Abstract
Background
CD4+ T cells are involved in the pathogenesis of immunoglobulin A nephropathy (IgAN); T helper (Th) 1, Th17 and Th22 cells promote the occurrence and amplification of inflammatory reactions, while regulatory T (Treg) cells produce the opposite effects. However, whether Th9 cells, a subset of CD4+ T cells, participate in IgAN development is still unknown.
Methods
Human peripheral blood mononuclear cells (PBMCs) were isolated from IgAN patients for Th9 cells detection by flow cytometry. Wild-type (WT) mouse was used to establish an IgAN mouse model while C3aR and C5aR inhibitor treated IgAN mouse. Kidney disease and function was assessed by histology and albumin-to-creatinine ratio. C3aR and C5aR expression was examined by immunohistochemical (IHC) assay. Th9 cell proportions in the blood of IgAN mouse was detected. C3a, C5a and interleukin (IL)-9 levels were tested by ELISA. Moreover, co-culture system between human mesangial cells (HMCs) and CD4+ T cells were constructed with or without C3a, C5a and anti-CCL20 mAb stimulation for transwell assay to examine Th9 cell chemotaxis.
Results
We observed the numbers of Th9 cell and the levels of IL-9 were increased in IgAN patients and IgAN mice. Furthermore, C3a and C5a level in serum and kidney, C3aR and C5aR expression was increased in IgAN mice compared to WT mice. Most interestingly, C3aR and C5aR inhibitor could reduce kidney damage, Th9 cell numbers and IL-9 levels. We also observed that C3a and C5a enhanced CCL20 production in HMCs. Notably, C3a and C5a also increased the recruitment of Th9 cells and IL-9 levels by HMCs through enhancing the CCL20-CCR6 pathway.
Conclusions
Our results support that C3a and C5a increase the production of CCL20 by HMCs and consequently augment Th9 cell recruitment and IL-9 levels, resulting in IgAN exacerbation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immunoglobulin A nephropathy (IgAN), the most common primary glomerulonephritis globally, is characterized by dominant deposition of immunoglobulin A (IgA) 1 in the glomerular mesangium [1]. Although there has been much recent progress in understanding the pathogenesis of IgAN, it is not certain that all patients with IgAN share a single common process leading to mesangial IgA deposition. Accumulating evidence indicates that changes in several T helper (Th) cell subsets, such as decreased numbers of regulatory T (Treg) cells and increased percentages of Th1, Th17, Th22, T follicular helper (Tfh), and γδ T cells, are involved in the pathogenesis of IgAN and are correlated with clinical severity [2, 3].
Interleukin (IL)-9 producing CD4+ T cells (Th9 cells), which are induced to differentiate by transforming growth factor (TGF)-β and IL-4 [4], participate in a variety of immune responses involved in autoimmunity and allergic inflammation [5]. Previous studies have demonstrated that serum IL-9 levels are increased in active systemic lupus erythematosus and closely associated with the production of antibodies against double-stranded DNA [6, 7]. In addition, IL-9 can induce an inflammatory response by recruiting lymphocytes to the kidneys in a mouse model of hepatic and renal fibrosis induced by carbon tetrachloride treatment [8]. However, whether Th9 cells play a role in IgAN pathogenesis has not been investigated.
In addition to IgA1 deposition, IgAN is characterized by glomerular deposits of C3, which indicates the involvement of complement activation in this disease [9]. Liu et al. found that renal C3a, C3aR, C5a and C5aR expression was significantly related to the activity and severity of renal injury in IgAN [10]. Additionally, a study reported that local renal C3 could result in IL-17A-mediated inflammatory cell infiltration into the kidneys and further drive fibrogenic responses in a unilateral ureteral obstruction mouse model [11]. Our previous study suggested that a C5aR inhibitor could reverse both kidney damage and Th1, Th17 and Treg cell dysfunction in IgAN mice infected with respiratory syncytial virus (RSV) [2]. Overall, complement activation orchestrates CD4+ T cell responses in IgAN. However, the roles of C3a–C3aR and C5a–C5aR in Th9 cell infiltration and recruitment in IgAN development are relatively unclear.
Ye et al. proposed that the recruitment of Th9 cells into a malignant pleural effusion was induced by the CCL20-CCR6 pathway, as high CCR6 expression was observed on the Th9 cell surface [12, 13]. Interestingly, CCL20 has been shown to be obviously highly expressed in both the serum of IgAN patients and IgA1-treated human mesangial cells (HMCs) [14]. According to our previous research, CCL20 generated by HMCs is high in IgAN mice and participates in the processes of recruiting Th17 and Th22 cells [15, 16]. Notably, C3aR-deficient mice show reduced levels of CCL2, CCL5 and other chemokines associated with inflammatory cell recruitment in muscle injury and regeneration [17]; in other words, C3a is important in chemokine production. Therefore, it is reasonable to speculate that C3a and C5a may regulate the effect of HMC-generated CCL20 and thus impact Th9 cell recruitment.
Recently, renal function and proteinuria in two rapidly progressing IgAN patients were improved by an anti-C5 antibody [18, 19]. The oral C5aR1 antagonist CCX168 has been shown to be a promising component of induction therapy for ANCA-associated nephritis [20]. These results indicate the possibility of targeting C3a–C3aR and C5a–C5aR to treat IgAN. Although the C5a-C5aR1 axis can regulate Th1, Th17 and Treg cells in IgAN mice [2], the relationship between C3a–C3aR/C5a–C5aR and Th9 cells is still unknown. Furthermore, how C3a and C5a affect Th9 cell recruitment has not been described. In view of the above considerations, this study was designed to clarify the possible effects of C3a and C5a on CCL20 generated by HMCs during the process of Th9 cell recruitment to the local kidney area in IgAN.
Materials and methods
Patients
All selected factors were the demographic, clinical, and biochemical characteristics and pathology of patients with IgAN. Patients were eliminated if they had an acute infection or other complications or were treated with glucocorticoid or immunosuppressive treatments. Healthy controls were obtained from healthy volunteer donors in Xiangya Hospital.
Mice and animal model
Seven- to eight-week-old female BALB/c mice were purchased from the Experimental Animal Center of Central South University (Changsha, Hunan, China). All mice were fed and housed under desired temperature and humidity conditions in a specific pathogen-free environment. All studies were completed in accordance with Institutional Animal Care Guidelines.
Twenty-four BALB/C mice were randomly divided into four groups (n = 6 per group): a control group (Control), an IgAN group (IgAN), a C3aR antagonist-treated IgAN group (C3aRA-IgAN), and a C5aR antagonist-treated IgAN group (C5aRA-IgAN). The IgAN mouse model was established as previously described [2, 15, 21]. BSA (Roche) diluted in acidified water (800 mg/kg body weight) was administered every other day via intragastric gavage. A subcutaneous injection of 0.1 ml carbon tetrachloride in castor oil (mixed at a ratio of 1–5) was administered once a week, combined with intraperitoneal (i.p.) injection biweekly, and LPS (50 µg, Sigma) was intravenously injected twice in weeks six and eight. In the C3aRA-IgAN group, the mice were treated with a C3aR antagonist (SB 290157, Santa Cruz) by i.p. injection. In the C5aRA-IgAN group, the mice were treated with a C5aR antagonist (W54011, Abcam) by caudal vein injection. The control mice received an equal amount of PBS. All mice were sacrificed at 9 weeks, and samples were collected. Before sample collection, all mice were housed in metabolic cages for 24 h to collect urine samples. The urine samples were evaluated to measure the albumin-to-creatinine ratio (ACR).
Histological analyses
Upper left kidney tissue samples were embedded in paraffin and then cut into 2-μm-thick sections. The sections were processed by periodic acid-Schiff (PAS) staining and then examined by light microscopy.
Additionally, mouse renal tissue samples were fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer and embedded in Epon-Araldite. The specimens were then cut into ultrathin Sects. (70 nm) and stained with uranyl acetate and lead citrate. The sections were observed by transmission electron microscopy.
Paraffin-embedded sections were subjected to immunohistochemistry (IHC; for C3aR and C5aR) and immunofluorescence (IF; for IgA). The sections were mounted, dewaxed, and rehydrated in an alcohol gradient and PBS. Antigen retrieval was performed with a citrate buffer (pH 6.0). Endogenous peroxidase activity was blocked with 3% H2O2 for 20 min. After blocking nonspecific binding with a diluted normal goat serum working fluid for 60 min, the sections were incubated for 16–20 h at 4 °C with an anti-C3aR antibody (NBP2-15649, Novus) or an anti-C5aR antibody (ab117579, Abcam). After an overnight incubation with the primary antibody, the corresponding secondary antibody was used with an SP goat IgG kit (ZSGB-Bio), chromogenic reactions were performed with a DAB liquid (ZSGB-Bio), and the sections were counterstained with Mayer’s hematoxylin (ZSGB-Bio). For IF, the dewaxing, rehydration and antigen retrieval protocol was performed as described for IHC. Sections were incubated with an anti-mouse IgA antibody (ab97234, Abcam) overnight at 4 °C. Normal rabbit, goat and rat IgG antibodies were used as isotype controls.
The integrated densities and areas of C3aR and C5aR were evaluated using the ImageJ program according to the software instructions, and the mean density was calculated by determining the ratio of the integrated density to the area. The calculated mean density for each group was used for statistical analysis.
Cell culture
CD4+ T cells were isolated from peripheral blood mononuclear cells (PBMCs) collected from IgAN patients by human CD4 microbeads (130-045-101) purchased from Miltenyi Biotec. HMCs were purchased from ScienCell™ Research Laboratories and cultured in mesangial cell medium (4201, ScienCell™ Research Laboratories) in an incubator at 37℃ in 5% CO2. The HMCs were stimulated with IgA (Sigma), recombinant human C3a (A118, CompTech), recombinant human C5a (pro-2300-b, ProSpec-Tany) or an anti-CCL20 monoclonal antibody (mAb; AF360, R&D), and the supernatants were collected for CCL20 detection and transwell experiments.
CD4+ T cell chemotaxis assays
Polycarbonate filters (8-µm pore) in 24-well Transwell chambers (Corning Costar) were used to perform a migration assay. CD4+ T cells purified from the PBMCs of IgAN patients (2 × 105) were added into the top chamber and resuspended in RPMI 1640 medium at a final volume of 100 μl. Supernatants acquired from HMCs treated with IgA, C3a, C5a or an anti-CCL20 mAb were placed in the bottom chamber in a volume of 600 μl, and the chambers were incubated at 37 °C in 5% CO2 for 12 h. After the incubation, the nonmigratory cells in the upper chamber were scraped off, and the membrane was washed gently in PBS. The migratory cells on the bottom surface of the transwell membrane were collected for Th9 cell and CCR6 detection by flow cytometry.
Flow cytometry
Blood samples from the mice in different groups (100 μl) were collected before sample harvest, and then a red blood cell lysis buffer (C3702, Beyotime Biotechnology) was used to remove the red blood cells. The washed cells were used for flow cytometry analysis.
For Th9 cell detection, isolated cells (PBMCs from IgAN patients, cells in blood samples from mice and CD4+ T cells from transwell experiments) were suspended in RPMI 1640 medium with 10% fetal calf serum (FCS) and activated by a leukocyte activation cocktail (BD Bioscience). After stimulation, the cells were incubated with anti-CD16/32 antibodies (BioLegend) to block nonspecific staining. For the blood samples from mice, the cells were incubated with anti-mouse CD3 (APC, eBioscience) and anti-mouse CD4 (FITC, eBioscience) antibodies for 30 min in the dark at 4 °C and then permeabilized with Cytofix/Cytoperm (eBioscience) at 4 °C for 30 min. Intracellular staining was performed with an anti-IL-9 antibody (PE, BD Biosciences). For the PBMCs from IgAN patients and CD4+ T cells from transwell experiments, the cells were incubated with anti-human CD3 (BV510, BD Biosciences), anti-human CD4 (BB515, BD Biosciences) and anti-human IL-9 (BV421, BD Biosciences) antibodies as described above. Finally, the cells were analyzed with a Becton Dickinson FACSCalibur instrument using Cell Quest software.
Enzyme-linked immunosorbent assay
Serum and kidney IL-9 (eBioscience), C3a, C5a (RayBiotech) and CCL20 (Lianke Biotech) levels were tested by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s protocols.
RNA preparation and real-time PCR
Total RNA was isolated using Trizol (Takara, Japan). After quantification of RNA concentrations with a Nanodrop instrument (Thermo Scientific, Germany), the RNA samples underwent reverse transcription at equal concentrations using a Takara First Strand cDNA Synthesis kit (Takara, Japan) and were then subjected to real-time PCR analysis using Power SYBR Green (Applied Biosystems, ABI 7100, Germany). CCL20 expression in HMCs was assessed by real-time PCR. The forward and reverse primers for CCL20 were as follows: 5′‐TGC TGT ACC AAG AGT TTG CTC‐3′ and 5′‐CGC ACA CAG ACA ACT TTT TCT TT-3′.
Statistical analysis
Data are shown as the mean ± SEM. Multigroup comparisons were performed with one-way analysis of variance (ANOVA), and two-group comparisons were assessed by the least significant difference (LSD) t test (Prism software, GraphPad). Significance was set as p < 0.05.
Results
Result 1: increased Th9 cell proportions and IL-9 levels in IgAN patients and mice
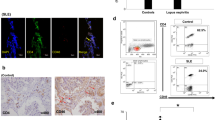
To determine whether Th9 cells participate in the immune-mediated pathogenesis of IgAN, we detected Th9 cells and IL-9 levels in IgAN patients and IgAN mice. Compared to healthy controls, the IgAN patients showed obvious elevations in Th9 cell and serum IL-9 levels (Fig. 1a, c, and d). To further explore the possible pathogenic mechanism involving Th9 cells in IgAN in vivo, an IgAN mouse model was established as described in our previous research. Tendencies toward rising Th9 cells (Fig. 1b, e) and serum and kidney IL-9 levels (Fig. 1f, g) were observed in the IgAN mice compared to control mice. Most importantly, kidney IL-9 expression was observed in the IgAN mice, and these IL-9 levels were even higher than the serum IL-9 levels, which suggested that the increased Th9 cell accumulation in the kidneys might be due to recruitment from the peripheral blood.
Increased Th9 cell proportions and IL-9 levels in IgAN patients and mice. a Representative plots of Th9 cells in PBMCs populations from IgAN patients evaluated by flow cytometry. b Representative plots of Th9 cells in the blood of an IgAN model mouse assessed by flow cytometry. c The numbers of Th9 cell in healthy control and IgAN patients (Ncontrol = 15, NIgAN = 30). d The serum IL-9 levels of IgAN patients assessed by ELISA. (E) The proportions of Th9 cells in IgAN mice and control mice. f, g Serum (f) and kidney (g) IL-9 levels in control and IgAN mice
Result 2: C3a–C3aR and C5a–C5aR axes were activated in IgAN mice
To explore the roles of C3a–C3aR and C5a–C5aR in IgAN mice, we examined the expression of C3a and C5a in the serum and kidneys by ELISA and that of C3aR and C5aR in the kidneys by IHC. Consistent with the study of IgAN patients by Liu [10], this study also found significant increases in the C3a and C5a levels in the serum and kidneys and obvious expression of C3aR and C5aR in kidney tissues in IgAN mice. Similarly, positive staining for C3aR and C5aR was detected mainly in the mesangial area, Bowman’s capsule cells and tubular epithelial cells in IgAN mouse kidney tissues (Fig. 2). In contrast, the expression of C3a and C5a and the intensity of C3aR and C5aR staining were reduced by C3aRA or C5aRA treatment in IgAN mice (Fig. 2).
C3a–C3aR and C5a–C5aR axes were activated in IgAN mice. a, c Representative images of C3aR expression (a) and C5aR expression (c) in kidney tissue samples evaluated by immunohistochemistry (400 ×). b, d The mean densities of C3aR expression (b) and C5aR expression (d) in kidney tissue samples evaluated by ImageJ software. e–h Serum C3a (e), kidney C3a (f), serum C5a (g) and kidney C5a (h) levels in different groups evaluated by ELISA. The results are shown as the mean ± SEM of repeated experiments performed in triplicate; n = 5 per group, t test
Result 3: kidney damage and Th9 cell and IL-9 levels could be reduced by C3aRA or C5aRA
To further explore the potential effects of C3aRA and C5aRA on kidney damage, we observed morphological changes in kidney tissue samples from IgAN mice. As shown in Fig. 3a–c, there was minor mesangial proliferation detected by PAS staining, IgA deposition detected by IF staining, and electron-dense deposits observed by transmission electron microscopy in the glomerular mesangial region and focal segmental foot process effacement in IgAN mice, and C3aRA or C5aRA treatment could ameliorate the aforementioned kidney damage. There was no mesangial proliferation, IgA deposition or electron-dense deposits in control mice. In addition to kidney damage assessed by histological analysis, the ACR was evaluated in mouse urine samples to detect changes. As shown in Fig. 3e, the ACR changes exhibited a tendency similar to that of the kidney damage changes. Combining the influences of C3aRA and C5aRA on kidney damage and the ACR, it was clear that C3aRA and C5aRA could rescue kidney dysfunction during IgAN development.
Kidney damage and Th9 cell and IL-9 levels could be reduced by C3aRA or C5aRA. a Representative images of pathological changes in PAS-stained kidney tissue samples from different mice (400×). b IgA deposition in a local kidney area detected by immunofluorescence staining (200 ×). c Ultrathin kidney sections stained with uranyl acetate and lead citrate and then examined by transmission electron microscopy. N = 6 per group. d Representative plots of Th9 cells in C3aRA- and C5aRA-treated IgAN mice. e 24-h urine samples collected before sample harvest to assess the ACR. f, g Serum (f) and kidney IL-9 (g) levels in different groups assessed by ELISA. h Th9 cell proportions in the blood of IgAN-, C3aRA- and C5aRA-treated IgAN mice. The results are shown as the mean ± sem of repeated experiments performed in triplicate; n = 6 per group, t test
The above findings indicated that C3aRA and C5aRA could repair kidney dysfunction, but the detailed mechanism was not clear. To clarify whether the protection mediated by C3aRA and C5aRA was attributable to the effects of C3a and C5a on Th9 cells in IgAN mice, we detected Th9 cell percentages in the blood by flow cytometry and IL-9 levels in the serum and kidney tissue samples by ELISA for each group. As shown in Fig. 3f–h, the Th9 cell percentages and IL-9 levels were reduced in the C3aRA- and C5aRA-treated IgAN mice compared to the IgAN mice. Collectively, our results might demonstrate that C3aRA and C5aRA alleviate pathological changes in the kidneys by decreasing Th9 immune responses.
Result 4: C3a and C5a enhanced the recruitment of Th9 cells via CCL20 produced by HMCs
Our group previously reported that CCL20 was expressed relatively strongly in the glomerular mesangium area and renal tubules in kidney tissue samples from IgAN patients [22]. To further assess the effects of C3a and C5a on CCL20, we used IgA with or without recombinant human C3a and C5a proteins to stimulate HMCs. As expected, we found that CCL20 expression was significantly elevated in the IgA-treated HMCs compared to control HMCs and further increased by C3a and C5a stimulation (Fig. 4a, b).
C3a and C5a enhanced the recruitment of Th9 cells via CCL20 produced by HMCs. a, b CCL20 mRNA expression (a) and protein expression (b) in HMCs treated with IgA, C3a and C5a. c–f CD4+ T cells were seeded in the upper chamber of a Transwell system, and supernatants from HMCs cultured with or without IgA, C3a, C5a and an anti-CCL20 antibody were placed in the bottom chamber of the Transwell system. After 12 h, the cells on the bottom surface of the membrane were collected, and Th9 cell proportions and CCR6 expression on the Th9 cell surface were detected by flow cytometry. (C) Representative plots of Th9 cells and CCR6 expression in different groups. Th9 cell proportions (d) and CCR6 expression (e) in different groups after transwell experiments. f IL-9 levels in supernatants collected from the bottom chamber and tested by ELISA. Data are shown as the mean ± sem of repeated experiments performed in triplicate; n = 3 per group, t test
The chemokine CCL20, as the unique ligand of CCR6, can recruit Th9 cells to lesion areas [13]. Our in vivo results demonstrated that C3aRA and C5aRA could lead to a decrease in Th9 cell levels in IgAN mice. In view of the findings mentioned above, we speculated that C3a and C5a can mediate the recruitment of Th9 cells via the CCL20-CCR6 pathway. To test this hypothesis, we performed an in vitro transwell assay to examine Th9 cell chemotaxis. A coculture system utilizing HMCs and CD4+ T cells was constructed in the absence or presence of C3a, C5a and anti-CCL20 mAb. We found that the supernatants of the HMCs treated with C3a and C5a exhibited potent chemoattraction of circulating Th9 cells, whereas that of the HMCs treated with the anti-CCL20 mAb significantly suppressed Th9 cell chemotaxis (Fig. 4d). Notably, we also found that C3a and C5a not only enhanced chemotaxis but also increased IL-9 expression levels, while the anti-CCL20 mAb exhibited the opposite tendency (Fig. 4f). Additionally, CCR6 expression on Th9 cells was increased by C3a and C5a stimulation but decreased by anti-CCL20 mAb treatment (Fig. 4e). Taken together, our results indicated that C3a and C5a produced a powerful enhancing effect on Th9 cell recruitment by promoting CCL20 production in HMCs.
Discussion
T cell infiltration is closely related to IgAN progression, which may suggest that T cells participate in renal injury in IgAN [23, 24]. CD4+ T cells play varied and multifaceted roles in IgAN development [3]. Our previous study indicated that the proportions of Th1, Th17 and Th22 cells in the kidneys and blood were all remarkably augmented, while the Treg cell proportions were reduced in IgAN mice [2, 15, 22]. However, whether Th9 cells are involved in IgAN pathogenesis is still unknown.
Th9 cells are characterized by production of IL-9, a pleiotropic cytokine with diverse effects [25]. Indeed, Th9 cells provide both beneficial and deleterious effects, which depend on the model and context of their induction. Th9 cells have been observed to produce strong anticancer immunity and induce allergic inflammation [25, 26]. To date, Th9 cells and IL-9 overexpression have been closely associated with the pathogenesis of systemic lupus erythematosus, lupus nephritis, granulomatosis with polyangiitis, experimental atherosclerosis and systemic sclerosis [27,28,29,30]. Thus, the development of strategies to therapeutically modulate Th9 cells is a crucial and clinically relevant issue.
Although Th9 cells are well explored in other immune-associated diseases of the kidneys, this research is the first study to focus on the numbers of Th9 cells and levels of IL-9 in IgAN patients and an IgAN mouse model. Th9 cell percentages were increased in the PBMCs of IgAN patients and IgAN mice compared with healthy control people and control mice, respectively, and IL-9 levels were also increased in the IgAN patients and mice; it was further found that the increased Th9 cell percentages and IL-9 levels were closely correlated with kidney damage in IgAN development. Therefore, reducing Th9 cell numbers has become a worthy and beneficial goal for clinical treatment.
Consistent with previous studies of patients with IgAN [10], our study found significant elevations in serum and kidney C3a and C5a levels and obvious increases in C3aR and C5aR expression levels in kidney tissue samples from IgAN mice, which suggested that C3a–C3aR and C5a–C5aR were activated during IgAN development. Both C3aR and C5aR1 deficiencies obviously inhibit the expression of TGF-β in IgAN mice [31], which suggests that C3a and C5a play important roles in promoting the differentiation of Th9 cells from CD4+ T cells. Additionally, our previous studies demonstrated that there were decreased percentages of Th1 and Th17 cells and an increased proportion of Treg cells in IgAN mice treated with a C5aR inhibitor compared to control mice [2]. Therefore, we speculated that the regulation of Th9 cells might be mediated by the C3a–C3aR and C5a–C5aR axes. To test this possibility, we detected Th9 cell percentages and IL-9 levels in IgAN mice treated with C3aRA or C5aRA. Our results showed that the Th9 cell proportions and serum and kidney IL-9 levels were decreased when C3aRA or C5aRA was used to treat the IgAN mice, which indicated that C3a–C3aR and C5a–C5aR might be closely related to the accumulation of Th9 cells in IgAN.
However, how C3a and C5a mediate the process of recruiting Th9 cells into glomeruli in IgAN remains largely unknown. Our previous study demonstrated that Th22 cells could be recruited into the kidneys via the CCL20-CCR6 axis [22, 32]. Lu et al. reported that HMCs treated with IgA produced CCL20 and consequently increased localized inflammatory Th17 cell recruitment to the kidneys to promote further lesion development in IgAN [14]. Additionally, research has shown that the recruitment of Th9 cells into a malignant pleural effusion can be induced by the CCL20 pathway [13]. Therefore, it is reasonable to speculate that independent of the recruitment of other subpopulations of CD4+ T cells, Th9 cell recruitment is related to the CCL20 pathway activated by HMCs during IgAN development. In addition, further investigation into the potential roles of the complement molecules C3a and C5a in CCL20-CCR6-induced chemotaxis is worthwhile.
To address these issues, we used IgA and recombinant C3a and C5a proteins to stimulate HMCs. According to our results, C3a or C5a stimulation could lead to increased expression of CCL20 in HMCs. Given our previous results, which indicated that CCL20 was highly expressed in the mesangial area of the kidneys in IgAN patients [22], we concluded that CCL20 produced by HMCs might participate in the accumulation of Th9 cells in IgAN and that this regulated process might be mediated by C3a and C5a. Combining the data indicating that C3aRA and C5aRA can reduce Th9 cell proportions in an in vitro model and that C3a and C5a stimulation enhance CCL20 production by HMCs, it is reasonable to speculate that C3a and C5a affect Th9 cell recruitment through the CCL20-CCR6 pathway.
To elucidate the mechanism underlying Th9 cell accumulation in glomeruli in IgAN, we performed an in vitro migration assay using CD4+ T cells isolated from the PBMCs of IgAN patients and supernatants from HMCs cultured in the presence or absence of C3a, C5a and an anti-CCL20 antibody. The results showed that the supernatants of the C3a- or C5a-treated HMCs could induce Th9 cell migration, but the anti-CCL20 mAb significantly inhibited the ability of the supernatants to stimulate Th9 cell chemotaxis. Moreover, CCR6 expression in Th9 cells detected by flow cytometry also exhibited a tendency similar to that of Th9 cell chemotaxis. Therefore, HMC-produced CCL20 might be able to chemoattract Th9 cells into glomeruli in IgAN, and C3a and C5a could facilitate the CCL20-CCR6 pathway.
In summary, this work builds on previous studies and expands the roles of C3a–C3aR and C5a–C5aR in IgAN beyond the role involved in regulating Th9 cell recruitment. Our data showed that the numbers of Th9 cells and levels of IL-9 were significantly increased in the IgAN group compared to the control group and that the overrepresentation of Th9 cells in glomeruli might be attributed to enhanced local proinflammatory cytokine release and CCL20 generation by HMCs. Furthermore, C3a and C5a are likely to increase the production of CCL20 by HMCs and thus augment Th9 cell recruitment and IL-9 levels, resulting in IgAN exacerbation, which indicates that C3a and C5a activation may be responsible for the immunopathology that occurs in IgAN.
References
Suzuki H, Kiryluk K, Novak J, Moldoveanu Z, Herr AB, Renfrow MB, Wyatt RJ, Scolari F, Mestecky J, Gharavi AG, Julian BA (2011) The pathophysiology of IgA nephropathy. J Am Soc Nephrol 22(10):1795–1803. https://doi.org/10.1681/ASN.2011050464
Hu X, Feng J, Zhou Q, Luo LS, Meng T, Zhong Y, Tang W, Deng S, Li X (2019) Respiratory syncytial virus exacerbates kidney damages in IgA nephropathy mice via the C5a–c5ar1 axis orchestrating Th17 cell responses. Front Cell Infect Microbiol 9:151. https://doi.org/10.3389/fcimb.2019.00151
Ruszkowski J, Lisowska KA, Pindel M, Heleniak Z, Dębska-Ślizień A, Witkowski JM (2019) T cells in IgA nephropathy: role in pathogenesis, clinical significance and potential therapeutic target. Clin Exp Nephrol 23(3):291–303. https://doi.org/10.1007/s10157-018-1665-0
Putheti P, Awasthi A, Popoola J, Gao W, Strom TB (2010) Human CD4 memory T cells can become CD4+IL-9+ T cells. PLoS ONE 5(1):e8706. https://doi.org/10.1371/journal.pone.0008706
Olson MR, Kaplan MH (2019) TH9 immunodeficiency in patients with hyper-IgE syndrome. J Allergy Clin Immunol 143(3):935–936. https://doi.org/10.1016/j.jaci.2018.10.044
Ouyang H, Shi Y, Liu Z, Feng S, Li L, Su N, Lu Y, Kong S (2013) Increased interleukin-9 and CD4+IL-9+ T cells in patients with systemic lupus erythematosus. Mol Med Rep 7(3):1031–1037. https://doi.org/10.3892/mmr.2013.1258
Yang J, Li Q, Yang X, Li M (2015) Interleukin-9 Is Associated with Elevated Anti–Double-Stranded DNA Antibodies in Lupus-Prone Mice. Mol Med 21:364–370. https://doi.org/10.2119/molmed.2014.00237
de Lira Silva NS, Borges BC, da Silva AA, de Castilhos P, Teixeira TL, Teixeira SC, Dos Santos MA, Servato JPS, Justino AB, Caixeta DC, Tomiosso TC, Espindola FS, da Silva CV (2019) The deleterious impact of interleukin 9 to hepatorenal physiology. Inflammation 42(4):1360–1369. https://doi.org/10.1007/s10753-019-00997-0
Floege J, Daha MR (2018) IgA nephropathy: new insights into the role of complement. Kidney Int 94(1):16–18. https://doi.org/10.1016/j.kint.2018.03.009
Liu L, Zhang Y, Duan X, Peng Q, Liu Q, Zhou Y, Quan S, Xing G (2014) C3a, C5a renal expression and their receptors are correlated to severity of IgA nephropathy. J Clin Immunol 34(2):224–232. https://doi.org/10.1007/s10875-013-9970-6
Liu Y, Wang K, Liang X, Li Y, Zhang Y, Zhang C, Wei H, Luo R, Ge S, Xu G (2018) Complement C3 Produced by Macrophages Promotes Renal Fibrosis via IL-17A Secretion. Front Immunol 9:2385. https://doi.org/10.3389/fimmu.2018.02385
Ye ZJ, Yuan ML, Zhou Q, Du RH, Yang WB, Xiong XZ, Zhang JC, Wu C, Qin SM, Shi HZ (2012) Differentiation and Recruitment of Th9 Cells Stimulated by Pleural Mesothelial Cells in Human Mycobacterium tuberculosis Infection. PLoS ONE 7(2):e31710. https://doi.org/10.1371/journal.pone.0031710
Bu XN, Zhou Q, Zhang JC, Ye ZJ, Tong ZH, Shi HZ (2013) Recruitment and phenotypic characteristics of interleukin 9-producing CD4+ T cells in malignant pleural effusion. Lung 191(4):385–389. https://doi.org/10.1007/s00408-013-9474-4
Lu G, Zhang X, Shen L, Qiao Q, Li Y, Sun J, Zhang J (2017) CCL20 secreted from IgA1-stimulated human mesangial cells recruits inflammatory Th17 cells in IgA nephropathy. PLoS ONE 12(5):e0178352. https://doi.org/10.1371/journal.pone.0178352
Meng T, Li X, Ao X, Zhong Y, Tang R, Peng W, Yang J, Zou M, Zhou Q (2014) Hemolytic Streptococcus May Exacerbate Kidney Damage in IgA Nephropathy through CCL20 Response to the Effect of Th17 Cells. PLoS ONE 9(9):e108723. https://doi.org/10.1371/journal.pone.0108723
Gan L, Li X, Zhu M, Chen C, Luo H, Zhou Q (2018) Acteoside relieves mesangial cell injury by regulating Th22 cell chemotaxis and proliferation in IgA nephropathy. Ren Fail 40(1):364–370. https://doi.org/10.1080/0886022X.2018.1450762
Zhang C, Wang C, Li Y, Miwa T, Liu C, Cui W, Song WC, Du J (2017) Complement C3a signaling facilitates skeletal muscle regeneration by regulating monocyte function and trafficking. Nat Commun 8(1):2078. https://doi.org/10.1038/s41467-017-01526-z
Rosenblad T, Rebetz J, Johansson M, Bekassy Z, Sartz L, Karpman D (2014) Eculizumab treatment for rescue of renal function in IgA nephropathy. Pediatr Nephrol 29(11):2225–2228. https://doi.org/10.1007/s00467-014-2863-y
Ring T, Pedersen BB, Salkus G, Goodship TH (2015) Use of eculizumab in crescentic IgA nephropathy: proof of principle and conundrum? Clin Kidney J 8(5):489–491. https://doi.org/10.1093/ckj/sfv076
Dick J, Gan PY, Ford SL, Odobasic D, Alikhan MA, Loosen SH, Hall P, Westhorpe CL, Li A, Ooi JD, Woodruff TM, Mackay CR, Kitching AR, Hickey MJ, Holdsworth SR (2018) C5a receptor 1 promotes autoimmunity, neutrophil dysfunction and injury in experimental. Kidney Int 93(3):615–625. https://doi.org/10.1016/j.kint.2017.09.018
Xiao C, Zhou Q, Li X, Li H, Zhong Y, Meng T, Zhu M, Sun H, Liu S, Tang R, Pu J, Xu Y, Xiao P (2017) Losartan and dexamethasone may inhibit chemotaxis to reduce the infiltration of Th22 cells in IgA nephropathy. Int Immunopharmacol 42:203–208. https://doi.org/10.1016/j.intimp.2016.11.025
Gan L, Zhu M, Li X, Chen C, Meng T, Pu J, Luo H, Shao F, Zhou Q (2018) Tonsillitis exacerbates renal injury in IgA nephropathy through promoting Th22 cells chemotaxis. Int Urol Nephrol 50(7):1285–1292. https://doi.org/10.1007/s11255-018-1792-2
Myllymäki JM, Honkanen TT, Syrjänen JT, Helin HJ, Rantala IS, Pasternack AI, Mustonen JT (2017) Severity of tubulointerstitial inflammation and prognosis in immunoglobulin A nephropathy. Kidney Int 71(4):343–348. https://doi.org/10.1038/sj.ki.5002046
Faria B, Henriques C, Matos AC, Daha MR, Pestana M, Seelen M (2015) Combined C4d and CD3 immunostaining predicts immunoglobulin (Ig)A nephropathy progression. Clin Exp Immunol 179(2):354–361. https://doi.org/10.1111/cei.12461
Kaplan MH (2013) Th9 cells: differentiation and disease. Immunol Rev 252(1):104–115. https://doi.org/10.1111/imr.12028
Flemming A (2018) TH9 cells tackle advanced tumours. Nat Rev Immunol 18(8):479. https://doi.org/10.1038/s41577-018-0032-4
Witko-Sarsat V, Thieblemont N (2018) Granulomatosis with polyangiitis (Wegener granulomatosis): A proteinase-3 driven disease? Jt Bone Spine 85(2):185–189. https://doi.org/10.1016/j.jbspin.2017.05.004
Li Q, Ming T, Wang Y, Ding S, Hu C, Zhang C, Cao Q, Wang Y (2017) Increased Th9 cells and IL-9 levels accelerate disease progression in experimental atherosclerosis. Am J Transl Res 9(3):1335–1343
Guggino G, Lo Pizzo M, Di Liberto D, Rizzom A, Cipriani P, Ruscitti P, Candore G, Gambino CM, Sireci G, Dieli F, Giacomelli R, Triolo G, Ciccia F (2017) Interleukin-9 over-expression and T helper 9 polarization in systemic sclerosis patients. Clin Exp Immunol 190(2):208–216. https://doi.org/10.1111/cei.13009
Yap DY, Lai KN (2015) Pathogenesis of renal disease in systemic lupus erythematosus-the role of autoantibodies and lymphocytes subset abnormalities. Int J Mol Sci 16(4):7917–7931. https://doi.org/10.3390/ijms16047917
Zhang Y, Yan X, Zhao T, Xu Q, Peng Q, Hu R, Quan S, Zhou Y, Xing G (2017) Targeting C3a/C5a receptors inhibits human mesangial cell proliferation and alleviates immunoglobulin A nephropathy in mice. Clin Exp Immunol 189(1):60–70. https://doi.org/10.1111/cei.12961
Gan L, Zhou Q, Li X, Chen C, Meng T, Pu J, Zhu M, Xiao C (2018) Intrinsic renal cells induce lymphocytosis of Th22 cells from IgA nephropathy patients through B7-CTLA-4 and CCL-CCR pathways. Mo cell Biochem 441(1–2):191–199. https://doi.org/10.1007/s11010-017-3185-8
Acknowledgments
This research was supported by the National Natural Science Foundation of China (81270786, 81670027, 81800641) and Natural Science Foundation of Hunan Province (2017JJ3495).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there is no conflict of interests.
Ethical approval
All procedures of human study performed in the present study was authorized by the Medical Ethics Committee of the Xiangya Hospital of Central South University according to the declaration of Helsinki (No. 201703582). All animal model experiments in this project were approved by the Animal Experimental Ethics Committee of Hunan Province (No. 201603376).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hu, X., Feng, J., Deng, S. et al. Anaphylatoxins enhance Th9 cell recruitment via the CCL20-CCR6 axis in IgA nephropathy. J Nephrol 33, 1027–1036 (2020). https://doi.org/10.1007/s40620-020-00708-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-020-00708-1