotherapy is associated with acute kidney injury. Its use is restricted to patients with eGFR > 45 mL/min [58]. In a phase 3 clinical trial comparing allopurinol with placebo, to allopurinol with lesinurad 200 mg or 400 mg, 19% of subjects had an eGFR 30–60 mL/min. Mean allopurinol dose was 300 mg/day. Statistically, more patients in the lesinurad subgroups achieved target sUA, and renal function remained stable across all treatment groups [59].
Pegloticase: Expensive but Key in Refractory Cases
Pegloticase is a PEGylated recombinant form of urate-oxidase enzyme and is approved for the treatment of refractory gout. It is administered intravenously at a dose of 8 mg every 2 weeks, with no dosage adjustment required in CKD. Long-term efficacy and safety up to 3 years has been reported in 149 patients in an open-label extension study. The drug was well tolerated, and severe infusion reactions were related in 90% patients to sUA exceeding 6 mg/dl prior to receiving the infusion. Treatment was associated with sustained improvement in tophi size and number of gout flares [60]. A post hoc analysis of two replicate phase 3 trials and their long-term open-label extension that included 83 patients with moderate to severe CKD found no difference in pegloticase safety according to degree of CKD [61].
Conclusion
Gout is the most common cause of inflammatory arthritis in men, and its incidence has been rising across the world as the population continues to age and patients survive their chronic comorbidities [62]. Poorly controlled gout impairs quality of life, overall function, and work productivity [63] so that adequate treatment is imperative. Careful assessment of each patient’s comorbidities, with particular attention to concomitant kidney disease, and medications taken for the co-management of heart disease, hypertension, and diabetes mellitus is necessary in selecting the safest medication regimen for each patient. Furthermore, patient education and engagement is critical in sustaining medication adherence and long-term treatment success. The added CKD comorbidity increases the complexity of medical treatment in both acute and maintenance phases but successful outcomes can be achieved.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Elfishawi MM, Zleik N, Kvrgic Z, Michet CJ Jr, Crowson CS, Matteson EL, et al. The rising incidence of gout and the increasing burden of comorbidities: a population-based study over 20 years. J Rheumatol. 2018;45(4):574-9 Retrospective study reporting on the rising incidence of comorbidities in gout patients over the last two decades.
Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the health professionals follow-up study. Arch Intern Med. 2005;165(7):742–8.
Choi HK, Ford ES, Li C, Curhan G. Prevalence of the metabolic syndrome in patients with gout: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2007;57(1):109–15.
Khanna D, et al. American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken), 2012. 2012;64(10):1447–61.
Colcrys Prescribing Information. Deerfield, I.T.P.A. and I.D. 2015.
Terkeltaub RA, et al. High versus low dosing of oral colchicine for early acute gout flare: twenty-four-hour outcome of the first multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison colchicine study. Arthritis Rheum. 2010;62(4):1060–8.
Sriperumbuduri S, Hiremath S. The case for cautious consumption: NSAIDs in chronic kidney disease. Curr Opin Nephrol Hypertens. 2018;28(2):163–70.
Novick TK, Surapaneni A, Shin JI, Ballew SH, Alexander GC, Inker LA, et al. Prevalence of opioid, gabapentinoid, and NSAID use in patients with CKD. Clin J Am Soc Nephrol. 2018;13(12):1886–8.
Plantinga L, Grubbs V, Sarkar U, Hsu CY, Hedgeman E, Robinson B, et al. Nonsteroidal anti-inflammatory drug use among persons with chronic kidney disease in the United States. Ann Fam Med. 2011;9(5):423–30.
Ritter J, Kerr LD, Valeriano-Marcet J, Spiera H. ACTH revisited: effective treatment for acute crystal induced synovitis in patients with multiple medical problems. J Rheumatol. 1994;21(4):696–9.
Connell JM, et al. Effects of ACTH and cortisol administration on blood pressure, electrolyte metabolism, atrial natriuretic peptide and renal function in normal man. J Hypertens. 1987;5(4):425–33.
Daoussis D, Antonopoulos I, Andonopoulos AP. ACTH as a treatment for acute crystal-induced arthritis: update on clinical evidence and mechanisms of action. Semin Arthritis Rheum. 2014;43(5):648–53.
Ghosh P, et al. Treatment of acute gouty arthritis in complex hospitalized patients with anakinra. Arthritis Care Res. 2013;65(8):1381–4.
Ottaviani S, Moltó A, Ea HK, Neveu S, Gill G, Brunier L, et al. Efficacy of anakinra in gouty arthritis: a retrospective study of 40 cases. Arthritis Res Ther. 2013;15(5):R123.
Thueringer JT, Doll NK, Gertner E. Anakinra for the treatment of acute severe gout in critically ill patients. Semin Arthritis Rheum. 2015;45(1):81–5.
Aouba A, et al. Efficacy of anakinra for various types of crystal-induced arthritis in complex hospitalized patients: a case series and review of the literature. Mediat Inflamm. 2015;2015:792173.
Desmarais J, Chu CQ. Utility of anakinra in acute crystalline diseases: a retrospective study comparing a university hospital with a veterans affairs medical center. J Rheumatol. 2018;46(7):748–50.
Liew JW, Gardner GC. Use of anakinra in hospitalized patients with crystal-associated arthritis. J Rheumatol. 2019;46(7):748–50.
Loustau C, Rosine N, Forien M, Ottaviani S, Juge PA, Lioté F, et al. Effectiveness and safety of anakinra in gout patients with stage 4-5 chronic kidney disease or kidney transplantation: a multicentre, retrospective study. Joint Bone Spine. 2018;85(6):755–60.
Perez-Ruiz F, Chinchilla SP, Herrero-Beites AM. Canakinumab for gout: a specific, patient-profiled indication. Expert Rev Clin Immunol. 2014;10(3):339–47.
Bardin T. Canakinumab for the patient with difficult-to-treat gouty arthritis: review of the clinical evidence. Joint Bone Spine. 2015;82(Suppl 1):eS9–16.
Putterman C, et al. Colchicine intoxication: clinical pharmacology, risk factors, features, and management. Semin Arthritis Rheum. 1991;21(3):143–55.
Justiniano M, Dold S, Espinoza LR. Rapid onset of muscle weakness (rhabdomyolysis) associated with the combined use of simvastatin and colchicine. J Clin Rheumatol. 2007;13(5):266–8.
Wason S, Mount D, Faulkner R. Single-dose, open-label study of the differences in pharmacokinetics of colchicine in subjects with renal impairment, including end-stage renal disease. Clin Drug Investig. 2014;34(12):845–55.
Solak Y, Atalay H, Biyik Z, Alibasic H, Gaipov A, Guney F, et al. Colchicine toxicity in end-stage renal disease patients: a case-control study. Am J Ther. 2014;21(6):e189–95.
Finkelstein Y, et al. Colchicine poisoning: the dark side of an ancient drug. Clin Toxicol (Phila). 2010;48(5):407–14.
Schlesinger N, et al. Canakinumab reduces the risk of acute gouty arthritis flares during initiation of allopurinol treatment: results of a double-blind, randomised study. Ann Rheum Dis. 2011;70(7):1264–71.
Sundy JS, Schumacher HR, Kivitz A, Weinstein SP, Wu R, King-Davis S, et al. Rilonacept for gout flare prevention in patients receiving uric acid-lowering therapy: results of RESURGE, a phase III, international safety study. J Rheumatol. 2014;41(8):1703–11.
Murrell GA, Rapeport WG. Clinical pharmacokinetics of allopurinol. Clin Pharmacokinet. 1986;11(5):343–53.
Hande KR, Noone RM, Stone WJ. Severe allopurinol toxicity. description and guidelines for prevention in patients with renal insufficiency. Am J Med. 1984;76(1):47–56.
Arellano F, Sacristan JA. Allopurinol hypersensitivity syndrome: a review. Ann Pharmacother. 1993;27(3):337–43.
Ramasamy SN, Korb-Wells CS, Kannangara DR, Smith MW, Wang N, Roberts DM, et al. Allopurinol hypersensitivity: a systematic review of all published cases, 1950-2012. Drug Saf. 2013;36(10):953–80.
• Stamp LK, Barclay ML. How to prevent allopurinol hypersensitivity reactions? Rheumatology (Oxford). 2018;57(suppl_1):i35–41 Insightful review of the spectrum of allopurinol hypersensitivity reaction and associated risk factors.
Stamp LK, et al. Starting dose is a risk factor for allopurinol hypersensitivity syndrome: a proposed safe starting dose of allopurinol. Arthritis Rheum. 2012;64(8):2529–36.
Vazquez-Mellado J, et al. Relation between adverse events associated with allopurinol and renal function in patients with gout. Ann Rheum Dis. 2001;60(10):981–3.
Khanna D, et al. American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res. 2012;64(10):1431–46.
Day RO, et al. Successful use of allopurinol in a patient on dialysis. BMJ Case Rep. 2012;2012:bcr0220125814.
Dalbeth N, Stamp L. Allopurinol dosing in renal impairment: walking the tightrope between adequate urate lowering and adverse events. Semin Dial. 2007;20(5):391–5.
Riedel AA, Nelson M, Joseph-Ridge N, Wallace K, MacDonald P, Becker M. Compliance with allopurinol therapy among managed care enrollees with gout: a retrospective analysis of administrative claims. J Rheumatol. 2004;31(8):1575–81.
Bose B, et al. Effects of uric acid-lowering therapy on renal outcomes: a systematic review and meta-analysis. Nephrol Dial Transplant. 2014;29(2):406–13.
Goicoechea M, et al. Allopurinol and progression of CKD and cardiovascular events: long-term follow-up of a randomized clinical trial. Am J Kidney Dis. 2015;65(4):543–9.
Xin W, Mi S, Lin Z. Allopurinol therapy improves vascular endothelial function in subjects at risk for cardiovascular diseases: a meta-analysis of randomized controlled trials. Cardiovasc Ther. 2016;34(6):441–9.
Alem MM. Allopurinol and endothelial function: a systematic review with meta-analysis of randomized controlled trials. Cardiovasc Ther. 2018;36(4):e12432.
Singh JA, Ramachandaran R, Yu S, Curtis JR. Allopurinol use and the risk of acute cardiovascular events in patients with gout and diabetes. BMC Cardiovasc Disord. 2017;17(1):76.
•• White WB, et al. Cardiovascular safety of febuxostat or allopurinol in patients with gout. N Engl J Med. 2018;378(13):1200–10 Multicenter, randomized, double-blind, non-inferiority trial evaluating the cardiovascular safety of allopurinol vs. febuxostat in patients with gout, showing non-inferiority of febuxostat in terms of the primary outcome of composite cardiovascular events, but demonstrating an significantly higher all cause mortality and cardiovascular mortality in patients on febuxostat, leading to the febuxostat label change.
Khosravan R, Grabowski BA, Wu JT, Joseph-Ridge N, Vernillet L. Pharmacokinetics, pharmacodynamics and safety of febuxostat, a non-purine selective inhibitor of xanthine oxidase, in a dose escalation study in healthy subjects. Clin Pharmacokinet. 2006;45(8):821–41.
Hoshide S, et al. PK/PD and safety of a single dose of TMX-67 (febuxostat) in subjects with mild and moderate renal impairment. Nucleosides Nucleotides Nucleic Acids. 2004;23(8-9):1117–8.
Mayer MD, Khosravan R, Vernillet L, Wu JT, Joseph-Ridge N, Mulford DJ. Pharmacokinetics and pharmacodynamics of febuxostat, a new non-purine selective inhibitor of xanthine oxidase in subjects with renal impairment. Am J Ther. 2005;12(1):22–34.
Kamel B, Graham GG, Williams KM, Pile KD, Day RO. Clinical pharmacokinetics and pharmacodynamics of febuxostat. Clin Pharmacokinet. 2017;56(5):459–75.
Becker MA, Schumacher HR Jr, Wortmann RL, MacDonald P, Eustace D, Palo WA, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005;353(23):2450–61.
Becker MA, Schumacher HR, Espinoza LR, Wells AF, MacDonald P, Lloyd E, et al. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: the CONFIRMS trial. Arthritis Res Ther. 2010;12(2):R63.
•• Saag KG, et al. Impact of febuxostat on renal function in gout patients with moderate-to-severe renal impairment. Arthritis Rheum. 2016;68(8):2035–43 Double-blind, randomized, placebo-controlled trial demonstrating efficacy and safety of febuxostat in patients with gout and moderate to severe CKD (eGFR 15-50 ml//min/1.73m2).
Uloric (febuxostat) [prescribing information]. Deerfield, I.T.P.A., Inc; 2019.
Akimoto T, et al. Febuxostat for hyperuricemia in patients with advanced chronic kidney disease. Drug Target Insights. 2014;8:39–43.
Stocker SL, et al. Pharmacokinetic and pharmacodynamic interaction between allopurinol and probenecid in patients with gout. J Rheumatol. 2011;38(5):904–10.
Miner JN, Tan PK, Hyndman D, Liu S, Iverson C, Nanavati P, et al. Lesinurad, a novel, oral compound for gout, acts to decrease serum uric acid through inhibition of urate transporters in the kidney. Arthritis Res Ther. 2016;18(1):214.
Gillen M, et al. Effects of renal function on pharmacokinetics and pharmacodynamics of lesinurad in adult volunteers. Drug Des Devel Ther. 2016;10:3555–62.
Zurampic (lesinurad) [prescribing information]. Wilmington, D.A.P.L.F.
Bardin T, Keenan RT, Khanna PP, Kopicko J, Fung M, Bhakta N, et al. Lesinurad in combination with allopurinol: a randomised, double-blind, placebo-controlled study in patients with gout with inadequate response to standard of care (the multinational CLEAR 2 study). Ann Rheum Dis. 2017;76(5):811–20.
Becker MA, Baraf HS, Yood RA, Dillon A, Vázquez-Mellado J, Ottery FD, et al. Long-term safety of pegloticase in chronic gout refractory to conventional treatment. Ann Rheum Dis. 2013;72(9):1469–74.
Yood RA, et al. Effect of pegloticase on renal function in patients with chronic kidney disease: a post hoc subgroup analysis of 2 randomized, placebo-controlled, phase 3 clinical trials. BMC Res Notes. 2014;7:54.
Roddy E, Choi HK. Epidemiology of gout. Rheum Dis Clin N Am. 2014;40(2):155–75.
Wood R, Fermer S, Ramachandran S, Baumgartner S, Morlock R. Patients with gout treated with conventional urate-lowering therapy: association with disease control, health-related quality of life, and work productivity. J Rheumatol. 2016;43(10):1897–903.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Pascale Schwab is a site investigator for the VA Cooperative Studies Program sponsored trial, STOP-Gout, which is a comparative effectiveness trial comparing allopurinol to febuxostat in patients with gout.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Comorbidities
Rights and permissions
About this article
Cite this article
Desmarais, J., Schwab, P. Gout Management in Chronic Kidney Disease: Pearls and Pitfalls. Curr Treat Options in Rheum 5, 326–335 (2019). https://doi.org/10.1007/s40674-019-00132-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40674-019-00132-y