Abstract
Aim
In this study by Foessleitner et al., both the maternal microbiome in the third trimester of pregnancy and the factors that influence the development of the child’s microbiome after cesarean delivery were investigated.
Methods
Maternal vaginal and rectal swabs were collected at inclusion in the last trimester of pregnancy and on the day of the cesarean section. In addition, placental and intrauterine swabs as well as infant dermal, buccal, and meconium swabs were taken during the cesarean section immediately after birth and subsequently on the second/third day of life. All samples were analyzed for microbial composition using 16s rRNA amplicon sequencing.
Results
A total of 30 mothers and their newborns were included in the study, with microbiome samples available for all maternal, intrauterine cavity, and placenta samples, as well as for 18 out of the 30 newborns. The vaginal and rectal microbiome was stable over the course of the third trimester and showed no significant changes (permutational multivariate analysis of variance [PERMANOVA]; p > 0.05). Both the intraoperative samples (placental, intrauterine) and the neonatal swabs at the time of birth were consistently sterile. However, rapid infant microbial colonization subsequently occurred, with neonatal buccal mucosa and stool samples showing significantly different microbial colonization from their mothers as early as the second/third day of life (PERMANOVA; p < 0.01).
Conclusion
The conclusion of the presented study was therefore that the vaginal and rectal microbiome of healthy pregnant women does not change in the last trimester, the infant and the placenta are not microbially colonized at the time of birth, and the development of the newborn’s microbiome after birth appears to be influenced mainly by environmental exposure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
The human microbiome comprises a variety of microorganisms that colonize the body in different places and in different compositions. A well-known example of this is the intestinal microbiome [1]. The microbiome differs from person to person. The study presented by Foessleitner et al. [1] focuses on the maternal vaginal and rectal microbiome during pregnancy and the development of the newborn’s microbiome after birth. There is evidence that an altered composition of the vaginal microbiome in pregnant women increases the risk of vaginal infection and can lead to premature birth [2, 3]. The healthy vaginal microbiome is mainly dominated by Lactobacillus species, which can suppress the growth of pathogenic bacteria by producing lactic acid [4, 5]. However, current data show that not all Lactobacillus species have a protective effect on the vaginal flora. For example, the presence of Lactobacillus iners appears to favor bacterial vaginosis, which in turn increases the risk of premature rupture of the membranes and consequently premature birth [2, 6]. During pregnancy, the vaginal microbiome changes early on, in the sense of a decrease in diversity [3, 4]. However, findings from studies regarding the link between the altered vaginal microbiome and an infection, which in turn can lead to premature birth or early premature rupture of the membranes, are inconsistent [4,5,6,7,8].
Since it is generally assumed that the uterus and placenta are sterile, the prevailing doctrine is that the development of the infant microbiome begins at birth through exposure to a variety of germs: during vaginal birth through contact with vaginal and fecal maternal germs and after cesarean section through skin contact and the environment [9]. In recent years, however, studies have been published that claim to have demonstrated a microbiome of the placenta and thus suggest an intrauterine microbial colonization of the fetus [10, 11].
The mode of birth also appears to have an influence on the development of the infant microbiome. In newborns born by cesarean section, there was evidence of a higher exposure of pro-inflammatory cytokines [12]. This is likely to be a major risk factor for the later development of autoimmune diseases such as type 1 diabetes mellitus, allergic bronchial asthma, or various allergies [13]. After vaginal birth, on the other hand, the infant microbiome showed a higher presence of Bifidobacterium species and, in turn, fewer pathogenic opportunistic germs such as Enterococcus and Klebsiella species [14]. Breast milk also plays an important role in the development of the infant’s microbiome, with both infant formula, as an alternative to breast milk, and antibiotics being shown to be disruptive factors in the development of the child’s microbiome [15].
The aim of the presented study was to investigate the maternal vaginal and rectal microbiome in the third trimester of an unremarkable pregnancy, the placental and uterine microbiome at birth by cesarean section, and the subsequent development of the infant microbiome immediately postpartum and in the first days of life. This understanding of the physiological composition of the maternal microbiome during pregnancy and the development of the child’s microbiome should form the basis for recognizing and treating microbiome changes at an early stage in the future and subsequently for preventing premature rupture of the membranes and thus premature birth.
Methods
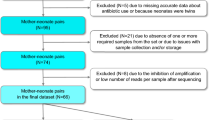
The study by Foessleitner et al. [1] is a prospective, longitudinal study that was conducted at the Medical University of Vienna at the Department of Obstetrics and Gynecology and the Department of Pediatrics and Adolescent Medicine between August 2020 and September 2021. Patients who presented between 32 + 0 and 37 + 0 weeks of pregnancy to schedule a planned cesarean section around the due date were included in the study. The following swabs were taken as part of the study: a maternal vaginal swab and a rectal swab at inclusion. At the time of the cesarean section, these were repeated, and an intraoperative swab from the fetal side of the placenta and a sterile swab from the uterine cavity were also collected. In the newborn, a buccal, a dermal, and a meconium swab were taken immediately after birth. These neonatal swabs were repeated on the second or third day of life. The sampling protocol is shown in Table 1.
The microbiome analysis was carried out in cooperation with the Joint Microbiome Facility (JMF) of the Medical University of Vienna and the University of Vienna and was performed using 16S rRNA amplicon sequencing. To guarantee the validity of the results, only samples with sufficient biomass were analyzed. The samples were analyzed longitudinally in order to detect potential differences in microbial composition between the different sampling times. Values of p < 0.05 were considered statistically significant.
Results
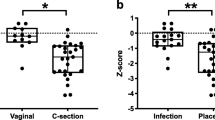
Of a total of 37 pregnant women screened, 30 mothers were included in the analysis. The average age at inclusion was 32.9 years. The birth took place at an average of 38.4 weeks’ gestation, and the newborns had an average birth weight of 3250.2 g. The maternal vaginal and rectal microbiome did not change during the third trimester and thus remained stable in their microbial composition (permutational multivariate analysis of variance [PERMANOVA]; p > 0.05). After exclusion of contamination, both the placental and intrauterine samples were sterile, meaning that no evidence of prepartum bacterial colonization was detected. Of the 18 newborns included in the analysis, 16 buccal, 15 dermal, and 14 meconium swabs showed no evidence of microbiome colonization on the day of birth. Afterwards, the neonatal microbiome developed rapidly in the first days of life. Already on the second or third day of life, the microbial colonization of the newborns was clearly different from the microbiome of the mothers, which was detected in the maternal vaginal and rectal swabs on the day of birth (PERMANOVA; p < 0.01). Furthermore, the infant microbiome was significantly different between buccal and fecal swabs (PERMANOVA; p < 0.01). The buccal microbiome mainly showed colonization with Staphylococcus and Streptococcus species, whereas the microbiome of the stool smears showed a significantly more diverse colonization with different genera of Actinobacteria, Firmicutes, and Proteobacteria. The diversity in the maternal and neonatal microbiome is shown graphically in Fig. 1 (adapted from Foessleitner et al. [1]).
Principal component analysis showing the microbial diversity of seven mother–child pairs. a Maternal microbiome compared with the infant microbiome on the second/third day of life and b infant buccal (blue) and stool smears (orange) on the second/third day of life. (Adapted from Foessleitner et al. [1], used under the Creative Commons CC-BY-NC license)
Discussion
The study by Foessleitner et al. [1] examined the composition of the vaginal and rectal microbiome in the third trimester of healthy pregnant women with an unremarkable course of pregnancy whose infants were delivered by elective cesarean section at term. Furthermore, the development of the infant’s microbiome in the first days of life was analyzed longitudinally. No significant change in the maternal vaginal and rectal microbiome was found during the last trimester. The vaginal microbiome of all pregnant women was dominated by Lactobacillus crispatus, with a low incidence of Gardnerella vaginalis. This composition has already been associated with term birth and a low incidence of preterm birth in other studies [5, 16]. Understanding the vaginal microbial colonization of healthy pregnant women is essential as this knowledge can be used in the future to identify disruptive factors that can lead to early premature rupture of the membranes [17]. It would also be desirable to define potential biomarkers in the microbiome which—if present—represent an increased risk of early premature rupture of membranes or premature birth [18]. There is a long-standing controversy regarding the microbial colonization of the placenta and uterine cavity [10, 11, 19,20,21]. Some studies have demonstrated microbial colonization of the placenta or uterine cavity, but the question that often arises is whether this is due to contamination of the samples at birth or during laboratory analyses [10, 19, 22]. In this study, two buccal, three dermal, and four meconium swabs showed microbial colonization on the day of birth, but these were dominated by a Staphylococcus species, which is often found on human skin. Therefore, it is more likely that this colonization originated from skin-to-skin contact after birth than being an intrauterine colonization, especially since no microbiome could be detected in the intrauterine and placental swabs of these mothers. Thus, the work of Foessleitner et al. supports the thesis that newborns do not exhibit microbial colonization before birth. Foessleitner and colleagues demonstrated that the development of the infant microbiome begins only after birth. In addition, they observed that the early neonatal microbiome on the second/third day of life differed significantly from that of their mothers. Therefore, it can be concluded that infant microbiome development is primarily influenced by exposure to the environment.
The strength of this study is the longitudinal study design, which made it possible to investigate the microbiome of the mother in the late phase of pregnancy and the early microbiome of the child after cesarean section of mother–child pairs. A limitation of the study is the lack of comparison of the neonatal microbiome development across different modes of delivery. However, there are already ample data indicating a difference in the infant microbiome after vaginal birth compared to that after cesarean section [9, 15, 23]. The small number of cases in this pilot study should also be mentioned as a limitation, which warrants confirmation of the results in larger studies with a higher number of cases.
Conclusion
In summary, the composition of the maternal microbiome in healthy women at the end of pregnancy was stable and without significant changes. The analysis of maternal and infant swabs on the day of birth showed no evidence of prepartum microbial colonization of the newborns in the context of an unremarkable pregnancy with elective delivery by cesarean section. After elective cesarean delivery, the neonatal microbiome appears to develop through postnatal exposure to the immediate environment. These findings provide a fundamental understanding of the physiological maternal microbiome in the third trimester, as well as the normal development of the infant microbiome after delivery by cesarean section, which will serve as a basis for further studies already underway at the Medical University of Vienna to investigate the microbiome in the context of early preterm rupture of the membranes. These studies will hopefully ultimately contribute to the identification of predictive parameters for early premature rupture of the membranes and subsequent premature birth, allowing this serious pregnancy complication to be detected early and hopefully prevented in the future.
References
Foessleitner P, Pjevac P, Granser S, Wisgrill L, Pummer L, Eckel F, et al. The maternal microbiome in pregnancy, delivery, and early-stage development of neonatal microbiome after cesarean section: A prospective longitudinal study. Acta Obstet Gynecol Scand. 2024;103:832–41.
Lamont RF, Nhan-Chang CL, Sobel JD, Workowski K, Conde-Agudelo A, Romero R. Treatment of abnormal vaginal flora in early pregnancy with clindamycin for the prevention of spontaneous preterm birth: a systematic review and metaanalysis. Am J Obstet Gynecol. 2011;205:177–90.
Di Simone N, Santamaria Ortiz A, Specchia M, Tersigni C, Villa P, Gasbarrini A, et al. Recent Insights on the Maternal Microbiota: Impact on Pregnancy Outcomes. Front Immunol. 2020;11:528202.
Stout MJ, Zhou Y, Wylie KM, Tarr PI, Macones GA, Tuuli MG. Early pregnancy vaginal microbiome trends and preterm birth. Am J Obstet Gynecol. 2017;356:e1–8.
Callahan BJ, DiGiulio DB, Goltsman DSA, Sun CL, Costello EK, Jeganathan P, et al. Replication and refinement of a vaginal microbial signature of preterm birth in two racially distinct cohorts of US women. Proc Natl Acad Sci USA. 2017;114:9966–71.
Hočevar K, Maver A, Vidmar Šimic M, Hodžić A, Haslberger A, Premru Seršen T, et al. Vaginal Microbiome Signature Is Associated With Spontaneous Preterm Delivery. Front Med. 2019;6:201.
Tabatabaei N, Eren AM, Barreiro LB, Yotova V, Dumaine A, Allard C, et al. Vaginal microbiome in early pregnancy and subsequent risk of spontaneous preterm birth: a case-control study. BJOG Int J Obstet Gynaecol. 2019;126:349–58.
Freitas AC, Bocking A, Hill JE, Money DM, VOGUE Research Group.. Increased richness and diversity of the vaginal microbiota and spontaneous preterm birth. Microbiome. 2018;6:117.
Bogaert D, van Beveren GJ, de Koff EM, Lusarreta Parga P, Balcazar Lopez CE, Koppensteiner L, et al. Mother-to-infant microbiota transmission and infant microbiota development across multiple body sites. Cell Host Microbe. 2023;31:447–460.e6.
Stinson LF, Boyce MC, Payne MS, Keelan JA. The Not-so-Sterile Womb: Evidence That the Human Fetus Is Exposed to Bacteria Prior to Birth. Front Microbiol. 2019;10:1124.
Li Y, Toothaker JM, Ben-Simon S, Ozeri L, Schweitzer R, McCourt BT, et al. In utero human intestine harbors unique metabolome, including bacterial metabolites. JCI Insight. 2020;5:e138751.
Huurre A, Kalliomäki M, Rautava S, Rinne M, Salminen S, Isolauri E. Mode of delivery—effects on gut microbiota and humoral immunity. Neonatology. 2008;93:236–40.
Cho CE, Norman M. Cesarean section and development of the immune system in the offspring. Am J Obstet Gynecol. 2013;208:249–54.
Reyman M, van Houten MA, van Baarle D, Bosch AATM, Man WH, Chu MLJN, et al. Impact of delivery mode-associated gut microbiota dynamics on health in the first year of life. Nat Commun. 2019;10:4997.
Kim H, Sitarik AR, Woodcroft K, Johnson CC, Zoratti E. Birth Mode, Breastfeeding, Pet Exposure, and Antibiotic Use: Associations With the Gut Microbiome and Sensitization in Children. Curr Allergy Asthma Rep. 2019;19:22.
Chu DM, Seferovic M, Pace RM, Aagaard KM. The microbiome in preterm birth. Best Pract Res Clin Obstet Gynaecol. 2018;52:103–13.
Paramel Jayaprakash T, Wagner EC, van Schalkwyk J, Albert AYK, Hill JE, Money DM, et al. High Diversity and Variability in the Vaginal Microbiome in Women following Preterm Premature Rupture of Membranes [PPROM]: A Prospective Cohort Study. PLoS ONE. 2016;11:e166794.
Klindworth A, Pruesse E, Schweer T, Peplies J, Quast C, Horn M, et al. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013;41:e1.
Kennedy KM, de Goffau MC, Perez-Muñoz ME, Arrieta MC, Bäckhed F, Bork P, et al. Questioning the fetal microbiome illustrates pitfalls of low-biomass microbial studies. Nature. 2023;613:639–49.
Rackaityte E, Halkias J, Fukui EM, Mendoza VF, Hayzelden C, Crawford ED, et al. Viable bacterial colonization is highly limited in the human intestine in utero. Nat Med. 2020;26:599–607.
Kennedy KM, Gerlach MJ, Adam T, Heimesaat MM, Rossi L, Surette MG, et al. Fetal meconium does not have a detectable microbiota before birth. Nat Microbiol. 2021;6:865–73.
Salter SJ, Cox MJ, Turek EM, Calus ST, Cookson WO, Moffatt MF. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC Biol. 2014;12:87.
Bäckhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe. 2015;17:690–703.
Funding
Open access funding provided by Medical University of Vienna.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S. Granser and P. Foessleitner declare that they have no competing interests, besides being authors of the original publication of the presented study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Notes
The article presented here is originally a German overview version and interpretation of the paper “The maternal microbiome in pregnancy, delivery, and early-stage development of neonatal microbiome after cesarean section: A prospective longitudinal study” by Foessleitner et al. [1] published in Acta Obstetricia et Gynecolgica Scandinavica in January 2024. Dr. Granser and Dr. Foessleitner are also authors of the original paper, and a table and parts of a graphic from this paper have been used with the authors’ permission and under the Creative Commons CC-BY-NC license. For the non-German-speaking readership, the article has been translated from German to English by the publisher.
Link to the original work: https://obgyn.onlinelibrary.wiley.com/doi/https://doi.org/10.1111/aogs.14773
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Granser, S., Foessleitner, P. The maternal microbiome in normal pregnancy and at delivery by cesarean section and the early developmental phase of the neonatal microbiome—presentation of a longitudinal pilot study. Allergo J Int (2024). https://doi.org/10.1007/s40629-024-00303-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40629-024-00303-x