Abstract
Objectives
The phenotype associated to reduced physical activity (PA) in dialysis patients is poorly documented. We here evaluate weekly PA in two independent cohorts.
Methods
Cross-sectional study with PA assessed by the number of steps/day measured by pedometer in two cohorts of prevalent dialysis patients: (1) peritoneal dialysis (PD) patients (n = 64; 62 ± 14 years; 70 % men) from Stockholm, Sweden using the pedometer for 7 consecutive days; (2) hemodialysis (HD) patients (n = 78; 63 ± 12 years; 65 % men) from a single center in Madrid, Spain using the pedometer for 6 consecutive days: 2 HD days, 2 non-HD midweek days and 2 non-HD weekend days. In both cohorts, comorbidities, body composition, nutritional status, and related biomarkers were assessed. Cohorts were not merged; instead data were analyzed separately serving as reciprocal replication analyses.
Results
Most patients (63 % of PD and 71 % of HD) were considered sedentary (<5,000 steps/day). PD patients had on average 4,839 ± 3,313 steps/day. HD patients had 3,767 ± 3,370 steps/day on HD-free days, but fewer steps/day on HD days (2,274 ± 2,048 steps/day; p < 0.0001). In both cohorts, and across increasing PA tertiles, patients were younger and had less comorbidities. Higher PA was also accompanied by better nutritional status (depicted by albumin, pre-albumin, creatinine and normalized protein catabolic rate in HD, and by albumin and subjective global assessment [SGA] in PD), higher lean body mass, and lower fat body mass (bioimpedance and/or dual-energy X-ray absorptiometry [DEXA]). Higher levels of PA were accompanied by lower levels of C-reactive protein in PD. Age and lean body mass were the strongest multivariate predictors of PA in both cohorts.
Conclusion
There is a high prevalence of sedentary behavior in dialysis patients. Better physical activity was consistently associated with younger age, lower presence of comorbidities and better nutritional status. Pedometers represent a simple and inexpensive tool to objectively evaluate physical activity in this patient population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Whereas mortality risk factors such as inflammation, mineral and bone disorders and protein-energy wasting (PEW) have been thoroughly investigated in chronic kidney disease (CKD), including dialysis patients, the role of physical activity/inactivity-an important issue for public health-has in general received less attention in studies on CKD patients. It is well established that a sedentary lifestyle is associated, inter alia, with high prevalence of cardiovascular events and increased mortality [1] while regular physical activity, even in small doses, bestows substantial health benefits [2]. Several of these known benefits, i.e. better control of diabetes and blood pressure [3], improved heart rate variability, and reduction in arterial stiffness [4], are particularly relevant for patients with end-stage renal disease (ESRD) in whom cardiovascular mortality accounts for approximately 40 % of all deaths [5].
Besides a positive impact on cardiovascular health, an active lifestyle is also linked to better nutritional status, and physical fitness and adequate walking capacity have been shown to be associated also with improved psychological well-being and enhanced health-related quality of life [6]. Such benefits of physical activity have been shown also in ESRD patients [7]. However, although most dialysis patients are rather sedentary and therefore are exposed to an increased risk, nephrologists are in general not well aware of the degree of physical activity (PA) performed by their patients; thus, counseling about this topic as an active part of their daily clinical practice is often lacking [8]. Furthermore, few facilities offer structured exercise programs [9].
Since walking represents a substantial part of daily PA, pedometers to assess the daily spontaneous activity of patients should be an attractive tool to quantitate PA in a simple and inexpensive way. Additionally, pedometers have been validated for use in research and clinical practice [10]. The aim of this study was to evaluate in two dialysis populations PA as assessed by pedometers and to determine associations between degree of PA and phenotype characteristics among these patients.
Materials and methods
Patients and study design
This cross-sectional study was performed in two cohorts of patients from two different countries who were treated with two different dialysis modalities. Because of these differences as well as differences in assessment, cohorts were not merged. Instead, we analyzed them separately and present them as replication cohorts.
Hemodialysis cohort
The hemodialysis (HD) cohort consisted of prevalent clinically stable patients treated at the Severo Ochoa Hospital, Leganes, Madrid, Spain who had been on HD for more than 2 months and without hospital admissions in the month preceding the investigation. Patients with physical limitations (amputation) and neurological impairment were excluded because of the difficulty of assessing PA. From 110 eligible patients, 78 patients [mean age 63 ± 12 years; 65 % men; median dialysis vintage 32 (interquartile range (IQR) 4–127) months] met the inclusion criteria and accepted to participate. Twenty-eight (36 %) patients were diabetics and 36 (46 %) patients had a history of cardiovascular disease (CVD).
Peritoneal dialysis cohort
The peritoneal dialysis (PD) cohort consisted of prevalent PD patients at the Karolinska University Hospital, Stockholm, Sweden. From a cohort of 84 patients, measurement of PA was available in 64 patients [mean age 62 ± 14 years; 70 % men; median dialysis vintage 9 (4–36) months] who were included in the current study. PD treatment was provided as continuous ambulatory peritoneal dialysis (CAPD) in 74 % of the patients and automated PD (APD) in 26 %. In this group, 15 (23 %) patients were diabetics, and 26 (41 %) patients had a history of CVD.
Informed consent was obtained from all patients prior to their inclusion in the study. The study protocol was approved by the Ethics Committees of the Severo Ochoa Hospital and Karolinska Institutet/Karolinska University Hospital, respectively.
Clinical data including comorbidities
Each patient’s medical chart was reviewed to extract demographic and clinical data, and comorbidities. The presence of CVD and diabetes mellitus (DM) was recorded. CVD was defined as cardiac, cerebrovascular (including stroke) or peripheral vascular disease. Blood pressure was presented as mean blood pressure, defined as: diastolic pressure + (systolic pressure−diastolic pressure)/3. The dose of erythropoietin-stimulating agents (ESAs) was recorded.
Anthropometric evaluation and assessment of nutritional status
Body weight, body mass index (BMI) and anthropometric measurements were recorded. BMI was calculated as dry body weight in kg/(height in meters)2. Dry weight was defined as the lowest weight a patient could tolerate after dialysis without developing hypotension, and was determined at routine dialysis assessment. In HD patients, the normalized protein catabolic rate (nPCR), a measure of net protein catabolism, adjusted for body size, was used as an estimate of dietary protein intake assuming that the patients were in nitrogen balance [11]. The formula used was: nPCR = (0.0136 × F) + 0.251, where F is equal to Kt/V × [(predialysis blood urea nitrogen (BUN)−postdialysis BUN) ÷ 2] [12]. In PD patients, nutritional status was estimated by subjective global assessment (SGA) rated as 1–4, with score four denoting severe malnutrition (i.e. PEW). For the purposes of analysis, PEW was defined as an SGA score >1 [13].
Laboratory analysis
Blood samples were collected in the morning before the midweek dialysis session in the HD cohort, while in the PD patients blood samples were collected on the day of their monthly routine control. The plasma was separated within 30 min, and samples were kept frozen at −70 °C if not analyzed immediately. Circulating high-sensitivity C-reactive protein (hsCRP) was measured by a high-sensitivity nephelometry assay, and cholesterol, creatinine, albumin and pre-albumin concentrations were measured by routine methods at the Department of Laboratory Medicine, Karolinska University Hospital, Huddinge and the Department of Biochemistry, Severo Ochoa Hospital, respectively.
Body composition assessment
In HD patients, body composition was assessed by bioimpedance (Body Composition Monitor, BCM, Fresenius Medical Care, Bad Homburg, Germany). The measurement was performed immediately before the second dialysis session of the week. Lean body mass (LBM) and fat body mass (FBM) were expressed as percentages and normalized by height yielding a lean tissue index (LTI) and fat tissue index (FTI). Total body water (TBW), extracellular water (ECW), intracellular water (ICW) and the relation ECW/ICW were calculated. Other parameters obtained were body cell mass in kg and phase angle (Phi50).
In PD patients, body composition was assessed by means of dual-energy X-ray absorptiometry, DEXA (Lunar Corp., Madison, WI, USA), estimating LBM and FBM. The lean body mass index (LBMI) and fat body mass index (FBMI) were calculated according to Kyle et al. [14] and expressed as kg/m2. Hydration status was assessed using multi-frequency bioimpedance (Xitron 4000B, Xitron Tech, San Diego, CA, USA).
Physical activity measurement
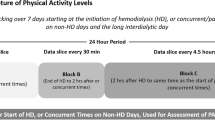
Physical activity was estimated by the number of steps/day, measured by a pedometer. The use of pedometers is considered a valid option for assessing PA in research [10]. We used the pedometer-determined PA classification for healthy adults according to Tudor-Locke and Bassett as a benchmark of PA degree. This classification considers that more than 10,000 steps per day is the goal for an active life [15]. Participants with PA recorded below 5,000 steps/day are considered sedentary [16]. In the HD group, the PA measurement was performed using a Geonaute-onstep-400® pedometer; each patient was requested to wear the device for six consecutive days (two HD days, two HD-free midweek days, and two HD-free weekend days). Information on PA was obtained from the device memory, calculating and recording the average daily steps both on HD days and HD-free days (midweek and weekend days). In the PD cohort, PA was measured using the SenseWear-Pro2-Armband device (Sense Wear Pro2 Armband, Bodymedia Inc., Pittsburgh, PA, USA). Patients were asked to use the device for seven consecutive days. Information was obtained from the device memory, and an average of steps/day was recorded.
Statistical analyses
Data are expressed as mean ± standard deviation (SD) or median (range of 10th–90th percentile) or percentage, as appropriate. Statistical significance was set at the level of p < 0.05. Comparisons between two groups were assessed with the nonparametric Wilcoxon test for continuous variables and a χ2 test for nominal variables. Patients were divided into three groups according to the tertiles of PA for each cohort. Differences among three groups were analyzed using the nonparametric ANOVA Kruskal–Wallis test. Spearman rank correlation analysis was used to determine associations between various selected parameters.
We used multivariate logistic analysis to study the association between PA and other parameters. Patients were divided into two groups based on PA, comparing the lowest tertile versus the other two tertiles in both cohorts. The cut-off point in HD patients was 1,618 steps/day and for PD patients it was 2,769 steps/day. We included age, gender, hemoglobin (Hb) and LTI (HD)/LBMI (PD) in the model to predict the physical activity. All statistical analyses were performed using statistical software SAS version 9.4 (SAS Campus Drive, Cary, NC, USA).
Results
Physical activity
HD patients performed on average 3,767 ± 3,370 steps/day on HD-free days. The step count was significantly lower on HD days (2,274 ± 2,048 steps/day, p < 0.0001) (Fig. 1). PD patients performed on average 4,839 ± 3,313 steps/day. There were no differences in steps/day between APD and CAPD modalities (median [10th–90th percentiles] 5,676 [904–11,419] vs. 4,194 [1,027–9,794] steps/day, p = 0.32, APD vs. CAPD, respectively). In both cohorts, only 9 % of patients (7 patients in the HD group and 6 in the PD group) reached the goal of 10,000 steps/day. The majority of patients, 55 (71 %) of the HD group and 40 (63 %) of the PD group, had step counts below 5,000 steps, and thus met the criterion for a sedentary lifestyle. Furthermore, 33 (42 %) patients in the HD cohort and 18 (28 %) in the PD cohort performed less than 2,500 steps/day. In the HD cohort, PA was higher in men than in women (4,389 ± 3,779 vs. 2,592 ± 2,030; p < 0.01). In the PD cohort, no difference was observed between sexes (5,070 ± 3,404 vs. 4,289 ± 3,106; p = 0.39).
Baseline characteristics and nutritional status
For further analyses we divided both cohorts according to tertiles of PA level. Baseline clinical and biochemical characteristics are shown in Tables 1, 2. In both cohorts, across increasing PA activity tertiles patients were younger and had less comorbid conditions. There was no statistically significant impact of sex or dialysis vintage across the tertiles of PA.
PA was significantly associated with a better nutritional status. In the HD group, this association was depicted by higher values of albumin, pre-albumin, creatinine and nPCR. In the PD patients, this association was represented by lower levels of albumin and higher prevalence of SGA-malnutrition. Although higher levels of physical activity were accompanied by lower hsCRP concentrations in both cohorts, this difference attained statistical significance in PD patients only (Fig. 2).
Physical activity and body composition
Higher levels of PA were associated with a better body composition. In both cohorts, and across increasing tertiles of PA, patients had more lean body mass and less fat body mass (Tables 3, 4). The associations were weaker (for LBM) or showed non-statistically significant trends (for FBM) among PD patients. Regarding hydration status, more active patients tended to be less overhydrated even though this association was not statistically significant. However, there was a significant inverse association between physical activity levels and the ratio of extracellular/intracellular water.
Multivariate analysis
In the HD cohort, univariate associations with PA included: age (rho = −0.31, p = 0.005), sex (rho = −0.21, p = 0.06), CVD (rho = −0.16, p = 0.15), albumin (rho = 0.38, p < 0.001), creatinine (rho = 0.50, p < 0.001), nPCR (rho = 0.38, p = 0.002), hsCRP (rho = −0.20, p = 0.07), Hb (rho = 0.32, p = 0.004), ESA dose (rho = −0.36, p < 0.001) and LTI (rho = 0.52, p < 0.001). In the PD cohort, PA in univariate analysis was associated with: age (rho = −0.53, p < 0.001), sex (rho = 0.12, p = 0.32), CVD (rho = −0.30, p = 0.01), albumin (rho = 0.42, p < 0.001), creatinine (rho = 0.16, p = 0.19), SGA (rho = −0.34, p = 0.005), hsCRP (rho = −0.40, p = 0.001), Hb (rho = 0.46, p = 0.09), ESA dose (rho = −0.17, p = 0.17) and LBMI (rho = 0.31, p = 0.01).
In multivariate stepwise regression analysis, older age, low Hb levels (only in the HD cohort) and low lean body mass were consistent factors independently associated with low PA in both cohorts (Table 5).
Discussion
This study, examining physical activity (PA) using pedometers in two different cohorts of dialysis patients, shows that dialysis patients in general are physically inactive compared even to sedentary healthy individuals [17] and that low PA is associated to older age, worse nutritional status and burden of comorbidities.
Although the pedometer is simple to handle and a validated instrument for research and clinical practice [10], reports on the use of pedometers in patients with renal disease are scarce. Consequently, the appropriate levels of PA among these usually elderly/fragile patients have not been established. While the classification proposed by Tudor-Locke et al. [15] for categorizing the degree of PA according to the number of steps per day is extensively used, it is likely that the target value proposed for the healthy population might not be valid for dialysis patients given their special characteristics and the fact that they are in general quite elderly. Further studies for defining the appropriate level of PA are required. According to the established classification, more than 60 % of both HD and PD patients did not reach the cut-off point of 5,000 steps per day in the current study and, accordingly, they were considered sedentary. Reports from the few studies that have been conducted measuring PA using this kind of device show that dialysis patients performed on average 3,500–4,500 steps/day [18–20] which is consistent with the data in the current study.
Dialysis patients are exposed to several factors that may reduce their PA such as elderly age and a high burden of comorbidities. Interestingly, a decline in PA is seen already at early stages of CKD although a further progression occurs as renal function deteriorates [21]. In addition, there are physical aspects related to the dialysis technique itself such as the hours of inactivity during HD sessions or the time needed for dialysate bag exchanges in PD patients. In the current study, older age, lower hemoglobin levels and lower lean body mass were associated with a low degree of PA. Overall, activity levels decline with age, but this decline is considerably more rapid for patients on dialysis than for sedentary controls [17]. Anemia is directly associated with renal failure, and many of the physical limitations observed in dialysis patients have been attributed to this circumstance. However, the precise role of anemia in the reduced functional capacity of these patients is not entirely clear. It could be hypothesized that anemia, besides leading to fatigue, could be associated with diminished muscle oxygenation, thereby reducing the strength and function and, subsequently, causing a reduction in physical performance. Previous studies have analyzed the relationship between PA and hemoglobin levels in patients with ESRD, but findings are not conclusive [7, 18, 22]. However, when ESAs are introduced for the treatment of anemia, the functional capacity of these patients improves greatly [23]. Anemia and ESA hypo-responsiveness are linked to inflammation and testosterone deficiency [24], factors both related to inactivity in dialysis patients. Furthermore, lower degree of PA was associated with higher hsCRP values in both cohorts in the current study. Previously, Mafra et al. [25] also found that inflamed dialysis patients are less active, while Viana et al. [26] reported that exercise could elicit anti-inflammatory effects in predialysis CKD patients.
In addition, low PA was related to poor nutritional status in our cohorts. Inflammation and malnutrition are considered, among others, important factors responsible for the progressive muscle wasting observed in dialysis patients [27, 28] which is associated with low PA, as observed also in our study.
The progressive loss of lean body mass is an independent risk factor for mortality in dialysis patients [29]. Probably the relationship between lean mass and PA goes both ways. Since the loss of muscle mass limits PA in patients on dialysis, by the same token, lack of activity entails muscle atrophy. Nevertheless, given the observational nature of the study, we cannot ascertain the direction of this relationship in our patients. However, improving PA as an intervention for preventing loss of muscle mass appears to be a logical approach. Some studies have been made in this direction with encouraging results [30].
Limitations of our study include its cross-sectional design, which precludes conclusions regarding causality, and the low sample size, which makes our capacity to make inferences weak. In addition, because of our inclusion criteria (free from serious mobility problems and neurological disorders) and the convenience nature of both cohorts (willingness to participate) there could have been a selection bias and thus we may underestimate the true prevalence of inactivity in the dialysis population. Although the relation between comorbidities and physical activity was assessed, the severity of comorbidities was not graded. Finally, the lack of information on the mean time to referral to the nephrologist before the start of dialysis should also be acknowledged. Strengths of the study include the fact that we obtained an objective measure of PA using instruments validated for this purpose and that results were consistent among two different populations in two different countries with patients who used two different dialysis modalities.
To conclude, our study shows that both HD and PD patients have a high prevalence of sedentary behavior. A lower degree of PA was consistently associated with older age, higher number of comorbidities, lower hemoglobin levels as well as worse nutritional status and body composition derangements. Our study also shows that pedometers, which are inexpensive and easy-to-use tools, can be used to objectively assess PA in dialysis patients. Whether exercise programs would be indicated on the basis of the current pedometer data cannot be established from our study, but they can be a way to raise awareness about the issue and increase the patient motivation to exercise. Considering the negative effects putatively associated with inactivity in patients on dialysis, including higher mortality [7, 22], increasing PA should be one of the goals of managing patients undergoing dialysis care. However, observational and interventional studies are first needed to determine among others the target value of steps/day in dialysis patients and whether changes in PA are associated with relevant end-points such as improved survival or quality of life, or both.
References
Menotti A, Puddu PE, Lanti M, Maiani G, Catasta G, Fidanza AA (2014) Lifestyle habits and mortality from all and specific causes of death: 40-year follow-up in the Italian Rural Areas of the Seven Countries Study. J Nutr Health Aging 18(3):314–321
Wen CP, Wai JPM, Tsai MK, Yang YC, Cheng TYD, Lee M-C et al (2011) Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet 378(9798):1244–1253
Brown RE, Riddell MC, Macpherson AK, Canning KL, Kuk JL (2013) The joint association of physical activity, blood-pressure control, and pharmacologic treatment of hypertension for all-cause mortality risk. Am J Hypertens 26(8):1005–1010
Edwards NM, Daniels SR, Claytor RP, Khoury PR, Dolan LM, Kimball TR et al (2012) Physical activity is independently associated with multiple measures of arterial stiffness in adolescents and young adults. Metabolism 61(6):869–872
Carrero JJ, de Jager DJ, Verduijn M, Ravani P, Meester JD, Heaf JG et al (2011) Cardiovascular and noncardiovascular mortality among men and women starting dialysis. Clin J Am Soc Nephrol 6(7):1722–1730
Davies CA, Vandelanotte C, Duncan MJ, van Uffelen JGZ (2012) Associations of physical activity and screen-time on health related quality of life in adults. Prev Med 55(1):46–49
O’Hare AM, Tawney K, Bacchetti P, Johansen KL (2003) Decreased survival among sedentary patients undergoing dialysis: results from the dialysis morbidity and mortality study wave 2. Am J Kidney Dis 41(2):447–454
Johansen KL, Sakkas GK, Doyle J, Shubert T, Adams Dudley R (2003) Exercise counseling practices among nephrologists caring for patients on dialysis. Am J Kidney Dis 41(1):171–178
Tentori F, Elder SJ, Thumma J, Pisoni RL, Bommer J, Fissell RB et al (2010) Physical exercise among participants in the Dialysis Outcomes and Practice Patterns Study (DOPPS): correlates and associated outcomes. Nephrol Dial Transplant 25(9):3050–3062
Tudor-Locke C, Williams JE, Reis JP, Pluto D (2002) Utility of pedometers for assessing physical activity: convergent validity. Sports Med Auckl NZ 32(12):795–808
Depner TA, Daugirdas JT (1996) Equations for normalized protein catabolic rate based on two-point modeling of hemodialysis urea kinetics. J Am Soc Nephrol 7(5):780–785
Daugirdas JT (1989) The post: pre dialysis plasma urea nitrogen ratio to estimate Kt/V and NPCR: validation. Int J Artif Organs 12(7):420–427
Gama-Axelsson T, Lindholm B, Bárány P, Heimbürger O, Stenvinkel P, Qureshi AR (2013) Self-Rated appetite as a predictor of mortality in patients with stage 5 chronic kidney disease. J Ren Nutr 23(2):106–113
Kyle UG, Genton L, Karsegard L, Slosman DO, Pichard C (2001) Single prediction equation for bioelectrical impedance analysis in adults aged 20–94 years. Nutrition 17(3):248–253
Tudor-Locke C, Craig CL, Brown WJ, Clemes SA, De Cocker K, Giles-Corti B et al (2011) How many steps/day are enough? for adults. Int J Behav Nutr Phys Act 28(8):79
Tudor-Locke C, Craig CL, Thyfault JP, Spence JC (2012) A step-defined sedentary lifestyle index: <5000 steps/day. Appl Physiol Nutr Metab 38(2):100–114
Johansen KL, Chertow GM, Ng AV, Mulligan K, Carey S, Schoenfeld PY et al (2000) Physical activity levels in patients on hemodialysis and healthy sedentary controls. Kidney Int 57(6):2564–2570
Zamojska S, Szklarek M, Niewodniczy M, Nowicki M (2006) Correlates of habitual physical activity in chronic haemodialysis patients. Nephrol Dial Transplant 21(5):1323–1327
Avesani CM, Trolonge S, Deléaval P, Baria F, Mafra D, Faxén-Irving G et al (2012) Physical activity and energy expenditure in haemodialysis patients: an international survey. Nephrol Dial Transplant 27(6):2430–2434
Nowicki M, Murlikiewicz K, Jagodzińska M (2010) Pedometers as a means to increase spontaneous physical activity in chronic hemodialysis patients. J Nephrol 23(3):297–305
Clyne N, Jogestrand T, Lins LE, Pehrsson SK (1994) Progressive decline in renal function induces a gradual decrease in total hemoglobin and exercise capacity. Nephron 67(3):322–326
Johansen KL, Kaysen GA, Dalrymple LS, Grimes BA, Glidden DV, Anand S et al (2013) Association of physical activity with survival among ambulatory patients on dialysis: the Comprehensive Dialysis Study. Clin J Am Soc Nephrol CJASN 8(2):248–253
Painter P, Moore GE (1994) The impact of recombinant human erythropoietin on exercise capacity in hemodialysis patients. Adv Ren Replace Ther 1(1):55–65
Carrero JJ, Bárány P, Yilmaz MI, Qureshi AR, Sonmez A, Heimbürger O et al (2012) Testosterone deficiency is a cause of anaemia and reduced responsiveness to erythropoiesis-stimulating agents in men with chronic kidney disease. Nephrol Dial Transplant 27(2):709–715
Mafra D, Deleaval P, Teta D, Cleaud C, Arkouche W, Jolivot A et al (2011) Influence of Inflammation on Total Energy Expenditure in Hemodialysis Patients. J Ren Nutr 21(5):387–393
Viana JL, Kosmadakis GC, Watson EL, Bevington A, Feehally J, Bishop NC et al (2014) Evidence for anti-inflammatory effects of exercise in CKD. J Am Soc Nephrol 25(9):2121–2130
Carrero JJ, Stenvinkel P, Cuppari L, Ikizler TA, Kalantar-Zadeh K, Kaysen G et al (2013) Etiology of the protein-energy wasting syndrome in chronic kidney disease: a consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J Ren Nutr 23(2):77–90
Carrero JJ, Chmielewski M, Axelsson J, Snaedal S, Heimbürger O, Bárány P et al (2008) Muscle atrophy, inflammation and clinical outcome in incident and prevalent dialysis patients. Clin Nutr 27(4):557–564
Gracia-Iguacel C, González-Parra E, Pérez-Gómez MV, Mahíllo I, Egido J, Ortiz A et al (2013) Prevalence of protein-energy wasting syndrome and its association with mortality in haemodialysis patients in a centre in Spain. Nefrologia 33(4):495–505
Ikizler TA (2011) Exercise as an anabolic intervention in patients with end-stage renal disease. J Ren Nutr 21(1):52–56
Acknowledgments
We thank the patients who participated in this study. From the Swedish side, we are indebted to Åsa Lindé for data and sample collection, and to our research staff Annika Nilsson, Anki Emmoth, Ulrika Jensen, Monica Ericsson and Ann-Christin Bragfors-Helin. From the Spanish side, we want to thank the collaboration of the whole staff at the hemodialysis unit. Gabriela Cobo benefits from a PhD scholarship from the Ecuadorian government, and the stay at Karolinska Institutet was supported by the Spanish Society of Nephrology. Patient data collection in Sweden was supported by an unrestricted grant from Amgen. This study also received support from The Swedish Medical Research Council and The Osterman and The Westman Foundations. Baxter Novum is the result of a grant from Baxter Healthcare Corporation to Karolinska Institutet.
Conflicts of interest
Gabriela Cobo: none; Paloma Gallar: none; Thiane Gama-Axelsson: none; Cristina Di Gioia: none; Abdul Rashid Qureshi: none; Rosa Camacho: none; Ana Vigil: none; Olof Heimbürger: none; Olimpia Ortega: none; Isabel Rodriguez: none; Juan Carlos Herrero: none; Peter Barany: none; Bengt Lindholm: employed by Baxter Healthcare Corporation; Peter Stenvinkel: none; Juan Jesús Carrero: none.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cobo, G., Gallar, P., Gama-Axelsson, T. et al. Clinical determinants of reduced physical activity in hemodialysis and peritoneal dialysis patients. J Nephrol 28, 503–510 (2015). https://doi.org/10.1007/s40620-014-0164-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-014-0164-y