Abstract
Germination, one of the earliest events in the plant life cycle, is a complex process in which the seed must physically recover from maturation drying, resume a sustained metabolic intensity, complete essential cellular events that allow for embryo emergence and prepare for subsequent seedling growth. Among the biochemical changes, compounds such as amino acids, proteins, soluble sugars and polyamines (PAs) presented relevant roles during seed development and germination. The aim of this work was to study the alterations in the content of free amino acids, PAs, soluble sugars and proteins during germination of Cedrela fissilis Vellozo (Meliaceae). The content of amino acids, soluble sugars and PAs were determined by high-performance liquid chromatography, and the soluble protein in ethanol content was quantified using a 2-D Quant Kit. A triphasic pattern of germination was observed, and germination was completed with radicle protrusion on day seven of incubation. A significant decrease in the soluble proteins in ethanol and increase in the total free amino acid content during germination (5–7 days) suggests that amino acids might be provided by the mobilization of stored proteins in mature seeds. Among soluble sugars, sucrose presented the highest content in mature seeds decreasing significantly during germination, whereas glucose and fructose were only detected in seedlings, suggesting that the degradation of sucrose to these monosaccharides is important for seedling growth. Endogenous free spermidine (Spd) and spermine (Spm) are significantly mobilized during germination, while the PA ratio [Put/(Spd + Spm)] significantly increased in seedlings, due to the significant increase in putrescine (Put) contents, which is involved with cell division for seedling growth. Our results revealed changes in the contents and forms of the studied compounds, suggesting the involvement of these biomolecules in C. fissilis seed germination and early seedling growth. The results provide additional data on the biochemical and physiological changes that occur during seed germination, particularly in endangered hardwood species from Brazilian Atlantic Forest.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Seed germination is one of the earliest events in the plant life cycle, and the transition from seed to seedling is one of the most drastic developmental processes in plants. Plants change from heterotrophic to autotrophic and from quiescent to active, and although they are initially protected within a seed coat, they are then exposed to ambient conditions (Donohue et al. 2010). Germination is a complex process in which the seed is physically capable to recover from drying, take up a sustained metabolic intensity, complete essential cellular events for the embryo emergence and, overall, adjust the whole metabolism for subsequent seedling growth (Obroucheva et al. 2006; Nonogaki et al. 2010).
To achieve germination, the seed employs three main phases: imbibition (phase I), germination sensu stricto (true germination process, phase II), and seedling growth (phase III) (Bewley 1997). Germination is considered complete when the radicle protrudes from the covering structures. Water uptake results in the resumption of respiratory activity and protein synthesis using extant mRNAs (phase I). Phase II is the most important phase, and it is associated with several cellular and biochemical events, including DNA repair and protein synthesis, the latter of which depends on the translation of de novo-synthesized RNA, as well as changes in the soluble sugar content (Bewley 1997).
Following the imbibition, in the dry seed, the re-initiation of metabolism and reestablishment of chemicals and cellular structural integrity require the integration of events involved in synthesis and protection. Protein synthesis and respiration initially involve components stored within the mature dry seed, although transcription and translation commence early in imbibition (Nonogaki et al. 2010). Alterations or modifications of hormones, especially abscisic acid (ABA) and gibberellin, play an important role during germination (Nonogaki et al. 2010). In woody species, compounds such as amino acids, proteins, carbohydrates and polyamines (PAs) are also important during seed development and germination (Astarita et al. 2003; Santa-Catarina et al. 2006; Dias et al. 2009; Balbuena et al. 2009; Pieruzzi et al. 2011).
Proteins that were synthesized and stored during seed maturation are degraded into free amino acids for biosynthesis and energy generation (Onomo et al. 2010; Alhadi et al. 2012; Tan-Wilson and Wilson 2012). Seed storage proteins can be divided into four classes based on their solubility. Albumins are water soluble, globulins are salt soluble, prolamines are soluble in ethanol solutions, and glutelins are soluble in alkali or acid (Shotwell and Larkins 1989; Krishnan and White 1995). Prolamines are stored in the form of polymers or aggregates, referred to as protein bodies (Mitsukawa et al. 1999). In Oryza sativa, these proteins are accumulated during endosperm development and further utilized as a source of nitrogen, carbon, and sulfur for the young developing seedling (Kim and Okita 1988). In woody species, these proteins have been mobilized during the germinative process (Dantas et al. 2008). The mobilization of seed storage proteins following seed imbibition (phase I) and germination (phase II) is crucial for the establishment of seedlings (phase III). In certain species, such as Ocotea catharinensis, alterations in the amino acid content during seed development and germination have been observed (Santa-Catarina et al. 2006; Dias et al. 2009). These results suggest the involvement of amino acids in the synthesis of proteins such as late embryogenesis abundant (LEA) proteins, which are important for desiccation tolerance (Santa-Catarina et al. 2006).
Carbohydrates are involved in cross-talk with hormone signaling networks to modulate critical growth processes, such as embryo establishment, seed germination and seedling growth (see Eveland and Jackson 2012). During these processes, carbohydrates act as substrates for intermediary metabolism, supplying the energy necessary for initial growth and seedling development (Peterbauer and Richter 2001; Tahir et al. 2011), or they act as signaling molecules (Smeekens et al. 2010). The mobilization of carbohydrates during germination is highly dynamic (Ferreira et al. 2009; Carrijo et al. 2010), and alterations in the profiles of these compounds have been observed in mature seeds and during germination (Borek et al. 2006).
In plants, the PAs putrescine (Put), spermidine (Spd), and spermine (Spm) act as regulatory molecules in several cellular processes, including cell division, cell differentiation and proliferation, cell death, DNA and protein synthesis and gene expression (Bouchereau et al. 1999; Kusano et al. 2008; Álcazar et al. 2010; Cai et al. 2015). These PAs have been implicated in abiotic and biotic plant stress responses as well as many physiological processes (Baron and Stasolla 2008; Kusano et al. 2008; Álcazar et al. 2010), including seed development and germination (Astarita et al. 2003; Santa-Catarina et al. 2006; Dias et al. 2009; Balbuena et al. 2009; Pieruzzi et al. 2011; Krasuska et al. 2014).
Cedrela fissilis Vellozo is a native woody species in the Atlantic Forest. Because of its economic importance, especially for wood production, this species has been heavily exploited and is currently included in the endangered category of the Red List of Threatened Species published by the International Union for Conservation of Nature (2014). C. fissilis seeds are considered orthodox (Carvalho et al. 2006), although their reduced viability during storage has been problematic for seed conservation and reforestation program development (Carvalho 2003). Therefore, studies on the biochemical aspects of seed development and germination could contribute to the conservation of this endangered species.
In this present work, the contents of amino acids, soluble proteins in ethanol, soluble sugars and PAs during germination and initial growth seedling in C. fissilis were analyzed, and the relationship between these compounds during germination was studied. The results illustrate the physiological changes that occur during the germination process in this species, and the data provided here will be useful for monitoring seed germination during storage and improve research on the somatic embryogenesis of woody species for use in conservation programs.
2 Materials and methods
2.1 Seed germination
Mature seeds of C. fissilis Vellozo were obtained in October 2011 from the Sementes Caiçara Nursery, which is located in Brejo Alegre, Sao Paulo State, Brazil (21°10′S and 50°10′W). Prior to imbibition, the seeds were surface-disinfected according to the method of Santa-Catarina et al. (2001). The seeds were washed with 250 ml distilled water and then immersed in 70 % (v/v) ethanol for 1 min and commercial bleach Qboa® (Anhembi SA, Osasco, Brazil) containing 2.0–2.5 % active chlorine supplemented with the fungicide Derosal® (Bayer, São Paulo, Brazil) (200 µl Derosal® per 1000 ml solution) for 30 min. The seeds were subsequently washed five times with distilled and autoclaved water and then placed in Petri dishes containing two layers of germibox paper with 10 ml distilled and autoclaved water containing Derosal® (200 µl Derosal®/1000 ml solution). The seeds were incubated in a growth chamber with a photoperiod of 16 h, light intensity of 22 μmol m2 s−1, and temperature of 25 ± 2 °C. The percentage of germination was obtained after 20 days from seven replicates with 10 seeds per replicate.
The germination phase establishment and sampling times were defined in previous assays. Germination process was monitored daily over 7 days and growth seedling observed until the 20th day. For the biochemical analyses, triplicate seed samples were collected prior (mature seed—time 0) and at 2, 5, 7, 10 and 17 days of imbibition (Fig. 1a). Each triplicate sample was obtained from a bulk sample of at least 100 seeds. The seed tegument was removed, and samples consisting of 200 mg fresh mass (FM) were collected for amino acid, protein and PA analyses, and samples consisting of 300 mg FM were collected for carbohydrate analyses. The samples were stored at −20 °C until further biochemical analysis.
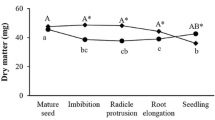
Morphological aspects of C. fissilis seeds during germination and early seedling development (a) and imbibition curve after 2, 5, 7, 10 and 17 days of imbibition (b). P1 phase I, characterized by the rapid absorption of water and reactivation of metabolism; P2 phase II, characterized by slow water absorption and completed with radicle protrusion; P3 phase III (final phase), characterized by continuous water absorption and seedling growth. DM dry mass, FM fresh mass, and WC water content. Capital letters denote significant differences in each analysis during the days of imbibition. Means followed by different letters are significantly different (P < 0.05) according to the SNK test (n = 7; CV FM = 4.6 %; CV DM = 8.5 %; CV WC = 7.0 %). CV coefficient of variation. Bars in a = 0.5 cm
The water content (WC) during germination was analyzed by evaluating the FM and dry mass (DM) of the seeds prior (mature seed) and at 2, 5, 7, 10 and 17 days after imbibition. For DM analyses, the seeds were weighed and maintained at 105 °C for 24 h. The WC was determined using the following formula: WC = FM − DM. Seven replicates with 10 seeds per replicate were used for each analysis.
2.2 Free amino acids and soluble proteins in ethanol determination
The amino acid content was analyzed according to the method of Santa-Catarina et al. (2006). Amino acid samples were prepared in biological triplicate and separated using high-performance liquid chromatography (HPLC) on a reversed-phase C18 column (Shim-pack CLC ODS, Shimadzu Corporation, Kyoto, Japan). The peak areas and retention times of each amino acid were evaluated through comparisons with known concentrations of aspartic acid, glutamic acid, asparagine, serine, glutamine, histidine, glycine, arginine, threonine, alanine, γ-aminobutyric acid (GABA), tyrosine, methionine, tryptophan, valine, phenylalanine, isoleucine, leucine, ornithine and lysine.
The contents of soluble proteins in ethanol were quantified using ethanolic extracts from the amino acid extractions and performed using the 2-D Quant Kit (GE Healthcare, Uppsala, Sweden) according to the manufacturer’s recommendations.
2.3 Soluble sugars determination
The soluble sugars content was determined according to the method of Filson and Dawson-Andoh (2009). The samples (300 mg FM) were ground in 1 ml extraction solution containing 80 % (v/v) ethanol (Merck), 3 % (m/v) polyvinylpolypyrrolidone (Sigma-Aldrich) and 1 % (m/v) ascorbic acid (Sigma-Aldrich) at 4 °C. The extracts were then incubated at 70 °C for 90 min. Following centrifugation at 20,000×g for 10 min, the supernatant was removed, and the pellets were then re-extracted with 1 ml extraction solution and centrifuged again at 20,000×g for 10 min. The supernatants were combined and filtered through a 20 µm membrane, and the carbohydrate content was identified using HPLC (Shimadzu) with an evaporative light scattering detector (ELSD-LT II—Shimadzu) at a temperature of 40 °C, nitrogen gas pressure of 350 MPa, gain of 9 and a filter setting of 4. A Prevail Carbohydrate ES (Alltech Associates, Deerfield, IL, USA) 5 µm (250 × 4.6 mm) HPLC column and Prevail Carbohydrate ES (Alltech Associates) 5 µm (7.5 × 4.6 mm) pre-column were used. The gradient was achieved by mixing decreasing proportions of absolute acetonitrile (Merck) with water. The acetonitrile gradient was programmed as follows: 80 % during the first 16 min, 80–70 % between 16 and 23 min and 70 % from 23 to 30 min. The flow rate was 1 ml min−1 at 25 °C. A 5 µl sample was injected, and the peak areas and retention times were measured by comparison with known quantities of carbohydrate standards containing sucrose, fructose, and glucose (Sigma-Aldrich).
2.4 Free polyamine (PA) determination
The free PA content was determined according to the method of Santa-Catarina et al. (2006). PA samples were prepared in biological triplicate. PAs were separated by HPLC using a reversed-phase C18 column (Shim-pack CLC ODS, Shimadzu Corporation, Kyoto, Japan), and the peak areas and retention times of each PA were evaluated by comparison with known concentrations of Put, Spd and Spm, which are the major PAs found in plants.
2.5 Statistical analyses
The data were analyzed by an analysis of variance (ANOVA) (P < 0.05) followed by the Student–Newman–Keuls (SNK) test (Sokal and Rohlf 1995) using the software program SAEG® v.9.1 (Fundação Arthur Bernardes, Viçosa, Brazil).
3 Results
3.1 Germination
During C. fissilis seed imbibition, a triphasic development pattern was observed (Fig. 1b). The first phase (P I) began at the mature seed and lasted until the second day of imbibition when rapid water absorption occurred. The second phase (P II) began on day two and was characterized by a reduction and stabilization of water absorption, and it was completed on day seven of incubation when radicle protrusion occurred. The third phase (P III) began after day seven and was characterized by continued water absorption and post-germinative events. During imbibition, the FM showed a pattern similar to that of the WC with a significant increase and higher values on the 17th day of seedling development (Fig. 1b). The DM value remained constant until day seven (Fig. 1b), and then a significant increase occurred. The germination percentage obtained in conditions of this experiment was 83 %.
3.2 Free amino acids and soluble proteins in ethanol
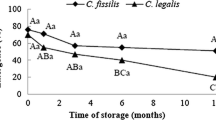
In C. fissilis, a significant increase in the total free amino acid content (Fig. 2) was observed on the 2nd day of imbibition (phase I) and similar values occurred during radicle protrusion (phase II) until the 10th day of imbibition. Moreover, a significant decrease in the total free amino acids occurred during seedling development in C. fissilis on the 17th day of imbibition.
Free amino acid contents (mg g−1 FM) and soluble proteins in ethanol (mg g−1 FM) in seeds of C. fissilis before (mature seed—time 0) and after 2, 5, 7, 10 and 17 days of imbibition. Lower-case letters denote significant differences in the soluble proteins in ethanol contents during the days of imbibition. Capital letters denote significant differences in the total free amino acid contents during the days of imbibition. Means followed by different letters are significantly different (P < 0.05) according to the SNK test (n = 3; CV total free amino acids = 20.2 %; CV soluble proteins in ethanol = 6.8 %). CV coefficient of variation
Among the analyzed amino acids, threonine was predominant during C. fissilis seed germination and early seedling growth (Table 1), and the contents of citrulline, glycine, lysine and ornithine were higher in the mature seed than at the onset of imbibition (Table 1). However, certain amino acids, such as aspartic acid, glutamic acid, glutamine, arginine, asparagine, serine, GABA, alanine, histidine, valine, leucine, phenylalanine and isoleucine, were significantly higher in the seedlings of C. fissilis (phase III) compared with the mature seed and phases I and II (Table 1). Among the amino acids, asparagine, aspartic acid, serine, GABA, valine, phenylalanine, leucine, isoleucine and alanine were observed in significant reductions on the 7th day during radicle protrusion followed by a significant increase during early seedling development on the 17th day (Table 1). The glutamine content did not change significantly during the imbibition phase (phase I), but an increase occurred after radicle protrusion, reaching the highest value on the 17th day of imbibition (Table 1).
A significant reduction was observed in the content of soluble proteins in ethanol content until 5th day, prior to radicle protrusion, and an increase occurred on the 7th day during radicle protrusion, with similar values maintained during early seedling growth (Fig. 2).
3.3 Soluble sugars contents
The soluble sugars sucrose, fructose and glucose were analyzed during C. fissilis germination and early growth seedling (Fig. 3). The higher sucrose content (Fig. 3a) was observed in mature C. fissilis seeds before imbibition; this value decreased significantly during germination (phases I and II) and early seedling development (phase III). However, fructose and glucose were only detected in C. fissilis after germination, from 10 to 17 days, during early seedling development.
Soluble sucrose (a), fructose (b) and glucose (c) contents (mg g−1 FM) in seeds of C. fissilis before (mature seed—time 0) and after 2, 5, 7, 10 and 17 days of imbibition. Capital letters denote significant differences in the content of each carbohydrate during the days of imbibition. Means followed by different letters are significantly different (P < 0.05) according to the SNK test (n = 3; CV sucrose = 8.5 %; CV fructose = 6.6 %; CV glucose = 17.1 %). CV coefficient of variation
3.4 Free polyamines (PAs) contents
The free PAs Put, Spd and Spm were identified in germination and early growth seedling in C. fissilis (Fig. 4a–c). The Spd contents decreased significantly during imbibition (phase I), maintained a similar content until seed germination on the 7th day (phase II), and increased significantly during seedling development (Fig. 4b). Free Put (Fig. 4a) presented the second-highest content among the PAs identified during germination process (phases I and II), and the content increased significantly in early seedling growth (Fig. 4a). In contrast, the Spm contents decreased significantly between imbibition (phase I), germination (phase II) and early seedling development (Fig. 4c). Among the free PAs, a higher content of Spd (Fig. 4b) occurred during germination than Put (Fig. 4a) and Spm (Fig. 4c).
Free PAs Put (a), Spd (b), Spm (c) contents (µg g−1 FM) and PA [Put/(Spd + Spm)−1] ratio (d) in seeds of C. fissilis before (mature seed—time 0) and after 2, 5, 7, 10 and 17 days of imbibition. Capital letters denote significant differences in each PA during the days of imbibition. Means followed by different letters are significantly different (P < 0.05) according to the SNK test (n = 3; CV Put = 10.8 %; CV Spd = 6.4 %; CV Spm = 9.8 %; CV PA ratio = 13.6 %). CV coefficient of variation
The PA ratio [Put/(Spd + Spm)] did not change throughout the germination process (phases I and II); however, on day 17 of seedling development, a significant increase in this ratio was observed (Fig. 4d) due of the increased Put contents (Fig. 4a).
4 Discussion
Seed germination is a complex process that starts with water absorption by quiescent seed and ends with radicle protrusion (Bewley 1997), and all of the subsequent events are classified as post-germinative (Gallardo et al. 2002). Germination in C. fissilis ends with radicle protrusion on the 7th day of imbibition (Fig. 1b) and a triphasic development pattern is observed (Bewley and Black 1994). In C. fissilis, the first phase (phase I) began with the mature seed and lasted until the second day of imbibition when rapid and significant water absorption occurred (Fig. 1b). According to Bove et al. (2001), rapid imbibition in phase I results in the re-initiation of basal metabolism. During this phase, which is known as physical imbibition, a step-by-step activation of metabolic pathways is caused by a gradual increase in hydration. When the level of hydration exceeds 60 %, the rate of hydration slows (phase II) and new physiological mechanisms enable cell expansion along the embryonic axes, which culminates in the onset of cell elongation (Bove et al. 2001). This process most likely occurs in preparation for the cellular elongation that results in radicle protrusion (phase II) at day seven in C. fissilis. In this phase, osmotically active substances, such as sugars, amino acids and potassium ions, accumulate, and acidification of the cell wall leads to a loosening of the bonds between cell-wall polymers. These events coincide with the activation of H+-ATPases in the plasmalemma, which results in a further increase in water uptake that might coincide with the weakening of the surrounding tissues (endosperm) as the embryonic axes elongate and germination is completed (Bove et al. 2001). In addition, the imbibition curve in C. fissilis shows significant increases in WC and FM during all phases, although these effects are more pronounced in phases I and III and increases in DM in phase III (Fig. 1b), most likely because of radicle elongation and subsequent seedling growth.
Certain biomolecules synthesized and stored during seed development, such as proteins and amino acids, are mobilized during germination and early seedling growth (Gallardo et al. 2002; Penfield et al. 2005; Catusse et al. 2008). In C. fissilis, a significant decrease in soluble proteins in ethanol and increase in total amino acids during the first 5–7 days were observed (Fig. 2), suggesting that amino acids might be produced by the mobilization of storage proteins in mature seeds during germination in C. fissilis. Proteins that were synthesized and stored in the mature seed are broken down into free amino acids for biosynthesis and energy generation (Tan-Wilson and Wilson 2012). The soluble proteins in ethanol could be prolamines (Shotwell and Larkins 1989; Krishnan and White 1995), being described as the source of energy for the seedling development in O. sativa (Kim and Okita 1988). Similarly to C. fissilis, in Caesalpinia pyramidalis, a woody species, the contents of prolamines decreased after the start of imbibition and increased in phase II (Dantas et al. 2008). Furthermore, the increase in total free amino acid content observed in C. fissilis might be associated with the role of these compounds as osmolytes, which are required to complete the germination process. According to Bove et al. (2001), physiological processes occur prior to root elongation to prepare the embryo for growth, and the level of osmotically active substances, such as amino acids, increases and may enhance the entry of water into the cell and contribute to root elongation.
In addition, storage nutrients in the cotyledon and/or endosperm, such as lipids, proteins or starch, begin to mobilize before germination ends and are used during post-germinative events to sustain the young plant in its early growth stages before it becomes autotrophic (Bove et al. 2001). Therefore, the significant decrease in the total free amino acid content in phase III from the 7th to 17th day (Fig. 2) might be associated with post-germinative events to support seedling growth in C. fissilis.
Among the analyzed amino acids, threonine, which is an essential amino acid and one of the products of the aspartic acid pathway, was predominant during C. fissilis seed germination process and early growth seedling (Table 1); this amino acid plays an important role during plant growth and development (Jander and Joshi 2010) and is involved in important metabolic pathways, including cellular energy production and photorespiration in plants (Joshi et al. 2006; Kang et al. 2006). Other free amino acids are predominant during germination in various species, such as asparagine in O. catharinensis (Dias et al. 2009) and GABA in Cola acuminata and Cola anomala (Onomo et al. 2010), suggesting that the predominance of a particular amino acid might be species-dependent.
The contents of certain free amino acids, such as aspartic acid, glutamic acid, glutamine, arginine and asparagine (Table 1), were significantly higher in seedlings in phase III. These results suggest that the increased amino acid content during early seedling growth in C. fissilis could be associated with nitrogen availability to support growth. This hypothesis is based on these amino acids acting as nitrogen donors and amino acid transporters in plant growth and their involvement in the biosynthesis of other amino acids, such as lysine, threonine, isoleucine and methionine (Azevedo et al. 2006). In addition, a significant reduction in glutamic acid content during imbibition and radicle protrusion in C. fissilis (Table 1) suggests that this amino acid is involved in the interconversion of other amino acids during imbibition and germination (phases I and II) and may be a precursor for glutamine and proline synthesis, which are used as substrates for respiratory enzymes during imbibition (Rocha et al. 2010). Glutamine content increased after radicle protrusion and reached high values during early seedling development of C. fissilis (Table 1); because glutamine is a nitrogen and transporter amino acid in plants, this behavior suggests that it is necessary for seedling growth (Oliveira et al. 2001).
GABA is a non-protein amino acid that is synthesized through glutamic acid decarboxylation (Satya-Naraian and Nair 1990) and the PA pathway (Bouchereau et al. 1999). This amino acid is associated with several processes in plants, including cell proliferation and embryo differentiation in carrot (Daucus carota) cultures (Kamada and Harada 1984); cellular differentiation and embryogenic competence acquisition during somatic embryogenesis development in Acca sellowiana (Booz et al. 2009); zygotic embryo development in O. catharinensis (Santa-Catarina et al. 2006); nitrogen storage in pea (Pisum sativum var. Esla), bean (Phaseolus vulgaris var. La Granja) and lentil (Lens culinaris var. Castellana) seeds (Rodriguez et al. 2008); and germination in C. acuminata, C. anomala (Onomo et al. 2010) and C. fissilis (Table 1). GABA is synthesized de novo during early seedling growth, and its significant reduction during phases I and II and significant increase in phase III (Table 1) suggests that this amino acid might play an important role in the events prior to radicle protrusion (germination) in C. fissilis. In addition these observations suggest an important role of GABA during seed development and germination, although its precise role remains poorly understood.
Tyrosine degradation is associated with alanine and glutamic acid production (Alhadi et al. 2012). A reduction in tyrosine content corresponded with an increase in alanine content on day five of imbibition in C. fissilis, which may have been caused by tyrosine degradation and alanine production. Furthermore, the increased tyrosine contents might have been associated with the inhibition of ABA synthesis, which is responsible for the suppression of germination (Ghelis et al. 2008). In addition, an increase in arginine content (Table 1) might be related to germination in C. fissilis as demonstrated by Alhadi et al. (2012). These authors showed that increased arginine contents promote the synthesis of gibberellins, which are germination promoters.
Carbohydrates are a source of cellular energy and act as osmolytes, which maintain the integrity of the plasma membrane, as well as signaling molecules in plants (Smeekens et al. 2010). The higher sucrose content observed in mature C. fissilis seeds (Fig. 3a), might be associated with the accumulation of this carbohydrate during embryonic development (Focks and Benning 1998). Higher sucrose contents and lower monosaccharide contents were observed in the mature orthodox seeds of certain species (Obendorf 1997; Kuo et al. 1998). During imbibition (phase I) and germination (phase II), a significant decrease in sucrose contents occurred in the seeds of C. fissilis (Fig. 3a). High contents of sucrose are considered important for the maintenance of osmotic status in cells and may favor the process of imbibition (Pastorini et al. 2003). The aforementioned authors demonstrated that sucrose is stored in the final stages of embryo development in Arabidopsis thaliana and subsequently used during germination. Specific growth and metabolic responses might be activated and/or modulated based on the nature of the sugar signal. Sucrose, the primary transport sugar in plants, can be sensed as a direct signal (Chiou and Bush 1998).
Alternatively, a signal may be produced because of its hexose cleavage products, such as glucose or uridine diphosphate (UDP)-glucose and fructose (Rolland et al. 2006; Li et al. 2011). The decrease in sucrose content observed in C. fissilis seedlings (phase III) might be associated with its degradation to fructose (Fig. 3b) and glucose (Fig. 3c), which were only detected after germination; however, these monosaccharides might be more relevant during seedling growth. Our results are consistent with those observed by Obroucheva et al. (2006) during germination in Aesculus hippocastanum. These authors demonstrated that sucrose content was higher in the beginning, whereas fructose and glucose increased at the end of germination (Obroucheva et al. 2006). Taken together, these results suggest a relationship between the contents of these soluble sugars and seed germination in certain species, including C. fissilis.
In plants, glucose has been highlighted as a signaling molecule in several processes associated with growth and development, such as germination, hypocotyl elongation, cotyledon expansion and leaf development (Rolland et al. 2006). In C. fissilis, glucose was only observed during early seedling development (Fig. 3c), suggesting that this sugar might not be required for germination process in this species. The exogenous addition of glucose has been shown to inhibit the germination and initial development of seedlings (Price et al. 2003; Gibson 2005; Hu et al. 2012). Studies have demonstrated that hexoses such as glucose and fructose show a greater signaling potential in the promotion of cell proliferation and organ growth. Thus, these sugars are observed during seedling growth, whereas sucrose is typically associated with differentiation and seed maturation (Borisjuk et al. 2004; Koch 2004).
In addition to soluble sugars, PAs are also important compounds that modulate several physiological and biochemical processes in plants (Kusano et al. 2008; Álcazar et al. 2010). Among the free PAs, Put, Spd and Spm were identified during C. fissilis germination process and early growth seedling (Fig. 4). The Spd and Spm contents were higher than Put in mature seeds before imbibition, suggesting that these PAs are accumulated in late seed development in this species. Similar results were observed in the seeds of Araucaria angustifolia (Astarita et al. 2003) and O. catharinensis (Santa-Catarina et al. 2006), and higher contents of Spd and Spm were observed in the final phase of seed development and might have been associated with cell elongation as well as desiccation tolerance (Astarita et al. 2003; Santa-Catarina et al. 2006). In O. catharinensis, a reduction in Put and increase in Spm might be important in the final stage of zygotic embryo development when cell elongation occurs (Santa-Catarina et al. 2006). In addition, Zea mays seeds showed high contents of Spd and Spm at the end of seed development, suggesting that these PAs are important during this phase (Cao et al. 2010). This pattern of PA content is similar in the seeds of several species, suggesting the role of these compounds in germination.
Free Spd contents (Fig. 4b) were higher throughout germination process (phases I and II) in C. fissilis than free Put (Fig. 4a) and Spm (Fig. 4c). These results suggest that the role of free Spd in germination of C. fissilis is similar to that observed in A. thaliana, where higher Spd contents were observed relative to other PAs (Puga-Hermida et al. 2006). Similar results were also observed in Malus domestica seeds (Sinska and Lewandowska 2006). In Arabidopsis, the addition of exogenous Put (but not Spd or Spm) resulted in the inhibition of germination (Mirza and Bagni 1991).
Furthermore, the significant increase in free Put (Fig. 4a) and decrease of Spm (Fig. 4c) contents in seedlings of C. fissilis on day 17 (Fig. 4a, c) might be associated with cell division processes during seedling growth. Matilla (1996) associated cellular differentiation with higher contents of Spd and Spm, whereas Put content appeared to be more closely associated with cell division. In addition, the activity of diamine oxidase (DAO), a Put-degradation enzyme, was significantly higher in the shoots relative to the other organs during seedling development in fava bean (Vicia faba) (Yang et al. 2011). Although DAO activity was not evaluated in this work, the reduction of Put contents (Fig. 4a) might be related to the degradation of Put and production of GABA (Table 1) during germination in C. fissilis since Put degradation by DAO leads to GABA biosynthesis (Bhatnagar et al. 2001).
The PA ratio [Put/(Spd + Spm)] could be used as a biochemical marker (Shoeb et al. 2001) of the different stages of embryo development (Santa-Catarina et al. 2006) and germination (Pieruzzi et al. 2011). A significant increase in this ratio (Fig. 4d) during early seedling development in C. fissilis occurred due of the higher Put content (Fig. 4a), indicating a high regeneration capacity of seedling. These results indicate that Put plays an important role in promoting the cell cycle and mitotic divisions necessary for seedling growth in C. fissilis, wherein Put is essential for cellular division as suggested by Maki et al. (1991). These authors demonstrated that the inhibition of Put biosynthesis halted cellular division and inhibited G1/S phase progression in suspension cultures of Catharanthus roseus.
This work is the first to report an association between free amino acids, free PAs, soluble sugars, as well as soluble proteins in ethanol, during seed germination process and early growth seedling of C. fissilis. Soluble proteins are mobilized during germination (phases I and II), which is related with the significant increase of total free amino acids contents at the same time. In addition, a significant degradation of sucrose was observed during seed germination (phase I and II), possibly to supply fructose and glucose for seedling growth (phase III). Moreover, endogenous Spd and Spm are significantly mobilized during germination (phase I), while Put seems important for seedling growth (phase III), possible by the promotion of cell divisions necessary at this time. We conclude that changes in the contents of these biomolecules are associated with seed germination and early seedling growth in this specie. These results will be useful to further studies on seed storage and germination in orthodox tree species, as well as to establish a network of these compounds in the regulation of germination, increasing our knowledge about this process in native endangered species from Brazilian Atlantic Forest. In addition, further researches related to enzymes involved in amino acid, PA and carbohydrate metabolism might contribute to a better understanding of the metabolism of these compounds during the germination of C. fissilis.
Abbreviations
- ABA:
-
Abscisic acid
- ANOVA:
-
Analysis of variance
- DAO:
-
Diamine oxidase
- DM:
-
Dry matter
- FM:
-
Fresh matter
- GABA:
-
γ-Aminobutyric acid
- HPLC:
-
High performance liquid chromatography
- OPA:
-
o-Phthaldialdehyde
- PA:
-
Polyamine
- Put:
-
Putrescine
- Spd:
-
Spermidine
- Spm:
-
Spermine
- WC:
-
Water content
References
Álcazar R, Altabella T, Marco F, Bortolotti C, Reymond M, Koncz C, Carrasco P, Tiburcio AF (2010) Polyamines: molecules with functions in planta biotic stress tolerance. Planta 231:1237–1249
Alhadi FA, Al-Asbahi AAS, Alhammadi ASA, Abdullah QAA (2012) The effects of free amino acids profiles on seeds germination/dormancy and seedlings development of two genetically different cultivars of Yemeni pomegranates. J Stress Physiol Biochem 8:114–137
Astarita LV, Handro W, Floh EIS (2003) Changes in polyamines content associated with zygotic embryogenesis in the Brazilian pine, Araucaria angustifolia (Bert.) O. Ktze. Rev Bras Bot 26:163–168
Azevedo RA, Lancien M, Lea MPJ (2006) The aspartic acid metabolic pathway, an exciting and essential pathway in plants. Amino Acids 30:143–162
Balbuena TS, Silveira V, Junqueira M, Dias LLC, Santa-Catarina C, Shevchenko A, Floh EIS (2009) Changes in the 2-DE protein profile during zygotic embryogenesis in the Brazilian Pine (Araucaria angustifolia). J Prot 72:337–352
Baron K, Stasolla C (2008) The role of polyamines during in vivo and in vitro development. In Vitro Cell Dev Biol Plant 44:383–395
Bewley JD (1997) Seed germination and dormancy. Plant Cell 9:1055–1066
Bewley JD, Black M (1994) Seeds: physiology of development and germination, 2nd edn. Plenum Press, New York
Bhatnagar P, Glasheen BM, Bains SK, Long SL, Minocha R, Walter C, Minocha SC (2001) Transgenic manipulation of the metabolism of polyamines in poplar cells. Plant Physiol 125(4):21–39
Booz MR, Kerbauy GB, Guerra MP, Pescador P (2009) The role of γ-aminobutyric acid (Gaba) in somatic embryogenesis of Acca sellowiana Berg. (Myrtaceae). Braz J Plant Physiol 21(4):271–280
Borek S, Ratajczak W, Ratajczak L (2006) Ultrastructural and enzymatic research on the role of sucrose in mobilization of storage lipids in germinating yellow lupine seeds. Plant Sci 170:441–452
Borisjuk L, Rolletschek H, Radchuk R, Weschke W, Wobus U, Weber H (2004) Seed development and differentiation: a role for metabolic regulation. Plant Biol 6:375–386
Bouchereau A, Aziz A, Larher F, Martin-Tanguy J (1999) Polyamines and environmental challenges: recent development. Plant Sci 140:103–125
Bove J, Jullien M, Grappin P (2001) Functional genomics in the study of seed germination. Genome Biol 3:1002.1–1002.5
Cai G, Sobieszczuk-Nowicka E, Aloisi I, Fattorini L, Serafini-Fracassini D, Del Duca S (2015) Polyamines are common players in different facets of plant programmed cell death. Amino Acids 47:27–44
Cao DD, Hua J, Zhua SJ, Hua WM, Knapp A (2010) Relationship between changes in endogenous polyamines and seed quality during development of sh2 sweet corn (Zea mays L.) seed. Sci Hortic 123:301–307
Carrijo LC, Borges EEL, Pontes CA, Lopes MR, Brune A (2010) α-Galactosidase activity and carbohydrate mobilization in seeds of Dalbergia nigra (Vell.) Alemão ex Benth.—Fabaceae (Brasilian Rosewood) during germination. Cerne 16:283–289
Carvalho PER (2003) Espécies arbóreas brasileiras. Colombo, Brasilia
Carvalho LR, Silva EAA, Davide AC (2006) Storage behaviour of forest seeds. Rev Bras Sementes 28:15–25
Catusse J, Job C, Job D (2008) Transcriptome and proteome wide analyses of seed germination. C R Biol 331:815–822
Chiou TJ, Bush DR (1998) Sucrose is a signal molecule in assimilate partitioning. Proc Natl Acad Sci USA 95:4784–4788
Dantas B, Corrreia J, Marinho L, Aragão C (2008) Biochemical changes during imbibition of Caesalpinia pyramidalis Tul. seeds. Rev Bras Sementes 30:221–227
Dias LLC, Santa-Catarina C, Silveira V, Pieruzzi FP, Floh EIS (2009) Polyamines, aminoacids, IAA and ABA contents during Ocotea catharinensis seed germination. Seed Sci Technol 37:42–51
Donohue K, Casas RR, Burghardt L, Kovach K, Willis CG (2010) Germination, postgermination, adaptation, and species ecological ranges. Annu Rev Ecol Evol Syst 41:293–319
Eveland AL, Jackson DP (2012) Sugars, signaling, and plant development. J Exp Bot 63:3367–3377
Ferreira CS, Piedade MTF, Tine MAS, Rossatto DR, Parolin P, Buckeridge MS (2009) The role of carbohydrates in seed germination and seedling establishment of Himatanthus sucuuba, an Amazonian tree with populations adapted to flooded and non-flooded conditions. Ann Bot 104:1111–1119
Filson PB, Dawson-Andoh BE (2009) Characterization of sugar from model and enzyme-mediated pulp hydrolyzates using high-performance liquid chromatography coupled to evaporative light scattering detection. Bioresour Technol 100:6661–6664
Focks N, Benning C (1998) Wrinkled1: a novel, low-seed-oil mutant of Arabidopsis with a deficiency in the seed-specific regulation of carbohydrate metabolism. Plant Physiol 118:91–101
Gallardo K, Job C, Groot SPC, Puype M, Demol H, Vandekerckhove J, Job D (2002) Importance of methionine biosynthesis for Arabidopsis seed germination and seedling growth. Biol Plant 116:238–247
Ghelis T, Bolbach G, Clodic G, Habricot Y, Miginiac E, Sotta B, Jeannette E (2008) Protein tyrosine kinases and protein tyrosine phosphatases are involved in abscisic acid-dependent processes in Arabidopsis seeds and suspension cells. Plant Physiol 148:1668–1680
Gibson S (2005) Control of plant development and gene expression by sugar signaling. Curr Opin Plant Biol 8:93–102
Hu M, Shi Z, Zhang Z, Zhang Y, Li H (2012) Effects of exogenous glucose on seed germination and antioxidant capacity in wheat seedlings under salt stress. Plant Growth Regul 68:177–188
International Union for Conservation of Nature (2014) The IUCN red list of threatened species. http://www.iucnredlist.org. Accessed 28 April 2014
Jander G, Joshi V (2010) Recent progress in deciphering the biosynthesis of aspartate-derived amino acids in plants. Mol Plant 3:54–65
Joshi V, Laubengayer KM, Schauer KM, Fernie A, Jander G (2006) Two Arabidopsis threonine aldolases are non-redundant and compete with threonine deaminase for a common substrate pool. Plant Cell 18:3564–3575
Kamada H, Harada H (1984) Changes in endogenous amino acids compositions during somatic embryogenesis in Daucus carota L. Plant Cell Physiol 25:27–38
Kang JH, Wang L, Giri A, Baldwin IT (2006) Silencing threonine deaminase and JAR4 in Nicotiana attenuata impairs jasmonic acid–isoleucine-mediated defenses against Manduca sexta. Plant Cell 18:3303–3320
Kim W, Okita T (1988) Nucleotide and primary sequence of a major rice prolamine. FEBS Lett 231:308–310
Koch K (2004) Sucrose metabolism: regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr Opin Plant Biol 7:235–246
Krasuska U, Ciacka K, Bogatek R, Gniazdowska A (2014) Polyamines and nitric oxide link in regulation of dormancy removal and germination of apple (Malus domestica Borkh.) embryos. J Plant Growth Regul 33:590–601
Krishnan H, White J (1995) Morphometric analysis of rice seed protein bodies (implication for a significant contribution of prolamine to the total protein content of rice endosperm). Plant Physiol 109:1491–1495
Kuo TM, Van Middlesworth JF, Wolf WJ (1998) Content of raffinose oligosaccharides and sucrose in various plant seeds. J Agric Food Chem 36:32–36
Kusano T, Berberich T, Tadeda C, Takahashi Y (2008) Polyamines: essential factors for growth and survival. Planta 228:367–381
Li P, Wind JJ, Shi X, Zhang H, Hanson J, Smeekens SC, Teng S (2011) Fructose sensitivity is suppressed in Arabidopsis by the transcription factor ANAC089 lacking the membrane-bound domain. Proc Natl Acad Sci USA 108:3436–3441
Maki H, Ando S, Kodama H, Komamine A (1991) Polyamines and the cell cycle of Catharanthus roseus cells in culture. Plant Physiol 96:1008–1013
Matilla AJ (1996) Polyamines and seed germination. Seed Sci Res 6:81–93
Mirza JI, Bagni N (1991) Effects of exogenous polyamines and difluoromethylornithine on seed germination and root growth of Arabidopsis thaliana. Plant Growth Regul 10:163–168
Mitsukawa N, Konishi R, Kidzu K, Ohtsuki K, Masumura T, Tanaka K (1999) Amino acid sequencing and cDNA cloning of rice seed storage proteins, the 13 kDa prolamins, extracted from type I protein bodies. Plant Biotechnol 16:103–113
Nonogaki H, Bassel GW, Bewley JD (2010) Germination—still a mystery. Plant Sci 179:574–578
Obendorf RL (1997) Oligosaccharides and galactosyl cyclitols in seed desiccation tolerance. Seed Sci Res 7:63–74
Obroucheva NV, Lityagina SV, Richter A (2006) Dynamics of carbohydrates in the embryo axes of horse chestnut seeds during their transition from dormancy to germination. Russ J Plant Physiol 56:768–778
Oliveira IC, Brenner E, Chiu J, Hsieh MH, Kouranov A, Lam HM, Shin MJ, Coruzzi G (2001) Metabolite and light regulation of metabolism in plants: lessons from the study of a single biochemical pathway. Braz J Med Biol 34:567–575
Onomo PF, Niemenak N, Ndoumou DO, Lieberei R (2010) Change in amino acids content during germination and seedling growth of Cola sp. Afr J Biotechnol 9:5632–5642
Pastorini LH, Bacarin MA, Trevizol FC, Bervald CMP, Fernandes HS (2003) Production and non-structural carbohydrates content in potato tubers obtained in two planting times. Hort Bras 21:660–665
Penfield S, Graham S, Graham I (2005) Storage reserve mobilization in germinating oil seeds: Arabidopsis as a model system. Biochem Soc Trans 33:380–383
Peterbauer T, Richter A (2001) Biochemistry and physiology of raffinose-family oligosaccharides and galactosyl cyclitols in seeds. Seed Sci Res 11:185–197
Pieruzzi FP, Dias LLC, Balbuena TS, Santa-Catarina C, Santos ALW, Floh EIS (2011) Polyamines, IAA and ABA during germination in two recalcitrant seeds: Araucaria angustifolia (Gymnosperm) and Ocotea odorifera (Angiosperm). Ann Bot 108:337–345
Price J, Li TC, Kang SG, Na JK, Jang JC (2003) Mechanisms of glucose signaling during germination of Arabidopsis. Plant Physiol 132:1424–1438
Puga-Hermida MI, Gallardo M, Rodriguez-Gacio MC, Matilla AJ (2006) Polyamine contents, ethylene synthesis, and BrACO2 expression during turnip germination. Biol Plant 50:574–580
Rocha M, Licausi F, Araujo W, Nunes-Nesi A, Sodek L, Fernie AR, Van Dongen JT (2010) Glycolysis and the tricarboxylic acid cycle are linked by alanine amino transferase during hypoxia induced by water logging of Lotus japonicus. Plant Physiol 152:1501–1513
Rodriguez C, Frias J, Vidal-Valverde C, Hernandez A (2008) Correlation between some nitrogen fractions, lysine, histidine, tyrosine and ornithine contents during the germination of peas, beans, and lentils. Food Chem 108:245–252
Rolland F, Baena-Gonzalez E, Sheen J (2006) Sugar sensing and signaling in plants: conserved novel mechanisms. Annu Rev Plant Biol 57:675–709
Santa-Catarina C, SdaC Maciel, Pedrotti EL (2001) In vitro germination and somatic embryogenesis from immature embryos of “canela sassafrás” (Ocotea odorifera Mez). Rev Bras Bot 24:501–510
Santa-Catarina C, Silveira V, Balbuena TS, Maranhão MEE, Handro W, Floh EIS (2006) IAA, ABA, polyamines and free amino acids associated with zygotic embryo development of Ocotea catharinensis. Plant Growth Regul 49:237–247
Satya-Naraian V, Nair PM (1990) Metabolism, enzymology and possible roles of 4-aminobutyrate in higher plants. Phytochemistry 29:367–375
Shoeb F, Yadav JS, Bajaj S, Rajam MV (2001) Polyamines as biomarkers for plant regeneration capacity: improvement of regeneration by modulation of polyamine metabolism in different genotypes of indica rice. Plant Sci 160:1229–1235
Shotwell M, Larkins B (1989) The molecular biology and biochemistry of seed storage proteins. In: Marcus A (ed) The biochemistry of plants, vol 15. Academic Press, San Diego, pp 297–345
Sinska I, Lewandowska U (2006) Polyamines and ethylene in the removal of embryonal dormancy in apple seeds. Physiol Plant 81:59–64
Smeekens S, Ma J, Hanson J, Rolland F (2010) Sugar signals and molecular networks controlling plant growth. Curr Opin Plant Biol 13:274–279
Sokal RR, Rohlf FJ (1995) Biometry, 3rd edn. Freeman and Co, New York
Tahir M, Vandenberg A, Chibbar RN (2011) Influence of environment on seed soluble carbohydrates in selected lentil cultivars. J Food Compos Anal 24:596–602
Tan-Wilson AL, Wilson KA (2012) Mobilization of seed protein reserves. Physiol Plant 145:140–153
Yang R, Chen H, Gu Z (2011) Factors influencing diamine oxidase activity and γ-aminobutyric acid content of fava bean (Vicia faba L.) during germination. J Agric Food Chem 59:11616–11620
Acknowledgments
Financial support was provided by the State of Rio de Janeiro Research Foundation—FAPERJ (Procs. E-26.110.846/2010, E-26/110.390/2012, E26/111.389-2012, E26/102.989/2012 and E26/010.001507/2014) and National Council of Scientific and Technological Development (Procs. 476465/2011-7, 305645/2013-7 and 444453/2014-8). The scholarships were supported by the FAPERJ to BVN and by the Coordination for the Improvement of Higher Education Personnel (CAPES) to VPMA and LZP.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aragão, V.P.M., Navarro, B.V., Passamani, L.Z. et al. Free amino acids, polyamines, soluble sugars and proteins during seed germination and early seedling growth of Cedrela fissilis Vellozo (Meliaceae), an endangered hardwood species from the Atlantic Forest in Brazil. Theor. Exp. Plant Physiol. 27, 157–169 (2015). https://doi.org/10.1007/s40626-015-0041-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40626-015-0041-7