Abstract
Atypical hemolytic uremic syndrome (aHUS) has gained increased visibility over several years as an important cause of renal failure. Unfortunately, diagnosis is often difficult because individual courses can be highly variable depending the causative genetic mutations. Here we present the case of a patient with a failed renal allograft and acute failure of a second allograft who was ultimately diagnosed with aHUS. Interestingly, he developed early de novo donor specific antibodies (DSA) after the second renal transplant in context of likely recurrent aHUS. Terminal complement inhibition with eculizumab resulted in prompt improvement of renal allograft function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atypical hemolytic uremic syndrome (aHUS) has gained increased visibility over several years as an important cause of renal failure. Unfortunately, diagnosis is often difficult because individual courses can be highly variable depending upon the causative genetic mutations [1–3]. The disease is often difficult to distinguish from other forms of microangiopathic hemolytic anemia [4]. Furthermore, it is thought that only a fraction of disease-causing mutations have been identified. Here we present the case of a patient with a failed renal allograft and acute failure of a second allograft who was ultimately diagnosed with genetically-proven aHUS.
Case report
First renal allograft
The patient was a 39 year-old male with a history of Alport syndrome, whose diagnosis was made at age 24. Full details of the original diagnosis are unavailable. The patient had attendant hearing loss. His sister had renal failure and a renal transplant, reportedly also due to Alport syndrome. There was no other family history of renal disease, which raised the possibility of non-X linked disease. There was a history of sarcoidosis in the father. Social history was notable for 30 pack-years of smoking (ongoing at the time of first transplant) without illicit drug use.
At age 34, the patient underwent deceased donor kidney transplantation. He was unsensitized at the time and had been on hemodialysis for 2 years prior to transplantation. Cold ischemic time was prolonged, and donor kidney biopsy was favorable, with 0/50 sclerosed glomeruli. Induction was with alemtuzumab. Six human leukocyte antigens (HLA) were mismatched, and initial allograft function was slow.Footnote 1 Nadir creatinine was 2.6 mg/dL. Due to slow graft function and multiple episodes of acute allograft injury, several biopsies were performed, one of which revealed a Banff 1B rejection (Fig. 1a) about 3.5 months post-transplant. Biopsy findings additionally were suspicious for thrombotic microangiopathy (Fig. 1b), which was attributed to calcinuerin inhibitor exposure. Additionally, there was 40–50% interstitial fibrosis and tubular atrophy. The patient was treated for acute cellular rejection with rabbit anti-thymocyte globulin and intravenous corticosteroids.
a Renal allograft biopsy 3.5 months following the first transplant showing a fibrin thrombus in the glomerulus (arrow). b Severe tubulitis in the same biopsy (arrowheads). c Renal allograft biopsy 13 days following the second transplant showing mild tubulitis (arrowheads). d Renal allograft biopsy 7 months following the second transplant showing fragmented red blood cells in the glomerulus (arrows)
Several laboratory values preceding and at the time of biopsy (in retrospect) suggested thrombotic microangiopathy. These included progressive thrombocytopenia (Supplemental Fig. 1a) and anemia (Supplemental Fig. 1b). At the time of biopsy, lactate dehydrogenase (LDH) was elevated to 292 L/U, and serum haptoglobin was below the threshold for detection. Peripheral blood smear, reviewed by hematopathology for anemia, revealed only severe normocytic, normochromic anemia with minimal polychromasia and occasional ovalocytes and burr cells. Mild thrombocytopenia with occasional large platelets was also noted. Reticulocyte count was elevated at 3.4%. Total bilirubin was 0.7 mg/dL (direct 0.1 mg/dL). INR was 1. Tacrolimus trough was not supra-therapeutic at 5.5 ng/mL.
The patient’s course was further complicated by an early urinary tract infection, ureteral stent migration and stricture requiring percutaneous nephrostomy, and early BK viruria without viremia or polyomavirus nephropathy. Serologic testing was negative for donor specific HLA antibodies (DSA) and anti-glomerular basement membrane antibodies. Tacrolimus levels remained therapeutic throughout. Hematology was consulted for pancytopenia (leukocyte count 1.5 × 103/μL; hemoglobin 7 g/dL, platelet count 144 × 103/μL at the time of above biopsy) that was thought multifactorial in etiology, with an unrevealing peripheral smear (as above) and negative parvovirus polymerase chain reaction test. Unfortunately, the patient developed progressive allograft failure requiring re-initiation of hemodialysis 4 months posttransplant. He subsequently developed acute allograft rejection prompting explant 6 months posttransplant and re-listing. Explant pathology revealed hemorrhagic necrosis.
Second renal allograft
By the time of the patient’s second transplant at age 39, he was highly sensitized (Class I and II panel reactive antibodies 99 and 100, respectively). The deceased donor was a 43 year-old Caucasian female. Kidney donor profile index (KDPI) was 43%. Three HLA antigens were mismatched (one HLA-A and two HLA-B mismatches) without any identified current or historic donor specific antibodies.Footnote 2 Pre-operative flow cytometric cross-match was negative, and induction was with alemtuzumab.
Renal allograft function was again slow, with a nadir creatinine of 2.5 mg/dL. As creatinine began to rise to 3.7 mg/dL, the patient underwent early allograft biopsy and DSA assessment on day thirteen posttransplant. Laboratory values at that time are listed in Table 1. Biopsy at that time showed borderline cellular rejection with some swollen endothelial cells (Fig. 1c). C4d staining was negative, and there was no peritubular capillaritis. Surprisingly, the patient developed de novo DSA directed against HLA-B7 and HLA-B27 with titers of 1:64 and 1:16, respectively. Testing was performed with single antigen beads (One Lambda, Canoga Park, CA). Prior serum samples were re-assessed by the histocompatibility laboratory and found to be negative for donor specific antibodies. Serial monitoring had consistently detected these antibodies, with varying strengths, in four serum samples over 6 weeks, suggesting the detection of these DSAs were not transient due to the strength/titer and detection of DSA multiple serum samples. These DSAs ultimately resolved with intravenous corticosteroid and intravenous immunoglobulin therapy. Testing for immunoglobulin subtypes and complement fixation was not performed. Three subsequent biopsies showed persistent borderline rejection despite ongoing corticosteroid treatment. Only one of these demonstrated weak C4d staining (3 months posttransplant), which was not treated aggressively as donor specific antibody titers were negative by that time. Serum creatinine stabilized around 3.4 mg/dL by 4 months post transplant with 30–40% interstitial fibrosis and tubular atrophy on corresponding biopsy. Despite a few supra-therapeutic tacrolimus troughs early on, levels generally remained within therapeutic range of 6–8 ng/mL.
Acute failure of the second renal allograft
Seven months posttransplant, the patient presented to the hospital with a respiratory illness characterized by headache, malaise, cough, dyspnea, and fever to 100.6 Fahrenheit. He was found to have an acute hypertensive crisis requiring nicardipine infusion and acute renal failure with a serum creatinine of 6.6 mg/dL. Physical examination was notable for bilateral pulmonary rales. There were no pericardial friction rubs. The allograft site was without bruits or tenderness. There were bilateral pulmonary infiltrates on chest radiography. Full laboratory results are reported in Table 1. They were notable for a hemoglobin of 8.4 g/dL (prior baseline around 11 mg/dL) and platelets of 101 K/μL (prior baseline around 180 K/μL). Urinalysis revealed 0–5 WBCs/hpf and 11–50 RBCs/hpf. Evaluation for infectious etiologies was unrevealing. Spot urine protein to creatinine ratio was about 1 gram. Peripheral smear showed no evidence of microangiopathic hemolytic anemia. Total complement activity (CH50) was normal, as were C3 and C4 levels. A direct Coomb’s test was negative. Allograft ultrasound was unrevealing. Donor specific antibody testing remained negative. Allograft biopsy (Fig. 1d) raised concern for hemolytic uremic syndrome. C4d staining remained negative with mild peritubular capillaritis. DSA testing was negative at the time of biopsy.
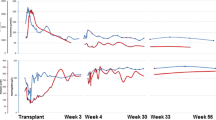
The patient’s serum creatinine peaked at 11.8 mg/dL (about 7.5 months posttransplant) with a blood urea nitrogen level of 145 mg/dL alongside mild uremic symptoms and severe peripheral edema. After extensive discussions with the patient, dialysis was withheld, and a trial of eculizumab was pursued alongside intravenous corticosteroids and conversion of tacrolimus to rapamycin. Eculizumab was started at 900 mg weekly for 4 weeks, followed by 1,200 mg every other week based on the protocol reported by Legendre et al. [5] Due to the need for urgent treatment of acute atypical hemolytic uremic syndrome, eculizumab treatment was not delayed for vaccination against meningococcus. The patient was therefore prescribed ciprofloxacin prophylaxis and subsequently vaccinated against meningococcus. The patient’s creatinine quickly declined in association with eculizumab therapy (Fig. 2). Platelets responded as well, increasing from about 100 K/μL (prior baseline around 180 K/μL) to nearly 250 K/μL almost immediately following treatment (Supplemental Fig. 2). Peripheral edema responded to oral diuretics. Serum creatinine stabilized around 5.5 mg/dL by 10 months posttransplant.
Serum creatinine for the patient’s second renal allograft plotted against time, showing improvement of renal function with eculizumab. Peak creatinine of 11.8 mg/dL occurred about 7.5 months posttransplant. Arrowheads indicate times of eculizumab administration (1/29, 2/5, 2/12, 2/19, 2/26, 3/11, and 3/25). The patient did not undergo hemodialysis
Genetic testing (Machaon Diagnostics, “aHUS genetic panel”), sent at the time of eculizumab initiation, revealed heterozygosity for a complement factor I (CFI) mutation (1246 A > C), which results in substitution of a leucine for an isoleucine at amino acid number 398 (I398L). The patient was further found to be heterozygous for the large deletion of complement factor related proteins 1 and 3. Basic complement levels were normal, as shown in Fig. 1. No additional functional complement or auto-antibody assessment was performed.
Upon further questioning of family history, the patient recalled an episode of allograft injury in his sister that had been attributed to tacrolimus and improved with transition to an alternative immunosuppressive regimen. Full details, however, were unavailable.
At the time of writing, serum creatinine stabilized around 5.3 mg/dL with 2–3 grams proteinuria on spot urine protein/creatinine ratio without microscopic hematuria. The patient remains on monthly eculizumab infusions. Follow-up biopsy revealed transplant glomerulopathy with mesangiolysis and focal and segmental glomerulosclerosis. In particular, some glomeruli had thickened capillary loops with duplication and/or collapse (Banff cg score 1) without glomerulitis (g score 0). There was no definite evidence of fragmented red blood cells or thrombi. Electron microscopic findings were consistent. Additionally, there was persistent tubulitis with interstitial inflammation. DSA testing remained negative.
Discussion
Numerous molecules act as brakes on alternative complement activation. Mutations in these molecules, particularly in complement factors H [6, 7] and I [8], are associated with atypical hemolytic uremic syndrome. Additionally, mutations in the Complement Factor H and Complement Factor H Related Protein gene cluster lead to a variety of autoantibodies and fusion proteins that result in complement-mediated diseases [9]. Regardless of the upstream initiation steps, the final common pathway leading to the clinical syndrome of aHUS is terminal complement activation [10, 11].
Many different mutations in CFI are associated with aHUS in heterozygous carriers [1, 12]. The I398L mutation, present in this patient, is a mutation at the serine protease catalytic site of CFI I and has been associated with aHUS in several individuals worldwide [13]. Molecular pathogenesis studies have shown that this mutation results in failed trafficking of CFI from the endoplasmic reticulum to the Golgi apparatus, with subsequent failure of cellular secretion. Additionally, the patient was found to be heterozygous for the large deletion of complement factor related proteins 1 and 3. Only homozygosity for these deletions, however, has been associated with Factor H autoantibodies [9, 14].
Eculizumab (Alexion Pharmaceuticals, Cheshire, CT) is a humanized monoclonal antibody directed against the terminal complement C5 protein, thereby blocking formation of the terminal complement complex [15]. It has been used to treat aHUS [16, 17] and recurrent aHUS in transplantation [18–20]. The first reported use of eculizumab to treat de novo aHUS in solid organ transplantation was in a recipient of a simultaneous kidney-pancreas transplant [21]. Alongside conversion from calcineurin inhibitor (CNI)-based immunosuppression to mammalian target of rapamycin (mTOR) inhibitor-based immunosuppression, eculizumab use restored renal function and reduced lactate dehydrogenase levels. Conversion from CNIs to mTOR inhibitors, however, remains controversial. mTOR inhibitor use in kidney transplant recipients has been associated with thrombotic microangiopathies, both alone [22, 23] and in combination with CNI-based regimens [24–26]. Subsequent studies have demonstrated successful use of eculizumab to treat aHUS in both solid organ and stem cell transplant recipients [27].
Of note, the patient reported by Chandran and colleagues, like our own, developed early de novo donor specific antibodies. This is an unusual phenomenon that we do not understand. De novo DSA generation in this case could have represented an undetected anamnestic immune response. However, DSA could not be detected in any prior serum sample evaluated. There is a clinicopathological correlation between complement activation and development of de novo DSA [28]. Smith and colleagues proposed a four-stage model of chronic alloantibody-mediated rejection in which de novo DSA precipitates complement activation and subsequent transplant glomerulopathy [29]. In this vein, DSA themselves have been shown to bind complement [30]. Yet, it is acknowledged that DSA may be a marker of sensitization in some circumstances [29]. Sensitized T cells are known to stimulate alloantibody-producing B cells [31, 32]. It is therefore conceivable to us that, in this case, sensitization may be have occurred in context of the following: ischemia–reperfusion injury at the time of transplant [33–35]; thrombotic microangiopathy-mediated release of Class I molecules expressed in damaged renal endothelia [36] or renal tubules [37]; or up-regulation of HLA molecules induced by inflammatory stimuli [38]. To our knowledge, however, there is no known link between complement activation and subsequent de novo DSA generation.
Conclusions
Genetic testing in this patient revealed a pathogenic Complement factor I mutation. The extent to which genetically-proven aHUS was responsible for failure of the patient’s native kidneys is unclear. Family history—transplantation and trouble tolerating tacrolimus in the patient’s sister—is suggestive. We do not know of an association between Alport syndrome and aHUS. Retrospective review suggests that the patient’s first kidney transplant may have failed as a result of undiagnosed genetic aHUS. Ideally, genetic testing would have been performed prior to the second transplant. Histology at the time revealed signs of early thrombotic microangiopathy, though the overriding clinical picture at the time was thought to be that of a Banff 1B acute cellular rejection. Several observations related to the possible role of genetic aHUS in the patient’s second allograft course are in order. First, the patient’s nadir creatinine of around 2.5 mg/dL was higher than expected. This suggests an early and ongoing renal insult, such as genetic aHUS precipitated by ischemia–reperfusion injury. Calcineurin inhibitor exposure may have been a contributing factor in a genetically susceptible individual. In line with the possibility of early and ongoing allograft damage is the observation of 30–40% interstitial fibrosis and tubular atrophy on biopsy 4 months posttransplant, which was not likely present in the donor kidney at procurement. Finally, it seems that a respiratory infection precipitated a more fulminant course at which time the patient’s underlying disease was diagnosed. Terminal complement blockade with eculizumab resulted in prompt improvement in this patient’s renal function. The patient is undergoing evaluation for a third renal transplant. Data regarding maintenance immunosuppression in a case such as this are scarce. The patient will likely require continued maintenance eculizumab therapy and strict avoidance of calcineurin inhibitors. This case teaches us an important lesson: maintain a high index of suspicion for this entity and a low threshold for genetic testing. In conclusion, we believe clinicians should be especially vigilant against genetic aHUS and its protean manifestations. Early treatment with terminal complement inhibitors is critical when genetic aHUS is suspected because turnaround time for genetic testing may be slow.
Notes
Recipient HLA typing was as follows: A11, A24, B18, B35, DR4, DR53 (there was no C, DQ or DP tying for the recipient). Donor HLA typing was as follows: A2, A68, B53, C4, DR13, DR16, DQ2, and DQ5 (there was no donor DP typing).
Recipient HLA typing was as follows: A11, A24, B18, B35, DR4, DR53 (there was no C, DQ or DP tying for the recipient). Donor HLA typing was as follows: A1, A24, B7, B62, C7, C10, DR4, DR53, DQ8, DP3, and DP14. There was no history of Class II donor specific antibodies pre- or post-transplant.
References
Sellier-Leclerc AL, Fremeaux-Bacchi V, Dragon-Durey MA, Macher MA, Niaudet P, Guest G, Boudailliez B, Bouissou F, Deschenes G, Gie S, Tsimaratos M, Fischbach M, Morin D, Nivet H, Alberti C, Loirat C (2007) Differential impact of complement mutations on clinical characteristics in atypical hemolytic uremic syndrome. J Am Soc Nephrol JASN 18(8):2392–2400. doi:10.1681/asn.2006080811
Noris M, Caprioli J, Bresin E, Mossali C, Pianetti G, Gamba S, Daina E, Fenili C, Castelletti F, Sorosina A, Piras R, Donadelli R, Maranta R, van der Meer I, Conway EM, Zipfel PF, Goodship TH, Remuzzi G (2010) Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin J Am Soc Nephrol CJASN 5(10):1844–1859. doi:10.2215/cjn.02210310
Dragon-Durey MA, Fremeaux-Bacchi V, Loirat C, Blouin J, Niaudet P, Deschenes G, Coppo P, Herman Fridman W, Weiss L (2004) Heterozygous and homozygous factor h deficiencies associated with hemolytic uremic syndrome or membranoproliferative glomerulonephritis: report and genetic analysis of 16 cases. J Am Soc Nephrol JASN 15(3):787–795
Laurence J (2012) Atypical hemolytic uremic syndrome (aHUS): making the diagnosis. Clin Adv Hematol Oncol H O 10(10 Suppl 17):1–12
Legendre CM, Licht C, Muus P, Greenbaum LA, Babu S, Bedrosian C, Bingham C, Cohen DJ, Delmas Y, Douglas K, Eitner F, Feldkamp T, Fouque D, Furman RR, Gaber O, Herthelius M, Hourmant M, Karpman D, Lebranchu Y, Mariat C, Menne J, Moulin B, Nurnberger J, Ogawa M, Remuzzi G, Richard T, Sberro-Soussan R, Severino B, Sheerin NS, Trivelli A, Zimmerhackl LB, Goodship T, Loirat C (2013) Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med 368(23):2169–2181. doi:10.1056/NEJMoa1208981
Rougier N, Kazatchkine MD, Rougier JP, Fremeaux-Bacchi V, Blouin J, Deschenes G, Soto B, Baudouin V, Pautard B, Proesmans W, Weiss E, Weiss L (1998) Human complement factor H deficiency associated with hemolytic uremic syndrome. J Am Soc Nephrol JASN 9(12):2318–2326
Warwicker P, Goodship TH, Donne RL, Pirson Y, Nicholls A, Ward RM, Turnpenny P, Goodship JA (1998) Genetic studies into inherited and sporadic hemolytic uremic syndrome. Kidney Int 53(4):836–844. doi:10.1111/j.1523-1755.1998.00824.x
Fremeaux-Bacchi V, Dragon-Durey MA, Blouin J, Vigneau C, Kuypers D, Boudailliez B, Loirat C, Rondeau E, Fridman WH (2004) Complement factor I: a susceptibility gene for atypical haemolytic uraemic syndrome. J Med Genet 41(6):e84
Skerka C, Chen Q, Fremeaux-Bacchi V, Roumenina LT (2013) Complement factor H related proteins (CFHRs). Mol Immunol 56(3):170–180. doi:10.1016/j.molimm.2013.06.001
de Jorge EG, Macor P, Paixao-Cavalcante D, Rose KL, Tedesco F, Cook HT, Botto M, Pickering MC (2011) The development of atypical hemolytic uremic syndrome depends on complement C5. J Am Soc Nephrol JASN 22(1):137–145. doi:10.1681/asn.2010050451
Le Quintrec M, Roumenina L, Noris M, Fremeaux-Bacchi V (2010) Atypical hemolytic uremic syndrome associated with mutations in complement regulator genes. Semin Thromb Hemost 36(6):641–652. doi:10.1055/s-0030-1262886
Maga TK, Nishimura CJ, Weaver AE, Frees KL, Smith RJ (2010) Mutations in alternative pathway complement proteins in American patients with atypical hemolytic uremic syndrome. Hum Mutat 31(6):E1445–E1460. doi:10.1002/humu.21256
Bienaime F, Dragon-Durey MA, Regnier CH, Nilsson SC, Kwan WH, Blouin J, Jablonski M, Renault N, Rameix-Welti MA, Loirat C, Sautes-Fridman C, Villoutreix BO, Blom AM, Fremeaux-Bacchi V (2010) Mutations in components of complement influence the outcome of factor I-associated atypical hemolytic uremic syndrome. Kidney Int 77(4):339–349. doi:10.1038/ki.2009.472
Zipfel PF, Edey M, Heinen S, Jozsi M, Richter H, Misselwitz J, Hoppe B, Routledge D, Strain L, Hughes AE, Goodship JA, Licht C, Goodship TH, Skerka C (2007) Deletion of complement factor H-related genes CFHR1 and CFHR3 is associated with atypical hemolytic uremic syndrome. PLoS Genet 3(3):e41. doi:10.1371/journal.pgen.0030041
Thomas TC, Rollins SA, Rother RP, Giannoni MA, Hartman SL, Elliott EA, Nye SH, Matis LA, Squinto SP, Evans MJ (1996) Inhibition of complement activity by humanized anti-C5 antibody and single-chain Fv. Mol Immunol 33(17–18):1389–1401
Rathbone J, Kaltenthaler E, Richards A, Tappenden P, Bessey A, Cantrell A (2013) A systematic review of eculizumab for atypical haemolytic uraemic syndrome (aHUS). BMJ open 3(11):e003573. doi:10.1136/bmjopen-2013-003573
Salant DJ (2011) Targeting complement C5 in atypical hemolytic uremic syndrome. J Am Soc Nephrol JASN 22(1):7–9. doi:10.1681/asn.2010111145
Al-Akash SI, Almond PS, Savell VH Jr, Gharaybeh SI, Hogue C (2011) Eculizumab induces long-term remission in recurrent post-transplant HUS associated with C3 gene mutation. Pediatr Nephrol (Berlin, Germany) 26(4):613–619. doi:10.1007/s00467-010-1708-6
Chatelet V, Fremeaux-Bacchi V, Lobbedez T, Ficheux M, Hurault de Ligny B (2009) Safety and long-term efficacy of eculizumab in a renal transplant patient with recurrent atypical hemolytic-uremic syndrome. Am J Transplant Off J Am Soc Transplant Am Soc Transplant Surg 9(11):2644–2645. doi:10.1111/j.1600-6143.2009.02817.x
Davin JC, Gracchi V, Bouts A, Groothoff J, Strain L, Goodship T (2010) Maintenance of kidney function following treatment with eculizumab and discontinuation of plasma exchange after a third kidney transplant for atypical hemolytic uremic syndrome associated with a CFH mutation. Am J Kidney Dis Off J Nat Kidney Found 55(4):708–711. doi:10.1053/j.ajkd.2009.08.011
Chandran S, Baxter-Lowe L, Olson JL, Tomlanovich SJ, Webber A (2011) Eculizumab for the treatment of de novo thrombotic microangiopathy post simultaneous pancreas-kidney transplantation–a case report. Transplant Proc 43(5):2097–2101. doi:10.1016/j.transproceed.2011.02.064
Sartelet H, Toupance O, Lorenzato M, Fadel F, Noel LH, Lagonotte E, Birembaut P, Chanard J, Rieu P (2005) Sirolimus-induced thrombotic microangiopathy is associated with decreased expression of vascular endothelial growth factor in kidneys. Am J Transplant Off J Am Soc Transplant Am Soc Transplant Surg 5(10):2441–2447. doi:10.1111/j.1600-6143.2005.01047.x
Reynolds JC, Agodoa LY, Yuan CM, Abbott KC (2003) Thrombotic microangiopathy after renal transplantation in the United States. Am J Kidney Dis Off J Nat Kidney Found 42(5):1058–1068
Langer RM, Van Buren CT, Katz SM, Kahan BD (2002) De novo hemolytic uremic syndrome after kidney transplantation in patients treated with cyclosporine-sirolimus combination. Transplantation 73(5):756–760
Robson M, Cote I, Abbs I, Koffman G, Goldsmith D (2003) Thrombotic micro-angiopathy with sirolimus-based immunosuppression: potentiation of calcineurin-inhibitor-induced endothelial damage? Am J Transplant Off J Am Soc Transplant Am Soc Transplant Surg 3(3):324–327
Fortin MC, Raymond MA, Madore F, Fugere JA, Paquet M, St-Louis G, Hebert MJ (2004) Increased risk of thrombotic microangiopathy in patients receiving a cyclosporin-sirolimus combination. Am J Transplant Off J Am Soc Transplant Am Soc Transplant Surg 4(6):946–952. doi:10.1111/j.1600-6143.2004.00428.x
Dhakal P, Giri S, Pathak R, Bhatt VR (2015) Eculizumab in transplant-associated thrombotic microangiopathy. Clin Appl Thromb/Hemost Off J Intl Acad Clin Appl Thromb Hemost. doi:10.1177/1076029615599439
Wiebe C, Gibson IW, Blydt-Hansen TD, Karpinski M, Ho J, Storsley LJ, Goldberg A, Birk PE, Rush DN, Nickerson PW (2012) Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am J Transplant Off J Am Soc Transplant Am Soc Transplant Surg 12(5):1157–1167. doi:10.1111/j.1600-6143.2012.04013.x
Smith RN, Kawai T, Boskovic S, Nadazdin O, Sachs DH, Cosimi AB, Colvin RB (2008) Four stages and lack of stable accommodation in chronic alloantibody-mediated renal allograft rejection in Cynomolgus monkeys. Am J transplant Off J Am Soc Transplant Am Soc Transplant Surg 8(8):1662–1672. doi:10.1111/j.1600-6143.2008.02303.x
Loupy A, Lefaucheur C, Vernerey D, Prugger C, Duong van Huyen JP, Mooney N, Suberbielle C, Fremeaux-Bacchi V, Mejean A, Desgrandchamps F, Anglicheau D, Nochy D, Charron D, Empana JP, Delahousse M, Legendre C, Glotz D, Hill GS, Zeevi A, Jouven X (2013) Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med 369(13):1215–1226. doi:10.1056/NEJMoa1302506
Steele DJ, Laufer TM, Smiley ST, Ando Y, Grusby MJ, Glimcher LH, Auchincloss H Jr (1996) Two levels of help for B cell alloantibody production. J Exp Med 183(2):699–703
Vella JP, Spadafora-Ferreira M, Murphy B, Alexander SI, Harmon W, Carpenter CB, Sayegh MH (1997) Indirect allorecognition of major histocompatibility complex allopeptides in human renal transplant recipients with chronic graft dysfunction. Transplantation 64(6):795–800
Sacks SH, Zhou W (2012) The role of complement in the early immune response to transplantation. Nat Rev Immunol 12(6):431–442. doi:10.1038/nri3225
Zhou W, Farrar CA, Abe K, Pratt JR, Marsh JE, Wang Y, Stahl GL, Sacks SH (2000) Predominant role for C5b-9 in renal ischemia/reperfusion injury. J Clin Invest 105(10):1363–1371. doi:10.1172/jci8621
Weisman HF, Bartow T, Leppo MK, Marsh HC Jr, Carson GR, Concino MF, Boyle MP, Roux KH, Weisfeldt ML, Fearon DT (1990) Soluble human complement receptor type 1: in vivo inhibitor of complement suppressing post-ischemic myocardial inflammation and necrosis. Science 249(4965):146–151
Evans PR, Trickett LP, Smith JL, MacIver AG, Tate D, Slapak M (1985) Varying expression of major histocompatibility complex antigens on human renal endothelium and epithelium. Br J Exp pathol 66(1):79–87
Bishop GA, Hall BM, Suranyi MG, Tiller DJ, Horvath JS, Duggin GG (1986) Expression of HLA antigens on renal tubular cells in culture. I. Evidence that mixed lymphocyte culture supernatants and gamma interferon increase both class I and class II HLA antigens. Transplantation 42(6):671–679
Locke JE, Zachary AA, Warren DS, Segev DL, Houp JA, Montgomery RA, Leffell MS (2009) Proinflammatory events are associated with significant increases in breadth and strength of HLA-specific antibody. Am J Transplant Off J Amn Soc Transplant Am Soc Transplant Surg 9(9):2136–2139. doi:10.1111/j.1600-6143.2009.02764.x
Acknowledgements
We would specifically like to thank Lisa Scholz, RN, MS for her continuity care of this patient. The authors would like to thank the faculty and staff of the Comprehensive Transplant Center of Northwestern Memorial Hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Gallon has a consulting and advisory relationship with Alexion Pharmaceuticals, Inc. The other authors declare no conflicts of interest.
Funding
None.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zwang, N.A., Ho, B., Kanwar, Y.S. et al. A case of atypical hemolytic uremic syndrome in a second renal transplant. J Nephrol 31, 165–172 (2018). https://doi.org/10.1007/s40620-016-0373-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-016-0373-7