Abstract
Introduction
Acute Kidney Injury (AKI) is increasingly common in people over 65 years of age, but its causes and management are poorly described. The purpose of this study was to describe the causes, management and prognosis of patients over 65 hospitalised for severe acute kidney injury (AKI) in all departments of a tertiary centre.
Method
The prospective IRACIBLE (IRA: AKI in French; CIBLE: target in French) cohort included 480 patients hospitalised at a university hospital over 18 months for severe AKI or subgroup of AKIN3 (Acute Kidney Injury Network classification) defined by an acute creatinine increase > 354 μmol/L or managed with acute renal replacement therapy (RRT). The history, aetiology of AKI, management, and prognosis were compared in three age groups: < 65, 65–75, and > 75 years.
Results
The study population included 480 subjects (73% men) with a median body mass index (BMI) of 26.6 kg/m2 [23.3, 30.9], 176 (37%) diabetic patients, 124 (26%) patients < 65 years, 150 (31%) 65–75 years and 206 (43%) > 75 years. Increasing age class was associated with more comorbidities, a significantly lower median estimated glomerular filtration rate (eGFR) 6 months before inclusion (82; 62; 46 ml/min/1.73 m2, p < 0.05) and aetiology of AKI, which was more often obstructive (12%; 15%; 23%, p = 0.03) or part of a cardio-renal syndrome (6%; 9%; /15%, p = 0.04). Older patients were less often managed in the intensive care unit (54%; 47%; 24%, p < 0.0001), were less frequently treated by RRT (52%; 43%; 31%, p < 0.001) and received fewer invasive treatments (6%; 9%; 22%, p < 0.0001). Older survivors returned home less often (80%; 73%; 62%, p = 0.05) in favour of transfers to rehabilitation services (10%; 13%; 22%) with higher mortality at 3 months (35%; 32%; 50%, p < 0.0001).
Conclusion
Older patients hospitalised for severe AKI have a specific profile with more comorbidities, lower baseline renal function, an aetiology of AKI of mainly extra-parenchymal causes and a complex pathway of care with an overall poor prognosis.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute Kidney Injury (AKI) is a syndrome characterized by a rapid reduction in renal function resulting in decreased clearance of toxins, water and electrolytes. According to the 2012 KDIGO (Kidney Disease: Improving Global Outcome) guidelines [1] it is defined by a change in creatinine and diuresis over a period of time with a prognostic classification in several stages of increasing severity in terms of morbi-mortality and costs [2, 3].
This pathology is increasingly common in the general population and particularly in older patients, as shown by several epidemiological studies throughout the world, whether for AKI requiring renal replacement therapy (RRT) [4,5,6] or not [7, 8].
The aetiologies of AKI are numerous and differ according to populations, regions and the country’s economic level [9]. They are usually classified as pre-renal (including hypovolemia, cardio-renal and hepato-renal syndromes), renal (including tubular necrosis and vascular, glomerular or interstitial causes), and post-renal (bladder or bi-ureteral obstruction) [10]. The aetiologies of AKI have mainly been described in Intensive Care Units (ICUs) where the principal causes are primarily related to septic, hypovolemic, or cardiogenic shock, to a lesser extent to drugs or toxic causes (in particular antibiotics and iodinated contrast agents), to obstructive causes and, even more rarely, to primary renal diseases and hepato-renal syndromes [11,12,13].
Increasing age is associated with an increase in frailty, defined as a decrease in physiological reserves exposing patients to a greater frequency of organ decompensation for moderate stresses, including AKI [14,15,16,17]. The increased risk of AKI in the elderly is thought to be due to various factors, some not modifiable such as the decrease in Glomerular Filtration Rate (GFR) associated with renal ageing [18,19,20], or the increase in the prevalence of comorbidities associated with the risk of AKI (diabetes mellitus, cardiovascular disease) [11, 21, 22], and others that are modifiable, such as polypharmacy [23, 24], exposure to nephrotoxic drugs [25,26,27], trauma [28, 29] or major surgery [30].
Although several studies have shown that the aetiologies of AKI in older patients are similar to those in the general population [31,32,33] there are some specificities such as the greater prevalence of drug and iatrogenic [27, 34,35,36] or obstructive causes [33, 37]. Finally, although the evidence of excess mortality in elderly patients with AKI compared to younger patients is not clear [38, 39], their prognosis is reported to be worse: poorer renal recovery when dialysis is required [40], increased risk of rehospitalisation [35, 41] and progression towards Chronic Kidney Disease (CKD) [42] or End-Stage Renal Disease (ESRD) [43].
The management of subjects with AKI has been studied and codified in the general population (1) but, so far, older patients with severe AKI have only been the subject of a few studies, mostly carried out in ICUs, thus including younger patients than in other hospitalisation sectors [44].
In this context, we compared the aetiology, care, and outcome of old patients (65–75) and very old patients (> 75) against non-old patients (< 65), in a population of adults hospitalised for severe AKI in all departments of a university hospital.
Patients and methods
Study population
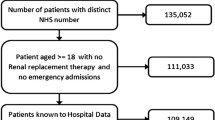
All adults (≥ 18 years) presenting severe AKI at or after admission to our university hospital from August 2016 to December 2017 were eligible. They were prospectively screened by physicians in the ICU, nephrologists in the nephrology department, and biochemistry laboratory results for the whole hospital. Inclusion criteria were severe AKI, defined by 2 of the 3 criteria for KDIGO stage 3 AKI, i.e. an acute increase in serum creatinine (SCr) above 354 μmol/L or acute indication for RRT, but not a 3-fold increase in SCr within 7 days with SCr below 354 μmol/L. Patients were divided into 3 age groups: under 65, 65–75 years, and over 75 years of age. The threshold of 65 years was retained as the international definition of older patients [15, 19, 32, 40, 45,46,47] and 75 years as the threshold defined for hospitalisation in geriatrics in France [48, 49].
Exclusion criteria were: Chronic Kidney Disease KDIGO stage 5, kidney transplant, planned dialysis for bilateral surgical nephrectomy, patients under curatorship/guardianship, refusal to participate and absence of health insurance. The exhaustiveness of inclusions during the study period was regularly verified by the biochemistry department, which sent information about all patients with SCr above 354 μmol/L. Patients requiring RRT for AKI were also screened weekly by the investigators throughout the study period.
The study flow chart is reported in Fig. 1.
Information
Patient characteristics
The following information was recorded at inclusion: age, sex, height, weight, body mass index [BMI], and pre-specified chronic illnesses (coronary heart disease, treated hypertension, heart failure, cardiac arrhythmia, cerebrovascular disease, peripheral vascular disease, chronic obstructive pulmonary disease or asthma, non-invasive ventilation except for supplemental oxygen, diabetes mellitus, chronic liver dysfunction, solid organ malignancies, and haemopathy). Comorbidities were defined according to the glossary of terms used for the REIN registry [50]. Charlson comorbidity score was calculated at inclusion. The presence of a consultation by a nephrologist and estimated GFR (eGFR) at least 6 months before inclusion were also recorded. In the absence of information on kidney function before hospitalisation, the GFR was estimated at a value of 75 mL/min/1.73 m2, as recommended [1].
The following laboratory values were recorded: SCr (enzymatic method), urea, potassium, and bicarbonate. Acute kidney injury cause was defined according to standard nephrology definitions [10] and the clinical context, as suggested by Kellum and Prowle [12]. The various causes considered were pre-renal kidney injury with a clinical context of real hypovolemia and rapid reversibility of AKI after fluid administration; obstructive kidney failure with documented urinary tract obstruction; and intrinsic kidney injury including acute tubular necrosis (ATN), glomerulopathy, vascular or acute interstitial nephritis, as assessed by a nephrologist based on medical history, clinical presentation, kidney imaging and urine analysis. Acute tubular necrosis was diagnosed in cases of sustained renal ischaemia, direct drug-induced tubular injury, rhabdomyolysis, cast nephropathy, or persistent AKI more than 72 h after hemodynamic correction. Acute kidney injury in the context of Type I cardio-renal syndrome was defined by acute heart failure complicated by AKI [51], and AKI with hepato-renal syndrome was defined by AKI with cirrhosis or liver failure [52]. AKI associated with sepsis was defined according to the 2016 definition [53] and AKI with multiple organ failure as failure of at least 3 organs. Finally, hospital-acquired AKI was defined by its occurrence ≥ 48 h after hospital admission, surgery-associated AKI by its occurrence within 72 h after surgery, and community-acquired AKI as not acquired during hospitalisation. Two investigators (C. Aglae and O. Moranne) reached a consensus about each probable cause of AKI. As mentioned by Kellum et al., this diagnosis is sometimes complex. Therefore, we first described causes according to their clinical syndrome, as in sepsis or multiple organ failure, and then as hypovolemic, renal, or obstructive [54, 55].
Trajectory and care
Patient trajectory during hospitalisation was recorded with information about the different departments of hospitalisation for patients. The “inclusion ward” was defined as the ward where the patient presented severe AKI requiring inclusion. Three main groups of department/ward were studied including the ICU and the nephrology ward (NW) which both had RRT availability, whereas the third group was “all other departments without RRT”. The ICU contains 2 units, 1 surgical and 1 medical, with different patient profiles. We also recorded the department, if any, before the “inclusion ward”, the inpatient’s death ward, the last ward where the patient stayed before discharge and the conditions of transfer to home or another facility such as hospital or rehabilitation centre for survivors.
Patients were sub-divided into 9 main groups according to the principal reason for admission: sepsis, cardiovascular, neurologic, digestive disease, respiratory distress, hypovolemia or haemorrhage, AKI, or other diagnoses. More than 1 condition could be selected in each case.
During hospitalisation, information regarding the inclusion ward, care plan including treatment with RRT, intravenous infusion of vasopressors, mechanical ventilation, decision to withhold/withdraw life-sustaining treatment, death during hospitalisation and dependence on dialysis at discharge were recorded in the patient’s electronic file.
When RRT was indicated, the attending physician specified the reason from the following pre-specified items: oligoanuria, hyperkalaemia, acute pulmonary oedema, metabolic acidosis, hypercalcaemia, volume overload with diuretic resistance or refractory shock. Several causes could be selected.
Statistical analysis
We report the incidence of hospitalisation for severe AKI in adults over the study period according to the overall number of stays in this tertiary university hospital for adults hospitalised in medical, surgical, and obstetric wards. The population characteristics, clinical presentation at inclusion, trajectory, and care are reported for the overall population and by age range. Quantitative values with Gaussian distribution are expressed as means with their standard deviations (SDs) and compared with the analysis of variance (ANOVA) test; those with non-Gaussian distribution are expressed as medians and their interquartile ranges (IQRs) and compared by age range using the Kruskal–Wallis nonparametric test. Qualitative values are expressed in numbers (with percentages) for qualitative values and compared by age range with the Chi-squared test. Significance was defined as P < 0.05 with a bilateral test. Statistical analyses were performed with SAS software version 9.3 (SAS Institute Inc, Cary, South Carolina, USA). The statistical comparison of the variables according to group is a global comparison between the three age groups.
Ethics
This study was approved by the Ethics Committee (REB) and the French Data Protection Authority (Commission Nationale Informatique & Libertés; CNIL number: 1963867v0) in accordance with the current French legislation (Toulouse E, ACCPM 2020). All patients or their legal representatives received clear information that they could object to the collection of information on their health records, and none expressed any opposition. The study is registered on the Clinical Trials website under the number NCT03192189.
Results
Socio-demographic characteristics and medical history
Sociodemographic data, medical history, and previous renal function are detailed according to age range in Table 1. Of the 507 eligible patients, 480 were included (median age 72 years [Q1-Q3 64, 83], 73% men) with 124 patients < 65 years (26%, median age 57 years; [Q1-Q3 51, 62]), 150 patients 65 to 75 years (31%, median age 70 years; [Q1-Q3 68, 72]), and 206 patients > 75 years (43%, median age 84 years; [Q1-Q3 80, 89]) (Fig. 1). The most commonly found medical histories were, for all participants, cardiovascular disease (cardiopathy 79%, hypertension 67%), diabetes (37%) and solid organ malignancies (27%). Among the whole cohort, median eGFR 6 months before inclusion was 59 ml/min/1.73 m2 [Q1-Q3 37.5,79] with 82 patients being followed by a nephrologist (17%). Diabetes (25%; 43%; 39%, p = 0.01) and cardiovascular diseases were significantly more common in older groups. The median eGFR 6 months before admission decreased with age: 82 ml/min/1.73 m2 in the < 65 year-old age group, 61.5 ml/min/1.73 m2 in the 65–75 year-old age group, 46 ml/min/1.73 m2 in the > 75 year-old age group (p < 0.0001). History of previous nephrological follow-up increased with age (10%; 18%; 20%, p < 0.001). However, for the whole cohort, data on previous renal function were unavailable for 21 patients (4%), and therefore the imputation was made with an eGFR of 75 ml/min/1.73 m2. Missing creatinine data was more frequent in younger patients: 11 for the < 65 year-old age group (9%), 9 for the 65–75 year-old age group (6%) and only 1 for the > 75 year-old age group (0.5%).
Other diseases in the clinical history, especially solid organ malignancies, were not found more frequently in older groups, and history of chronic liver dysfunction was more common among younger subjects.
Reasons for hospitalisation, clinical data, and aetiologies of AKI
Reasons for admission, hospital-acquired and surgery-associated AKI rate, clinical and laboratory data at inclusion, and causes of AKI are detailed in Table 2. The median eGFR 6 months before inclusion according to the aetiology of severe AKI is detailed in the Appendix (Fig. 1S). There was no difference in admission reasons for the three groups. Among the patients in the > 75 year-old age group, the most frequent causes were infectious (24%), gastrointestinal (21%), hypovolemia (19%), isolated AKI (18%) and cardiovascular (18%). Similarly, the rates of hospital-acquired and surgery-associated AKI did not vary significantly with age. On the other hand, clinical characteristics differed significantly between groups: older patients had a lower median BMI (26.1; 28.2; 25.6 kg/m2, p < 0.01) and a higher Charlson comorbidity index (4; 6; 8, p < 0.001).
The main AKI aetiologies for the three groups are shown in Fig. 1s. In the > 75 year-old age group, the main causes were obstructive (23%), followed by sepsis (20%) and hypovolemia (20%), then cardio-renal syndrome (15%), and finally acute tubular necrosis (14%). Some aetiologies increased with age: obstructive causes (12% for < 65 years, 15% for 65–75 years, 23% for > 75 years, p = 0.03), hypovolemia without nephrotoxic drugs (14/15/19%) and cardio-renal syndrome (6/9/15%, p = 0.04). Conversely, other etiologies decreased with increasing age, including intrinsic kidney injury (29% for < 65 years, 26% for 65–75 years, 18% for > 75 years, p < 0.05) and hypovolemia with nephrotoxic drug use (9%; 1% respectively).
Trajectory, care and outcome
Table 3 reports data on patient trajectory (including hospitalisation ward, length of stay and mode of discharge), treatment (invasive and supportive care), and outcome (vital and renal status at discharge and 3 months after inclusion). Complementary data concerning treatment in relation to age (Fig. 2S), and eGFR distribution at discharge are detailed in the Appendix (Table 1S). Trajectory differed according to age: patients aged > 75 were more often admitted from emergency (45%; 45%; 59% respectively) and surgical wards (2%; 6%; 5%) than from medical wards (16%; 17%; 10%). They were less often admitted to the ICU (54%; 47%; 24%) and Nephrology (27/%; 29%; 25%) but more often to other wards (19%; 23%; 50%). The other wards included, first, geriatrics (17%) and other medical wards (17%) for the > 75 year-old group, onco-haematology (7%) and other medical wards (7%) for the 65–75 year-old group. Patients aged < 65 admitted to other wards were first admitted to other medical wards (10%), and urology (5%).
Among the whole cohort, 194 patients (40%) received RRT for AKI, for whom a decision to withhold/withdraw life-sustaining treatment (including mechanical ventilation, dialysis, vasopressor use and/or ICU Care admission), hereinafter referred to as supportive care, was made for 67 patients (14%). This latter case was more frequent in older patients (6%; 9%; 22%, respectively p < 0.0001), who received less frequently RRT (p < 0.001), vasopressor support (p = 0.03) and mechanical ventilation (p < 0.001). Among the AKI patients treated with RRT, the indications for anuria , acute pulmonary oedema and diuretic resistance increased with age and, conversely, the indications for metabolic acidosis and hyperkalaemia decreased with age (p < 0.001).
The median length of stay was 13 days with no significant variation between groups (11; 14; 13 days, p = 0.51). Regarding in-hospital mortality, there was no significant difference according to age. On the other hand, 3-month mortality was significantly associated with age group in the crude analysis (35%; 32%; 50%, p < 0.0001). Finally, among patients discharged alive from the hospital, older patients were less likely to go home directly and were more often transferred to a rehabilitation centre or another hospital (Table 3).
Discussion
In our population of patients hospitalised for severe AKI, 43% of the subjects were over 75 years old. These older patients had specificities such as more comorbidities, particularly cardiovascular, diabetes and a significantly lower eGFR 6 months before hospitalisation for severe AKI. Moreover, the causes of AKI varied in older patients who had more obstructive aetiologies, cardio-renal syndrome, and hypovolemia. Finally, these older patients had fewer admissions to ICU and Nephrology, fewer invasive procedures, and received more supportive care. Mortality was higher at 3 months, and survivors were less likely to return home directly. These results emphasize that older patients are a unique population with various AKI aetiologies often related to their specific comorbidities and often worsened by a lower baseline eGFR. These findings highlight the importance of a multidisciplinary team approach upon admission to discuss the interest of invasive care early on, plan interventions on the identified vulnerabilities, and adapt the pathway of care. Finally, these older patients could greatly benefit from a robust aftercare process with remote nephrological follow-up and geriatric evaluation to reduce mid- and long-term mortality and prevent unnecessary procedures and further hospitalisations.
The study population included patients with severe AKI, on RRT or not, in all wards of a tertiary centre. Such data are complementary to data from epidemiological registry studies and cohorts of AKI requiring RRT [4,5,6] or AKI all stages except RRT in all hospital wards [8], as well as AKI managed in the ICU, requiring RRT or not [11, 13]. The overall characteristics of our population are similar to those reported in the literature, and show a predominance of men (54 to 65%), over 60 (60–70 years), with significant cardiovascular comorbidities (17% to 65%), diabetes (13% to 31%), cancer (14% to 20%), and prior CKD (16%36%) [4,5,6, 8, 11, 13]. In our cohort, patients were older (72 years) and showed a greater frequency of these comorbidities. Comparison of in-hospital mortality rates confirms our population’s specificity in terms of severity, at the interface between all-stages of AKI except RRT (10%–25% mortality) [8, 17, 36, 56] and AKI requiring RRT or ICU management (27%–60% mortality) [5, 6, 11, 13, 57]. Mortality after hospitalisation has not been described for these particular studies, but the increased risk of mid- and long-term mortality after AKI has been previously reported [58, 59].
Our population had similarities with the one described in a Spanish study conducted 30 years ago, in 1991–1992, by Pascual et al. [33] which included 103 patients aged over 80 with AKI defined by creatinine > 177 µmol/L, in all hospital wards. Sixteen% of these patients aged over 80 were followed in Nephrology and 3% were in the ICU. Unlike our study, theirs was multicentric. On the other hand, their inclusion criteria were patients seen for consultation in Nephrology, exposing them to a selection bias. Finally, renal function prior to AKI was not described. The distribution of aetiologies was similar to our study, with a high frequency of post-renal and pre-renal AKI (including hypovolemia and cardio-renal syndrome) [33], as well as a low prevalence of intrinsic AKI other than ATN. The most important difference is ATN which was much less frequent in our cohort. This probably results from our different classification, taking AKI into account as part of a clinical syndrome (cardio- or hepato-renal syndrome, and sepsis-associated AKI) [60].
Renal ageing makes older patients more prone to volume variations [19], which together with the high prevalence of cardiovascular disease and low baseline eGFR (Fig. 2S), may explain the excess of cardio-renal syndrome and hypovolemic AKI in our old patient population. Among hypovolemic AKI, the low number of nephrotoxic drugs in older patients compared to other studies, and their association with young age in our study is more surprising. This may be explained by several factors: more frequent evolution to ATN in older patients treated with nephrotoxic drugs, better management of nephrotoxic agents in old patients than in young patients and also the recruitment of patients with severe AKI. These results may also be explained by our choice not to include natriuretics and RAAS inhibitors in nephrotoxic drugs, whereas these were described in other studies [36].
Regarding management of these patients, our population differed from Pascual’s [33]. Although their results did not report a difference in death rates by age, they showed that older patients had a lower rate of hospitalisation in Nephrology and ICU, and less access to invasive treatments, including RRT. We were able to show more precisely that the use of RRT decreased little with age until patients were over 85, after which it dropped sharply, while conversely the proportion of patients on supportive care increased slowly from 55 years old, then sharply over 85 (Fig. 32S).
The differences in the indication for RRT reported by the physicians seem to be related to AKI aetiologies, with two typical clinical pictures: on the one hand, "volemic" older patients, with cardio-renal syndrome, resistance to diuretics, acute pulmonary oedema or anuria, and on the other hand, "metabolic" younger patients with multi-visceral failure complicated by acidosis and hyperkalaemia. It should be noted that, although our biological data at inclusion do not show an excess of hyperkalaemia in younger patients, they do show higher urea and bicarbonate levels with increasing age (Table 2). This could be explained by the increase in pre-renal causes (hypovolemia and cardio-renal syndrome) which are often associated with elevated urea levels.
Finally, as regards non-increasing in-hospital mortality in older patients with severe AKI, we found similar results to those of the study by Pascual [33] and several others, for surgery-associated AKI [61], AKI managed in Nephrology and ICU [62] or throughout the whole hospital [63]. It should be noted that these studies mainly focused on in-hospital mortality which, as illustrated here, is only part of the prognosis of a patient hospitalised for an acute event. In our study we report that older patients return home less directly, reflecting the greater frailty of this population and the need for specific care. Finally, we report higher mortality at 3 months for older patients and a non-significant trend for a higher frequency of dialysis dependence after an episode of severe AKI in our study population of median age 72 years, as other studies have also shown [40, 42].
The strengths of our study are the prospective nature of the cohort, and the inclusion of all adult patients with severe AKI, defined by robust criteria, in all wards of a tertiary centre, while most studies in a population of adult patients with severe AKI are limited to Nephrology or IC or are based on medico-administrative data, include all KDIGO stages of AKI, or are limited to AKI requiring RRT, often without specifying aetiology. Furthermore, the description of our population is very detailed and very few prior eGFR data (4%) were unavailable, due to the prospective design of this study. Finally, we describe the trajectory of these patients and the way they were managed at discharge, to better take into account the prospective organization of care.
Our study also has limitations. Firstly, a possible selection bias of our population due to the inclusion criteria which do not consider the totality of the KDIGO 3 AKI definition, with non-use of the criteria based on diuresis and serum creatinine tripling. This led to the non-inclusion of patients with decreased urinary output or tripling of creatinaemia without reaching the threshold of 353 µmol/l and not treated with RRT. However, this may also be a strength, with robust selection criteria to define severe AKI, easy screening and easy implementation of these selection criteria. The second limitation is the single-centre nature of the study, a tertiary centre without cardiac surgery or an organ transplant department. This limits the extrapolation of results to hospitals with such services.
Moreover, our results suggest that patients > 75 years old are very heterogeneous, probably with a dichotomy in the management of those over and under 85 years of age. Further studies would be useful to describe severe AKI in patients aged over 85, with a more precise description of geriatric parameters (such as dependence, chronic and/or acute cognitive impairment, iatrogenicity), and their association with the decision for supportive care and with outcomes.
Kidney ageing puts older patients at greater risk of severe AKI, primarily from pre- and post-renal causes. This type of damage predominates in elderly patients but does not correspond to all the aetiologies of AKI in the elderly, which are as variable as in younger subjects [31,32,33, 64]. Elderly patients should therefore be explored in the same way without limiting access to renal biopsy if necessary [65, 66]. Moreover, this frail and very comorbid elderly population requires multidisciplinary management during hospitalisation, associating nephrologists, intensivists, geriatricians, and other specialists to evaluate their functional reserves as completely as possible. This is essential to try and answer the complex question [45, 67] of the expected benefit of dialysis and ICU management, in order to avoid unreasonable care, implement interventions on the medico-psycho-social frailties and anticipate the subsequent care in the geriatric network. Indeed, the pathway of care of older patients is complex and often involves a rehabilitation centre before returning home, which is often difficult. Follow-up requires special organization adapted to this population [47, 68,69,70] and this is why early consultation with a geriatrician is highly recommended. In addition, given the frequency of previous CKD or age-related reduction of the kidney tissue, and insufficient previous nephrological follow-up in this population, consultation by a nephrologist is important for older patients, particularly with low GFR before hospitalisation as indicated by national and international recommendations [1, 71].
Conclusion
In this prospective single-centre study, patients aged over 65 hospitalised for severe AKI had more comorbidities, a lower baseline eGFR, and more frequent pre- and post-renal causes of AKI. Management is less often performed in Nephrology and the ICU, with less invasive treatments. Among survivors, the study reports more transfers to inpatient facilities before an eventual return home. This study emphasizes the specificity of this elderly population, and the importance of seeking an underlying lower eGFR whilst investigating the aetiologies of AKI. A multidisciplinary approach is necessary to discuss the option of invasive or non-invasive management, plan interventions focused on the vulnerabilities identified, and adapt the pathway of care. Nephrological follow-up and geriatric evaluation could probably help to reduce mortality, unnecessary procedures, and further hospitalisations.
References
Kellum JA, Lameire N, KDIGO AKI Guideline Work Group (2013) Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care Lond Engl. 17(1):204
Silver SA, Chertow GM (2017) The economic consequences of acute kidney injury. Nephron 137(4):297–301
Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW (2005) Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol JASN 16(11):3365–3370
Hsu RK, McCulloch CE, Dudley RA, Lo LJ, Hsu C (2013) Temporal changes in incidence of dialysis-requiring AKI. J Am Soc Nephrol JASN janv 24(1):37–42
Garnier F, Landais P, Moranne O (2019) Increased incidence of acute kidney injury requiring dialysis in metropolitan France. PLoS ONE 14(2):e0211541
Kolhe NV, Muirhead AW, Wilkes SR, Fluck RJ, Taal MW (2015) National trends in acute kidney injury requiring dialysis in England between 1998 and 2013. Kidney Int 88(5):1161–1169
Hsu C, McCulloch C, Fan D, Ordoñez J, Chertow G, Go A (2007) Community-based incidence of acute renal failure. Kidney Int 72(2):208–212
Kolhe NV, Muirhead AW, Wilkes SR, Fluck RJ, Taal MW (2016) The epidemiology of hospitalised acute kidney injury not requiring dialysis in England from 1998 to 2013: retrospective analysis of hospital episode statistics. Int J Clin Pract 70(4):330–339
Hoste EAJ, Kellum JA, Selby NM, Zarbock A, Palevsky PM, Bagshaw SM et al (2018) Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol 14(10):607–625
Lameire N, Van Biesen W, Vanholder R (2005) Acute renal failure. Lancet Lond Engl. 365(9457):417–430
Hoste EAJ, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN et al (2015) Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med 41(8):1411–1423
Kellum JA, Prowle JR (2018) Paradigms of acute kidney injury in the intensive care setting. Nat Rev Nephrol 14(4):217–230
Uchino S (2005) Acute renal failure in critically ill patients a multinational, multicenter study. JAMA 294(7):813
Chowdhury R, Peel NM, Krosch M, Hubbard RE (2017) Frailty and chronic kidney disease: A systematic review. Arch Gerontol Geriatr 68:135–142
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J et al (2001) Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56(3):M146-156
Rockwood K, Mitnitski A (2007) Frailty in relation to the accumulation of deficits. J Gerontol Ser A 62(7):722–727
Nash K, Hafeez A, Hou S (2002) Hospital-acquired renal insufficiency. Am J Kidney Dis Off J Natl Kidney Found. 39(5):930–936
O’Sullivan ED, Hughes J, Ferenbach DA (2017) Renal aging: causes and consequences. J Am Soc Nephrol JASN 28(2):407–420
Infante B, Franzin R, Madio D, Calvaruso M, Maiorano A, Sangregorio F et al (2020) Molecular mechanisms of AKI in the elderly: from animal models to therapeutic intervention. J Clin Med 9(8):2574
Delanaye P, Jager KJ, Bökenkamp A, Christensson A, Dubourg L, Eriksen BO et al (2019) CKD: a call for an age-adapted definition. J Am Soc Nephrol JASN 30(10):1785–1805
James MT, Grams ME, Woodward M, Elley CR, Green JA, Wheeler DC et al (2015) A Meta-analysis of the Association of Estimated GFR, Albuminuria, Diabetes Mellitus, and Hypertension With Acute Kidney Injury. Am J Kidney Dis Off J Natl Kidney Found 66(4):602–612
Michel A, Martín-Pérez M, Ruigómez A, García Rodríguez LA (2016) Incidence and risk factors for severe renal impairment after first diagnosis of heart failure: a cohort and nested case-control study in UK general practice. Int J Cardiol 207:252–257
Formica M, Politano P, Marazzi F, Tamagnone M, Serra I, Marengo M et al (2018) Acute kidney injury and chronic kidney disease in the elderly and polypharmacy. Blood Purif 46(4):332–336
Pazan F, Wehling M (2021) Polypharmacy in older adults: a narrative review of definitions, epidemiology and consequences. Eur Geriatr Med 12:443–452
Fusco S, Garasto S, Corsonello A, Vena S, Mari V, Gareri P et al (2016) Medication-induced nephrotoxicity in older patients. Curr Drug Metab 17(6):608–625
Robert L, Ficheur G, Gautier S, Servais A, Luyckx M, Soula J et al (2019) Community-acquired acute kidney injury induced by drugs in older patients: a multifactorial event. Clin Interv Aging 14:2105–2113
Haq MFU, Yip CS, Arora P (2020) The conundrum of contrast-induced acute kidney injury. J Thorac Dis 12(4):1721–1727
Porter CJ, Moppett IK, Juurlink I, Nightingale J, Moran CG, Devonald MAJ (2017) Acute and chronic kidney disease in elderly patients with hip fracture: prevalence, risk factors and outcome with development and validation of a risk prediction model for acute kidney injury. BMC Nephrol 18(1):20
Haines RW, Fowler AJ, Kirwan CJ, Prowle JR (2019) The incidence and associations of acute kidney injury in trauma patients admitted to critical care: a systematic review and meta-analysis. J Trauma Acute Care Surg 86(1):141–147
STARSurg Collaborative, Writing Committee, Data analysis, Steering committee, Advisory group, Regional leads et al (2018) Prognostic model to predict postoperative acute kidney injury in patients undergoing major gastrointestinal surgery based on a national prospective observational cohort study. BJS Open. 2(6):400–410
Selmi Y, Ariba YB, Labidi J (2019) Epidemiology, diagnosis, and etiology of acute kidney injury in the elderly: a retrospective analysis. Saudi J Kidney Dis Transplant Off Publ Saudi Cent Organ Transplant Saudi Arab 30(3):678–685
Brown CM, Scheven L, O’Kelly P, Dorman AM, Walshe JJ (2012) Renal histology in the elderly: indications and outcomes. J Nephrol 25(2):240–244
Pascual J, Liaño F (1998) Causes and prognosis of acute renal failure in the very old. Madrid Acute Renal Failure Study Group. J Am Geriatr Soc 46(6):721–725
Awdishu L, Mehta RL (2017) The 6R’s of drug induced nephrotoxicity. BMC Nephrol 18(1):124
Turgutalp K, Bardak S, Horoz M, Helvacı I, Demir S, Kiykim AA (2017) Clinical outcomes of acute kidney injury developing outside the hospital in elderly. Int Urol Nephrol 49(1):113–121
Hu W, Lian X, Lin J, Chen Y, Wu Y, Liu W et al (2021) The incidence, characteristics, and use of suspected nephrotoxic drugs in elderly patients with community-acquired acute kidney injury. Clin Interv Aging 16:35–42
Feest TG, Round A, Hamad S (1993) Incidence of severe acute renal failure in adults: results of a community based study. BMJ 306(6876):481–483
Ali T, Khan I, Simpson W, Prescott G, Townend J, Smith W et al (2007) Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol JASN 18(4):1292–1298
Lámeire N, Matthys E, Vanholder R, De Keyser K, Pauwels W, Nachtergaele H et al (1987) Causes and prognosis of acute renal failure in elderly patients. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc Eur Ren Assoc 2(5):316–322
Schmitt R, Coca S, Kanbay M, Tinetti ME, Cantley LG, Parikh CR (2008) Recovery of kidney function after acute kidney injury in the elderly: a systematic review and meta-analysis. Am J Kidney Dis Off J Natl Kidney Found. 52(2):262–271
Sawhney S, Marks A, Fluck N, McLernon DJ, Prescott GJ, Black C (2017) Acute kidney injury as an independent risk factor for unplanned 90-day hospital readmissions. BMC Nephrol 18(1):9
Chawla LS, Amdur RL, Amodeo S, Kimmel PL, Palant CE (2011) The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int 79(12):1361–1369
Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA et al (2009) Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol JASN 20(1):223–228
Aglae C, Muller L, Reboul P, Cariou S, Saber Davide B, Trusson R et al (2019) Heterogeneity of cause, care, and prognosis in severe acute kidney injury in hospitalized patients: a prospective observational study. Can J Kidney Health Dis 6:2054358119892174
Bagshaw SM, Adhikari NKJ, Burns KEA, Friedrich JO, Bouchard J, Lamontagne F et al (2019) Selection and receipt of kidney replacement in critically ill older patients with AKI. Clin J Am Soc Nephrol CJASN. 14(4):496–505
Druml W, Lax F, Grimm G, Schneeweiss B, Lenz K, Laggner AN (1994) Acute renal failure in the elderly 1975–1990. Clin Nephrol juin 41(6):342–349
Fønss Rasmussen L, Grode LB, Lange J, Barat I, Gregersen M (2021) Impact of transitional care interventions on hospital readmissions in older medical patients: a systematic review. BMJ Open 11(1):e040057
Salles N, Floccia M, Videau M-N, Diallo L, Guérin D, Valentin V et al (2014) Avoiding emergency department admissions using telephonic consultations between general practitioners and hospital geriatricians. J Am Geriatr Soc 62(4):782–784
Conti A, Renzi N, Catarzi S, Mazzucchelli M, Covelli A, Pampana A et al (2020) Bleeding events in patients 75 years of age and older under long-term anticoagulant therapy: a real-life study. Crit Pathw Cardiol 19(3):131–138
Couchoud C, Stengel B, Landais P, Aldigier J-C, de Cornelissen F, Dabot C et al (2006) The renal epidemiology and information network (REIN): a new registry for end-stage renal disease in France. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc Eur Ren Assoc. 21(2):411–418
Ronco C, Di Lullo L (2014) Cardiorenal syndrome. Heart Fail Clin 10(2):251–280
Ginès P, Guevara M, Arroyo V, Rodés J (2003) Hepatorenal syndrome. Lancet Lond Engl. 362(9398):1819–1827
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M et al (2016) The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315(8):801–810
Ronco C, Bellomo R, Kellum JA (2019) Acute kidney injury. The Lancet 394(10212):1949–1964
Darmon M, Ostermann M, Cerda J, Dimopoulos MA, Forni L, Hoste E et al (2017) Diagnostic work-up and specific causes of acute kidney injury. Intensive Care Med 43(6):829–840
Nie S, Feng Z, Tang L, Wang X, He Y, Fang J et al (2017) Risk factor analysis for aki including laboratory indicators: a nationwide multicenter study of hospitalized patients. Kidney Blood Press Res 42(5):761–773
Schiffl H (2006) Renal recovery from acute tubular necrosis requiring renal replacement therapy: a prospective study in critically ill patients. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc Eur Ren Assoc. 21(5):1248–1252
See EJ, Jayasinghe K, Glassford N, Bailey M, Johnson DW, Polkinghorne KR et al (2019) Long-term risk of adverse outcomes after acute kidney injury: a systematic review and meta-analysis of cohort studies using consensus definitions of exposure. Kidney Int 95(1):160–172
Ikizler TA, Parikh CR, Himmelfarb J, Chinchilli VM, Liu KD, Coca SG et al (2021) A prospective cohort study of acute kidney injury and kidney outcomes, cardiovascular events, and death. Kidney Int 99(2):456–465
Poston JT, Koyner JL (2019) Sepsis associated acute kidney injury. BMJ 364:k4891
Van Den Noortgate N, Mouton V, Lamot C, Van Nooten G, Dhondt A, Vanholder R et al (2003) Outcome in a post-cardiac surgery population with acute renal failure requiring dialysis: does age make a difference? Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc Eur Ren Assoc. 18(4):732–736
Silveira Santos da CG, Romani RF, Benvenutti R, Ribas do Zahdi JO, Riella MC, Mazza NM (2018) Acute kidney injury in elderly population: a prospective observational study. Nephron 138(2):104–112
Xu L, Wu Y, Chen Y, Li R, Wang Z, Li Z et al (2021) Is acute kidney injury age-dependent in older adults: an observational study in two centers from North China. BMC Geriatr 21(1):7
Hamroun A, Frimat M, Beuscart J-B, Buob D, Lionet A, Lebas C et al (2019) Spécificités des néphropathies du sujet âgé. Néphrol Thérap 15(7):533–552
Pinçon E, Rioux-Leclercq N, Frouget T, Le Pogamp P, Vigneau C (2010) Renal biopsies after 70 years of age: a retrospective longitudinal study from 2000 to 2007 on 150 patients in Western France. Arch Gerontol Geriatr déc 51(3):e120-124
Di Palma AM, d’Apollo AM, Vendemia F, Stallone G, Infante B, Gesualdo L (2010) Kidney biopsy in the elderly. J Nephrol 23(Suppl 15):S55-60
Butler CR, O’Hare AM (2019) Complex decision making about dialysis in critically ill older adults with AKI. Clin J Am Soc Nephrol CJASN. 14(4):485–487
Mesteig M, Helbostad JL, Sletvold O, Røsstad T, Saltvedt I (2010) Unwanted incidents during transition of geriatric patients from hospital to home: a prospective observational study. BMC Health Serv Res 10:1
Neiterman E, Wodchis WP, Bourgeault IL (2015) Experiences of older adults in transition from hospital to community. Can J Aging Rev Can Vieil. 34(1):90–99
Røsstad T, Garåsen H, Steinsbekk A, Håland E, Kristoffersen L, Grimsmo A (2015) Implementing a care pathway for elderly patients, a comparative qualitative process evaluation in primary care. BMC Health Serv Res 15:86
Stevens PE, Levin A, Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members (2013) Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 158(11):825–830
Acknowledgements
The authors wish to thank Nîmes University Hospital, France, for its structural, human, and financial support through the award obtained by our team during the internal call for tenders “Thématiques émergentes”. We also thank Teresa Sawyers, medical writer at the BESPIM, Nîmes University Hospital, France, for her expertise in reviewing and editing this paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declarations and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cardinale, A., Messikh, Z., Antoine, V. et al. Specificity of severe AKI aetiology and care in the elderly. The IRACIBLE prospective cohort study. J Nephrol 35, 2097–2108 (2022). https://doi.org/10.1007/s40620-022-01322-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-022-01322-z