Abstract
Introduction
We assessed the effect of recipient body mass index (BMI) on the outcomes of renal transplantation and the management of obese patients with end-stage renal disease across the UK.
Methods
We analyzed data of 25539 adult renal transplants (2007–2016) from the UK Transplant Registry. Patients were divided in BMI groups [underweight: < 18.5, normal: 18.5–24.9 (reference group), overweight: 25–29.9, class I obese: 30–34.9, class II/III obese: ≥ 35]. We also conducted a national survey of all UK renal transplant centers on the influence of BMI on decisions regarding management of renal transplant candidates.
Results
BMI ≥ 25 was an independent risk factor for delayed graft function and primary non-function (p ≤ 0.001). Underweight (p = 0.001), class I obese (p = 0.017) and class II/III obese recipients (p < 0.001) had poorer graft survival, however, 5- and 10-year graft survival rates were good. Patient survival was shorter for underweight recipients (p < 0.001) and longer for overweight (p = 0.028) and class I obese recipients (p = 0.013). The national survey revealed significant variability among transplant centers in BMI threshold for listing patients on transplant waiting list and limited support with conservative or surgical procedures for weight control.
Conclusions
Obesity alone should not be a barrier for renal transplantation. A national strategy is required to give all patients equal chances in transplantation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The World Health Organization defines overweight as a body mass index (BMI) between 25 and 29.9 kg/m2 and obesity as BMI ≥ 30 kg/m2, which is further divided in class I (BMI: 30–34.9 kg/m2), class II (BMI: 35–39.9 kg/m2) and class III or morbid obesity (BMI ≥ 40 kg/m2) [1]. Obesity is increasing throughout the world. It is expected that 2.7 billion adults and 70 million children will be overweight or obese in 2025 worldwide [2]. As obesity increases in the general population, it also increases amongst patients with end stage renal disease (ESRD) [3].
Kidney transplantation is the treatment of choice for most patients with ESRD. The effect of obesity on renal transplant long-term outcomes remains controversial, with some studies identifying an impact on graft failure, while others not [4,5,6,7,8,9]. In particular, three large studies from the USA have reported higher risk of graft loss in obese recipients [4, 8, 9], whereas another large study from the USA, as well as two others from the UK and Brazil showed no significant difference in graft survival between obese and non-obese recipients [5,6,7]. However, there is consensus amongst studies with regard to the increased risk of delayed graft function (DGF) in obese patients [4, 6, 9].
Although there are national guidelines regarding the management of obese patients in the general population in the UK, there is no national strategy for managing obese patients with ESRD and enlisting them for renal transplantation. There is a lack of a common policy for the proper treatment of obese candidates and unanimous enlisting criteria for this type of ESRD patients are not available at a national level so far [10, 11].
The aim of our study was to estimate the effect of recipient BMI on specific renal transplant outcomes, namely DGF rates, primary non-function (PNF) rates, graft survival and recipient survival, based on the most recent and comprehensive UK data gathered from the UK transplant registry. We also assessed through a national survey the various strategies among all UK renal transplant centres regarding enlisting criteria for obese ESRD patients and the available treatment options in terms of weight management that are provided by these centres. In addition, we tried to highlight the need for a national strategy concerning the management and enlisting of obese renal transplant candidates and make suggestions based on our findings obtained from the analysis of data at a national level.
Methods
Patients
Data from the UK Transplant Registry were provided by the National Health Service Blood and Transplant (NHSBT) for a 10 year period (1st January 2007–31st December 2016) for a total of 25,539 adult renal transplant recipients. In October 2018, data for the following parameters were collected retrospectively from a prospectively maintained database:
- 1.
Recipient characteristics: age, gender, ethnicity, BMI at transplant, cause of renal failure, number of previous renal transplants, time on waiting list and whether the transplant was preemptive or the patients had already started dialysis
- 2.
Donor characteristics: age and type (live or deceased after brain or circulatory death)
- 3.
Cold ischaemia time (time between cold perfusion of donor and reperfusion of kidney with recipient’s blood)
- 4.
HLA mismatch (level 1: 000, level 2: 0DR+0/1B, level 3: 0DR+2B or 1DR+0/1B, level 4: 1DR+2B or 2DR) [12] and transplant compatibility (ABO and HLA)
- 5.
Transplant outcomes: whether there was immediate function, DGF (defined as the need for dialysis during the first week post-transplant) or PNF (defined as permanent loss of allograft function starting immediately after transplantation), graft survival (time between transplantation and return to dialysis or retransplantation) and recipient survival (time between transplantation and recipient’s death).
In addition, we conducted a national survey to assess the current management of obese patients with ESRD in UK. A questionnaire of twelve questions was sent via email to each of the 23 UK renal transplant units. The questionnaire focused on enlisting criteria for patients for renal transplant in regards to BMI and on the weight management pathways for ESRD patients with BMI ≥ 35 kg/m2.
Our study conforms to the 2000 Declaration of Helsinki and the 2008 Declaration of Istanbul.
Statistical analysis
BMI at the time of transplant was grouped as < 18.5 kg/m2 (underweight), 18.5–24.9 kg/m2 (normal weight), 25–29.9 kg/m2 (overweight), 30–34.9 kg/m2 (class I obese) and ≥ 35 kg/m2 (class II/III obese). Normal weight recipients were considered as the reference group for all comparisons.
The characteristics of those that had immediate graft function, DGF or PNF were described using summary statistics and compared using t tests or ANOVA for parametric descriptors, Mann–Whitney U test or Kruskal–Wallis for non-parametric variables or Chi squared tests for categorical variables. Those that had PNF were compared to those with some function (either delayed or immediate) in terms of BMI group using logistic regression analysis, adjusting for all baseline variables in the model. This was then repeated to compare those with DGF to those with immediate function. The correlation between eGFR and BMI was tested using Spearman’s rank correlation coefficient after removing cases with PNF.
Graft survival was assessed for those patients that have shown some graft function, after removing patients categorized as PNF, in a survival analysis using a Cox regression model. Graft loss rates were presented by baseline variable. Continuous variables were categorized in order to present event rates but were used as continuous measures in the regression analysis. The analysis was primarily interested in the association between BMI and survival and we adjusted for the other baseline variables. This analysis was repeated for patient survival using the same variables but with all transplant patients, including those with PNF.
Data were analysed using Stata version 14.2. Two-sided tests were conducted and p < 0.05 was considered statistically significant.
Results
Out of the 25,539 patients included, BMI at the time of transplant was available for 20,864 cases. The mean BMI was 26.3 kg/m2 (SD: 4.7) and the median BMI was 25.9 kg/m2 (min–max: 12.4–52.2). The distribution of recipients in the various BMI groups was as follows: underweight: 538 (2.6%), normal weight: 8167 (39.1%), overweight: 7562 (36.2%), class I obese: 3790 (18.2%), class II obese: 723 (3.5%), class III or morbidly obese: 84 (0.4%). The mean surveillance period for the entire population of the study was 1715.5 days (SD: 1081.9) and the median surveillance period was 1519 days (range 0–4245).
Table 1 summarizes patient characteristics according to BMI group. Due to the large number of patients included in our analysis, BMI groups were found to have statistically significant differences in nearly all tested parameters. Notably, underweight group had a higher percentage of female recipients (59%) and lower percentage of diabetic recipients (2%). Moreover, there were higher rates of Asian (17%) and lower rates of White patients (74%) in this BMI group. It was also noticed that the rates of diabetic patients increased as BMI increased (from 2% to in underweight to 14% to class II/III obese recipients). In terms of donor type, the lowest rates of live donors were observed in class II/III obese recipients (30%), whereas underweight and normal weight recipients had the lowest rates of donors after circulatory death (22% and 23%, respectively).
Delayed graft function
There was a significant increase in DGF rates as BMI increased [BMI < 18.5 kg/m2: 68/477 (14.3%), BMI 18.5–24.9 kg/m2: 1238/7302 (17%), BMI 25-29.9 kg/m2: 1383/6756 (20.5%), BMI 30–34.9 kg/m2: 821/3353 (24.5%), BMI ≥ 35 kg/m2: 182/692 (26.3%)] (p < 0.001). BMI was an independent predictor of DGF compared to immediate graft function in logistic regression analysis (Table 2). Underweight and normal recipients had similar risk for DGF (OR: 0.81, 95% CI 0.6–1.08, p = 0.146). Overweight (OR: 1.17, 95% CI 1.06–1.29, p = 0.001), class I obese (OR: 1.57, 95% CI 1.41–1.76, p < 0.001) and class II/III obese recipients (OR: 1.73, 95% CI 1.41–2.13, p < 0.001) had higher risk for DGF, which increased as BMI increased. Additional independent risk factors for DGF in logistic regression analysis were male recipient’s gender, donor’s age, donation after brain or circulatory death, Black recipient’s race, diabetes mellitus in recipient, renal transplant after onset of dialysis, history of previous renal transplant, longer cold ischaemia time, level 4 of HLA mismatch and ABO or HLA incompatible renal transplant (Table 2).
Graft primary non-function
A significant increase in graft PNF rate was seen as BMI increased over 25 kg/m2 [BMI < 18.5 kg/m2: 9/486 (1.9%), BMI 18.5–24.9 kg/m2: 120/7422 (1.6%), BMI 25–29.9 kg/m2: 148/6904 (2.1%), BMI 30–34.9 kg/m2: 93/3446 (2.7%), BMI ≥ 35 kg/m2: 26/718 (3.6%)] (p < 0.001). BMI was an independent predictor of PNF in logistic regression analysis (Table 3). Underweight and normal weight recipients had similar risk for PNF (OR: 1.25, 95% CI 0.62–2.5, p = 0.531). Overweight (OR: 1.32, 95% CI 1.02–1.71, p = 0.035), class I obese (OR: 1.67, 95% CI 1.25–2.25, p = 0.001) and class II/III obese recipients (OR: 2.24, 95% CI 1.39–3.59, p = 0.001) exhibited higher risk for PNF compared to normal weight patients, which increased as the BMI increased. Additional independent risk factors for PNF of the graft in logistic regression analysis were donor’s age, donation after circulatory death, Asian or Black recipient’s ethnicity, renal transplant after onset of dialysis, history of previous renal transplant and longer cold ischaemia time (Table 3).
Graft function and survival
eGFR at 12 months after renal transplant was inversely correlated with BMI (r = − 0.131, p < 0.001). In addition, eGFR at 12 months after renal transplant showed a slight, but statistically significant, decrease as the BMI category increased (p < 0.001). In particular, underweight recipients had a median eGFR of 60 ml/min (min–max: 6–130), recipients with normal BMI had a median eGFR of 54 ml/min (min–max: 3–130), overweight recipients had a median eGFR of 51 ml/min (min–max: 3–130), recipients with class I obesity had a median eGFR of 48 (min–max: 3–124), and recipients with class II/III obesity had a median eGFR of 47 (min–max: 3–112). All pairwise comparisons provided statistically significant differences (p ≤ 0.001), except for the comparison between class I and class II/III obese recipients (p = 1).
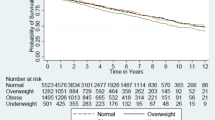
Kaplan–Meier analysis indicated a significant difference in graft survival between the BMI groups (Fig. 1 ). 5-year graft survival rates were as follows: underweight: 87%, normal weight: 90%, overweight: 91%, class I obese: 88%, class II/III obese: 86%. Nevertheless, differences were more obvious at 10 years after transplant: underweight: 72%, normal weight: 82%, overweight: 82%, class I obese: 80%, class II/III obese: 69%. Normal weight and overweight recipients had similar rates of graft loss with 21.72 graft losses and 19.87 graft losses per 1000 patient years, respectively. (Rate ratio: 0.91, 95% CI 0.82–1.01, p = 0.084). Underweight, class I obese and class II/III obese recipients had higher rates of graft loss. In particular, underweight recipients had 30 graft losses per 1000 patient-years (Rate ratio: 1.38, 95% CI 1.07–1.76, p = 0.011), class I obese recipients had 25.34 graft losses per 1000 patient-years (Rate ratio: 1.16, 95% CI 1.03–1.31, p = 0.016) and class II/III recipients had 32.94 graft losses per 1000 patient-years (Rate ratio: 1.5, 95% CI 1.22–1.85, p < 0.001).
BMI was an independent predictor of graft survival in the Cox regression multivariable analysis (Table 4). Normal weight and overweight recipients had similar risk of graft loss (HR: 0.98, 95% CI 0.87–1.1, p = 0.749). Underweight (HR: 1.53, 95% CI 1.18–1.98, p = 0.001), class I obese (HR: 1.19, 95% CI 1.03–1.36, p = 0.016) and class II/III obese patients (HR: 1.53, 95% CI 1.21–1.93, p < 0.001) had significantly shorter graft survival compared to normal weight patients. Additional independent risk factors for worse graft survival in Cox regression analysis were recipient’s age, Black recipient’s race, donor’s age, donation after brain or circulatory death, renal transplant after onset of dialysis, history of previous renal transplant, DGF, level 3 or level 4 of HLA mismatch and ABO or HLA incompatible renal transplant (Table 4).
Recipient survival
1.1% of recipients died within 90 days of the renal transplant operation. No significant differences were detected regarding the rates of 90-day postoperative mortality among different BMI categories. There was no significant difference in patient survival between the BMI groups in the Kaplan–Meier analysis (Fig. 2). In this regards, 5-year recipient survival rates were similar among BMI groups: underweight: 90%, normal weight: 91%, overweight: 90%, class I obese: 90%, class II/III obese: 90%. The rates at 10 years after transplant were as follows: underweight: 80%, normal weight: 80%, overweight: 79%, class I obese: 77%, class II/III obese: 74%. There were 20.21 deaths per 1000 patient-years in normal weight recipients. Only overweight recipients had a significantly higher risk of death, with 22.77 deaths per 1000 patient-years (Rate ratio: 1.13, 95% CI 1.01–1.26, p = 0.029). The differences between normal weight recipients and the other BMI groups were not statistically significant. In particular, there were 23.07 deaths per 1000 patient-years in underweight recipients (Rate ratio: 1.14, 95% CI 0.85–1.54, p = 0.379), 22.71 deaths per 1000 patient-years in class I obese recipients (Rate ratio: 1.13, 95% CI 0.99–1.29, p = 0.072) and 23.78 deaths per 1000 patient-years in class II/III obese recipients (Rate ratio: 1.18, 95% CI 0.92–1.51, p = 0.194). It worth mentioning that 78.3% of the recipients who died during follow-up they had a functioning renal transplant.
However, in Cox regression analysis underweight patients had shorter survival compared with normal weight patients (HR: 1.81, 95% CI 1.31–.51, p < 0.001), whereas overweight (HR: 0.87, 95% CI 0.78–0.99, p = 0.028) and class I obese patients (HR: 0.83, 95% CI 0.72–0.96, p = 0.013) exhibited a longer survival. Class II/III obese recipients had similar survival (HR: 1.09, 95% CI 0.83–1.42, p = 0.543) with the normal weight group (Table 5). Additional independent risk factors for worse recipient survival in Cox regression analysis were male recipient’s gender, recipient’s age, donor’s age, recipient history of diabetes mellitus, renal transplant after onset of dialysis, longer cold ischaemia time, DGF, PNF of the graft, ABO or HLA incompatible renal transplant and longer waiting time before renal transplant (Table 5).
National survey
All the 23 UK renal transplant centres responded to the survey and answered all 12 questions. The mean percentage of ESRD patients with BMI ≥ 35 by unit was 18.4% (8%–35%).
Questions for listing criteria. The cut-off BMI for enlisting patients on renal transplant waiting list is < 40 kg/m2 in 14 centres, < 35 kg/m2 in 6 centres, while 3 centres do not have a BMI cut-off. Seventeen centres have different BMI cut-offs for live and deceased donor transplants. Fourteen centers suspend patients from transplant waiting list if BMI exceeds the cut-off target. Four centers would exclude patients from marginal kidney offers and dual kidney transplants solely relied on recipients’ BMI.
Questions on weight management. All responders agreed that obesity affects the outcome of renal transplantation and 16 centres agreed that obesity management should be a core part of managing ESRD patients. However, 6 centres have no pathway for weight management of obese patients, 9 have only a dietician, 8 would consider bariatric surgery as a treatment option and only 1 centre refers obese patients regularly for bariatric surgery. During the last year, no patient had a bariatric procedure in 13 centres, less than 5 patients had a bariatric procedure in 9 centres and more than 5 patients were treated with this approach in just 1 centre. Regarding the types of bariatric procedures that are available, sleeve gastrectomy is available in 10 centres, duodenal bypass in 8 centres, and gastric band in 6 centres. Finally, only 4 centres support the opinion that transplant surgeons and nephrologists should be trained in obesity management, while another 3 centres consider this as an option (Table 6).
Discussion
To our knowledge this is the largest and most recent UK registry analysis evaluating the effects of obesity on kidney transplant outcomes. The results of this analysis indicate that high BMI is an independent risk factor for DGF, PNF and graft loss. Of note, the risk of DGF increases along with BMI increase and for class II/III obese recipients it becomes 1.73 times higher than that of normal BMI recipients. Previous studies have shown similar results [4, 6, 9]. We have also detected a similar association between BMI and PNF, with the risk being 2.24 times higher in class II/III obese recipients than that of normal weight individuals. Most importantly, our results suggest that graft survival is significantly shorter in underweight, class I and class II/III obese patients (HR 1.52, CI 1.21–1.92 for class II/III obese patients). However, 5-year graft survival rates were more or less similar amongst different BMI groups, ranging between 86% and 91%. The difference was more obvious at 10 years after transplant, but graft survival rates were still very good, with 69% of class II/III obese recipients having a functioning renal graft. In addition, when we examined eGFR at 12 months after transplant, we found that there is a slight decrease in renal graft function as BMI increases. Previous studies evaluating the effect of BMI on graft survival have shown conflicting results, with some of them suggesting an increased risk for graft loss for high BMI recipients [4, 8, 9, 13,14,15], whereas others have shown no significant difference [5, 6, 16]. In this regard, a large previous UK study showed that BMI has no effect on graft survival [7].
Underweight patients had shorter overall survival that may be attributed to the effects of malnourishment [17]. None of the other groups had inferior results compared to normal BMI patients in regards to patient survival, while overweight and class I obese patients showed a longer survival compared to normal weights. The results of previous studies on this front are also conflicting, with some of them suggesting worse outcomes for high BMI patients [13, 15, 18], whereas others failed to detect any difference [4,5,6, 14, 17]. The previously mentioned UK study also showed no association between BMI and patient survival. At this point, we should also mention that previous studies have demonstrated the survival benefit of obese transplanted patients when compared with obese patients remaining on dialysis [19].
Based on the results of the present analysis, we believe that ESRD patients should not be precluded from kidney transplantation based solely on high BMI. Other factors, such as age, availability of living donor, difficulties on dialysis and cardiovascular comorbidities, should also be taken into consideration, when considering such patients for listing. A multidisciplinary team consisting of patient’s nephrologist, surgeons and anaesthetists should take the final decision for enlisting the patients. If patients are accepted for transplantation, they should be well informed and appropriately consented for the increased risks to the graft related to high BMI, while taking also into account the generally higher rate of postoperative complications in obese patients [20].
Very interesting conclusions were extracted from the national survey we conducted. This showed significant variability on listing criteria among UK kidney transplant centers with BMI affecting all steps of enlisting process and organ selection. As a result, although patients share organs from the same pool, they have different chances for transplant depending on the center they are registered. Even when they go on the list, BMI alone can exclude them from specific organ offers. Furthermore, patients can spend a long time trying to lose weight and become eligible for transplantation and finally get transplanted with a higher than their centre’s acceptance weight. Unnecessary delays on enlisting patients also come with a cost for the healthcare system. The average cost of haemodialysis is approximately £34,000 per patient per year [21], whereas the cost of a renal transplantation is about £18,500 [22] and the cost of one year of immunosuppression therapy is around £6000 per patient [23].
We believe that a national strategy and national guidelines are necessary to give patients equal chances to get a renal transplant irrespective of their BMI. However, as some centres might not have the required support or volume of transplants in order to offer their recipients the best possible outcomes, it seems reasonable to refer them to the closest center that can effectively manage them. Concentrating this population in high volume centers could offer better long term support, endorse relevant research and allow the application of novel techniques that could improve outcomes [24]. The national survey has also shed light on the way renal transplant centres help high BMI ESRD patients to lose weight. Although all responders agreed that obesity affects renal transplant outcomes and most centres consider high BMI as a restricting factor for enlisting patients, there is only limited support offered to them with regard to weight reduction. As the survey showed, many hospitals have only a dietitian available, while others have no dedicated support at all. Furthermore, for those patients that need it, access to bariatric surgery is very difficult with only one centre providing bariatric surgery to more than 5 obese renal transplant candidates in a year’s time. This is the result of long waiting times for bariatric surgery, but also a reflection of the unwillingness to operate on these patients as they are considered high risk for bariatric procedures.
Experience so far has shown that conservative ways for weight loss demonstrate generally poor results. For ESRD patients, this is even more difficult due to dietetic restrictions and poor exercise tolerance [25]. Furthermore, obese ESRD patients have higher rates of diabetes and increased difficulties to control it [25]. Moreover, access formation can be especially challenging for these patients [26]. Based on the above, we believe that transplant centers should have multidisciplinary teams consisting of nephrologists and surgeons with a special interest in obese ESRD patients, as well as dietitians and psychologists to support the patients with weight control. They should also keep close links with bariatric teams to ensure bariatric surgery will be available for patients that need it.
We acknowledge the limitations of our study. First of all, there are inherent weaknesses associated with registry databases analysis, such as missing data (e.g. BMI at the time of transplant was available for 20,864 out of the 25,539 patients), and some possible erroneous entries that might have escaped our thorough screening. Second, the retrospective nature of the study made it impossible to adjust the regression analyses for other possible confounding factors, apart from diabetes mellitus and the cause of renal failure, for which data were available. This might have affected the associations between the various obesity groups and graft or patient survival. The lack of data regarding surgical complications, diabetes mellitus onset and donor/recipient weight ratio are a few examples of potential confounding factors that are missing from our database, but could affect graft and patient outcomes. Finally, the large number of patients involved, although it has enormously increased the power of the study, it has also led on the other hand to the identification of statistical significant associations that might have no clinical relevance.
In conclusion, management of obese ESRD patients poses challenges in terms of dialysis, transplantation and weight control. Appropriate support with specialized teams, treatment of patients in centers with significant experience and cooperation with bariatric surgeons could offer the best of the outcomes. The results of our analysis indicate that obesity should not be a barrier for kidney transplantation. A national strategy is required to ensure equity of access to donor organ pool.
References
Stommel M, Schoenborn CA (2010) Variations in BMI and prevalence of health risks in diverse racial and ethnic populations. Obesity (Silver Spring) 18:1821–1826
Swinburn B, Vandevijvere S (2016) WHO report on ending childhood obesity echoes earlier recommendations. Public Health Nutr 19:1–2
Potluri K, Hou S (2010) Obesity in kidney transplant recipients and candidates. Am J Kidney Dis 56:143–156
Cannon RM, Jones CM, Hughes MG, Eng M, Marvin MR (2013) The impact of recipient obesity on outcomes after renal transplantation. Ann Surg 257:978–984
Pieloch D, Dombrovskiy V, Osband AJ, Lebowitz J, Laskow DA (2014) Morbid obesity is not an independent predictor of graft failure or patient mortality after kidney transplantation. J Ren Nutr 24:50–57
Gusukuma LW, Harada KM, Baptista AP et al (2014) Outcomes in obese kidney transplant recipients. Transplant Proc 46:3416–3419
Krishnan N, Higgins R, Short A et al (2015) Kidney transplantation significantly improves patient and graft survival irrespective of BMI: a cohort study. Am J Transplant 15:2378–2386
Naik AS, Sakhuja A, Cibrik DM, Ojo AO, Samaniego-Picota MD, Lentine KL (2016) The impact of obesity on allograft failure after kidney transplantation: a competing risks analysis. Transplantation 100:1963–1969
Kwan JM, Hajjiri Z, Metwally A, Finn PW, Perkins DL (2016) Effect of the obesity epidemic on kidney transplantation: obesity is independent of diabetes as a risk factor for adverse renal transplant outcomes. PLoS One 11:e0165712
Pruthi R, Tonkin-Crine S, Calestani M et al (2018) Variation in practice patterns for listing patients for renal transplantation in the United Kingdom: a national survey. Transplantation 102:961–968
Tonkin-Crine S, Pruthi R, Taylor DM et al (2018) Assessing consensus between UK renal clinicians on listing for kidney transplantation: a modified Delphi study. Transplant Direct 4:e343
Johnson RJ, Fuggle SV, Mumford L, Bradley JA, Forsythe JL, Rudge CJ (2010) A New UK 2006 National Kidney Allocation Scheme for deceased heart-beating donor kidneys. Transplantation 89:387–394
Lafranca JA, IJermans JN, Betjes MG, Dor FJ (2015) Body mass index and outcome in renal transplant recipients: a systematic review and meta-analysis. BMC Med 13:111
Hill CJ, Courtney AE, Cardwell CR et al (2015) Recipient obesity and outcomes after kidney transplantation: a systematic review and meta-analysis. Nephrol Dial Transplant 30:1403–1411
Sood A, Hakim DN, Hakim NS (2016) Consequences of recipient obesity on postoperative outcomes in a renal transplant: a systematic review and meta-analysis. Exp Clin Transplant 14:121–128
Nicoletto BB, Fonseca NK, Manfro RC, Gonçalves LF, Leitão CB, Souza GC (2014) Effects of obesity on kidney transplantation outcomes: a systematic review and meta-analysis. Transplantation 98:167–176
Bhaskaran K, Dos-Santos-Silva I, Leon DA, Douglas IJ, Smeeth L (2018) Association of BMI with overall and cause-specific mortality: a population-based cohort study of 3.6 million adults in the UK. Lancet Diabetes Endocrinol 6:944–953
Ahmadi SF, Zahmatkesh G, Streja E et al (2014) Body mass index and mortality in kidney transplant recipients: a systematic review and meta-analysis. Am J Nephrol 40:315–324
Pelletier SJ, Maraschio MA, Schaubel DE et al (2003) Survival benefit of kidney and liver transplantation for obese patients on the waiting list. Clin Transpl 2003:77–88
Tsai A, Schumann R (2016) Morbid obesity and perioperative complications. Curr Opin Anaesthesiol 29:103–108
Baboolal K, McEwan P, Sondhi S, Spiewanowski P, Wechowski J, Wilson K (2008) The cost of renal dialysis in a UK setting—a multicentre study. Nephrol Dial Transplant 23:1982–1989
Treharne C, Liu FX, Arici M, Crowe L, Farooqui U (2014) Peritoneal dialysis and in-centre haemodialysis: a cost-utility analysis from a UK payer perspective. Appl Health Econ Health Policy 12:409–420
Muduma G, Shaw J, Hart WM, Odeyemi A, Odeyemi I (2014) Cost utility analysis of immunosuppressive regimens in adult renal transplant recipients in England and Wales. Patient Prefer Adherence 8:1537–1546
Oberholzer J, Giulianotti P, Danielson KK et al (2013) Minimally invasive robotic kidney transplantation for obese patients previously denied access to transplantation. Am J Transplant 13:721–728
Lesage J, Gill JS (2017) Management of the obese kidney transplant candidate. Transplant Rev (Orlando) 31:35–41
Miles Maliska C, Jennings W, Mallios A (2015) When arteriovenous fistulas are too deep: options in obese individuals. J Am Coll Surg 221:1067–1072
Acknowledgements
We would like to thank Ms Lisa Mumford for her valuable assistance in providing the data from NHSBT and her useful comments on their analysis.
Author information
Authors and Affiliations
Contributions
IDK and TK: Literature search, study design, data analysis and interpretation, manuscript preparation and revision; VB: Literature search, data analysis and interpretation, manuscript preparation; PP: Study design, data analysis and interpretation, manuscript preparation; CF: Data analysis and interpretation, manuscript revision; CC, BP, NKa, NK: Literature search, data interpretation, manuscript revision; FC: Literature search, study design, data analysis and interpretation, manuscript revision; IL: Literature search, study design, data analysis and interpretation, manuscript preparation and revision.
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that there is no financial support from any organisation for the submitted work, no financial relationships with any organisations that might have an interest in the submitted work and no other relationships or activities that could appear to have influenced the submitted work. All the authors declare that there are no conflicts of interest.
Research involving human participants
Our study conforms to the 2000 Declaration of Helsinki and the 2008 Declaration of Istanbul.
Informed consent
Not applicable. Retrospective study based on the UK Transplant Registry of NHSBT.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kostakis, I.D., Kassimatis, T., Bianchi, V. et al. UK renal transplant outcomes in low and high BMI recipients: the need for a national policy. J Nephrol 33, 371–381 (2020). https://doi.org/10.1007/s40620-019-00654-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-019-00654-7