Abstract
Background
Uremic pruritus as a symptom that affects hemodialysis (HD) patients can decrease the quality of life and increase morbidity in these patients. The aim of this study was to evaluate the effects of turmeric on uremic pruritus in HD patients.
Subjects and methods
This was a double-blind placebo-controlled trial conducted on 100 HD patients suffering from pruritus. Patients (mean age 53.3 ± 15.8 years) were randomized into two groups: turmeric and placebo. The pruritus score and biochemical determinants including high-sensitivity C-reactive protein (hs-CRP) were compared before and at the end of the study between the two groups.
Results
The mean decrease in hs-CRP was significantly higher in the turmeric than the placebo group (−0.8 ± 2.6 vs. 0.4 ± 8.7 mg/l, p = 0.012). Also reduction of pruritus scores was greater in the turmeric than the placebo group (13.6 ± 2.6 vs. 7.2 ± 2.6, p = 0.001). No side effect was observed during the study due to the use of turmeric.
Conclusions
This study demonstrates the possible efficacy of turmeric in decreasing hs-CRP and uremic pruritus in end stage renal disease patients. Future studies are needed to further evaluate the efficacy and safety of turmeric.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Uremic pruritus (UP) is one of the most common and bothersome symptoms affecting patients with end stage renal disease (ESRD) [1]. The prevalence of UP varies from 50 to 90 % in various studies [2]. Chronic pruritus can be persistent and distressing, with significant impact on quality of life and physical comfort with potential psychological, functional, and social impact and increased morbidity [3]. The pathogenic molecular basis of pruritus in chronic renal failure remains elusive, which limits the use of effective treatments. Treatments currently used for UP are oral antihistamines, gabapentin, ondansetron, thalidomide, naltrexone/nalbufine, ultraviolet (UV) light, and topi-cal tacrolimus [4–10]. Unfortunately, the results of different studies are not concordant and most of the mentioned treatments have had limited success.
Many hypotheses have been proposed to clarify the reasons for its occurrence, including xerosis and hypohidrosis, the presence of pruritogenic cytokines (histamine, kallikrein, interleukin (IL)-2, acetylcholine, and other substances that are released by histamine-mediated mast cell stimulation), secondary hyperparathyroidism and immune-inflammatory reactions [2, 11]. Previous studies have verified that inflammation is mainly associated with UP in ESRD patients. Patients with severe UP have higher high-sensitivity C-reactive protein (hs-CRP) levels [12]. On the other hand, several inflammatory cytokines promote the genesis of uremic pruritus, such as IL-2 [13].
Turmeric, a powder of the rhizomes of Curcuma longa L. (Zingiberaceae), commonly used as a dietary spice, is also used in Asian and Iranian medicine ordinarily for treatment of inflammation and skin wounds [14]. Curcumin (diferuloylmethane), the most active and non-toxic component of turmeric, is a polyphenol [15] that has been extensively studied for its therapeutic benefit, such as anti-inflammatory activities [16, 17]. Turmeric appears to be non-toxic to humans even at high doses [18]. However, there is a paucity of information on the effect of turmeric on hemodialysis (HD) patients. We, therefore, conducted this study to determine if there was any beneficial effect of turmeric on pruritus in HD patients.
Materials and methods
This study was a parallel, double-blind placebo-controlled trial carried out between August 2011 and June 2012. The trial was approved by the ethics committee of Shiraz University of Medical Sciences and carried out in accordance with the Declaration of Helsinki. The trial was registered at clinicaltrials.gov (NCT01037595).
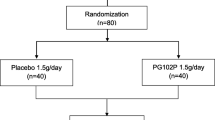
In accord with the convenience sampling method, all HD patients (n = 255) attending the Ebrahimi HD center in Shiraz were enrolled in this study. Inclusion criteria were: adults over 18 years of age who had been suffering from pruritus during the previous 6 weeks but did not respond to anti pruritic drugs, and who signed the informed consent. Patients with dermatologic, liver or metabolic diseases associated with pruritus and serum parathyroid >300 pg/ml were excluded. One hundred and one patients who met the study criteria were enrolled in the study. One patient before the start of the study dropped out due to renal transplantation. The remaining 100 patients were randomized into two groups: the trial group (n = 50) and the controls (n = 50); a factorial block randomization was used for allocation sequence (each block has equal numbers of A for trial and B for control). Our statistical consultant assigned by a table of random numbers a number 0 to 4 to AB block and 5–9 to BA block. The allocation sequence was concealed from the researcher enrolling and assessing participants in sequentially numbered, opaque, sealed envelopes. Clinical investigators, laboratory personnel, and patients were all masked to the treatment assignment.
Each patient in the trial group received a safe dose of turmeric (one capsule with each meal containing 500 mg turmeric, of which 22.1 mg was the active ingredient curcumin, 3 caps/day for 8 weeks) while the control group received starch capsules for the same 8-week period. The type and dose of the individualized drugs remained unchanged during the study. Each patient was given an order number and received the medications in the equivalent pre-packed bottles. All drugs and placebo tablets were similar in size, shape, weight and color. Drug compliance was evaluated by tablet counts. The membrane and general dialysis prescription were similar for all patients. Any medications with antipruritic effect were discontinued 1 week before the study.
All the patients were visited by a trained dermatologist who evaluated the pruritus complaint. The pruritus measurement was performed by the same person throughout the study (at the start, during, and end). The data collection technique was based on observation and interview. We evaluated patients in weekly visits at the dialysis center for side effects but the pruritus score was calculated before and at the end of the study. To assess pruritus, we used the Detailed Pruritus Score proposed by Duo [19], based on a combined score of severity and distribution of pruritus and sleep disturbance. Severity was scored as follows: sense of itching with no need to scratch = 1 point; scratching a few times without excoriation = 2; frequent need to scratch = 3; scratching with excoriation = 4; an itch that led to continuous unrest = 5 points (maximum daily score of 10 points—5 in the morning, 5 in the afternoon). Distribution of the pruritus was scored: 1 point for pruritus in less than two areas; 2 points for pruritus in more than two areas; and 3 points for widespread pruritus. The scores for severity and distribution were multiplied separately for the morning and afternoon. The maximum daily score was 30 points. Sleep disturbance was scored: 2 points for each wakeup because of pruritus (with a maximum of 10 points); and 1 point for each scratching with excoriation during the night (with a maximum of 5 points). Sleep disturbance and severity/distribution scores were added up to calculate the patient’s final score at the start and the end of the study.
Turmeric rhizome was obtained from the Indian market and powdered rhizomes were encapsulated by Medical and Natural Products Chemistry Research Center of Shiraz University of Medical Sciences, using hard gelatin capsules. Also starch capsules were made by the same Center. Curcumin as standard was obtained from Sigma-Aldrich (St. Louis, MO, USA). Acetonitrile, methanol and acetic acid were high pressure liquid chromatography (HPLC)-grade (Merck, Darmstadt, Germany). Reagent grade water (Purelab® UHQ, ELGA, High Wycombe, UK) was used throughout the study. Curcumin level of turmeric was measured by methods described elsewhere [20].
Blood samples from HD patients in a fasting state were collected from the arterial line im-mediately before the mid week dialysis session before heparin administration, and they were centrifuged and frozen at −70 °C before the measurements. Biochemical determinations included levels of serum albumin (Alb), lipid profile, blood urea nitrogen (BUN), serum creatinine (cr), calcium (Ca), phosphorus (P) before starting the study, and hs-CRP at the start and end of the study was measured. All measurements were performed at the Gastrohepatology Research Center Laboratory of Shiraz Medical University. The levels of hs-CRP were assayed with the nephelometric method.
Statistical analysis
Data were analyzed using the Statistical Package for the Social Sciences software, version 15.0 (SPSS Inc, Chicago, IL, USA). Association between categorical variables was analyzed using the Chi square test. Quantitative data were presented as mean ± standard deviation and compared by independent-samples t test in the two groups. Data variations were performed before and after administration of turmeric by paired-samples t test. Other tests were used as appropriate. All the tests were two-sided, and p values <0.05 were considered as significant.
Results
All dialysis patients attending the Ebrahimi HD referral center in Shiraz between August 2011 and June 2012 were assessed for eligibility. One hundred and one patients fulfilled the inclusion criteria and were randomized in double-blind fashion into two groups. One patient dropped out due to renal transplantation leaving a total of 100 patients (60 males and 40 females) who participated in this research project, randomized into the trial (turmeric) group (n = 50) and controls (placebo) (n = 50). The mean age of patients was 53.3 years ± 15.8 (standard deviation, SD). Average duration of HD treatment was 4.9 ± 4.1 months. Characteristics of the two groups are presented in Table 1.
Tables 2 and 3 present a comparison of the measured parameters between the two groups (turmeric vs. controls). Mean hs-CRP in the turmeric group changed from 10.8 ± 9.7 mg/l at baseline to 7.0 ± 8.9 (p = 0.003) while it changed from 9.4 ± 8.4 to 9.6 ± 9.5 mg/l (p = 0.831) in the placebo group. Mean decrease of hs-CRP was significantly higher in the turmeric group −0.8 ± 2.6 mg/l vs. 0.4 ± 8.7 in the placebo group (p = 0.012).
Mean pruritus scores in both groups at baseline and subsequent follow-up visits are shown in Table 2. The mean pruritus score between the two groups did not differ significantly at baseline (p = 0.068), but after treatment the mean reduction in pruritus score of the group treated with turmeric was significantly greater than that of the placebo group (p = 0.01); the mean difference in pruritus scores before and after treatment in the turmeric group at 8 weeks was 13.6 ± 2.6 vs. 7.2 ± 2.6 in the placebo group. Spearman’s correlation showed no significant correlation between the severity of pruritus and hs-CRP (p > 0.05).
Due to a possible role of dialysis duration, sex, and age as a confounding factor and their effect on response, after univariate analysis of variance these variables were controlled (dialysis duration with p-value: 0.293, sex with p-value: 0.749, age with p-value: 0.633) and the group variable still was significant (p-value 0.001).
Based on repeated interviews during the study, the patients mentioned no minor or major complaints attributable to the use of turmeric.
Discussion
Our findings revealed that turmeric reduces pruritus in patients with UP. Even though the decrease in symptoms was seen in both the turmeric and placebo groups, there was a significant difference between the pruritus scores in the two groups at the end of the study, indicating that turmeric is significantly more effective than placebo.
Previous studies showed that besides metabolic factors and dialysis clearance [21, 22], inflammation is the factor mainly associated with UP in HD patients [12]. UP has been shown to be associated with high levels of C reactive protein and inflammatory cytokines such an IL-2 or IL-6 [12, 13, 23]. Narita et al. [24] showed that higher β2-microglobulin independently predicts UP. And chronic inflammation stimulates the accumulation of β2-microglobulin in dialysis patients [25].
Our study showed that the hs-CRP level decreased significantly in ESRD patients who received turmeric as compared to placebo. hs-CRP has been confirmed to be one of the most reliable markers of inflammation in ESRD patients, independently predicting their overall mortality [26, 27]. Besides hs-CRP, ESRD patients with moderate/severe UP have a worse survival [12]. The skin of ESRD patients with UP has an increased number of mast cells, and these cells can release a variety of substances such as histamine, tumor necrosis factor (TNF) and IL, common markers of inflammatory processes [28, 29].
Fallahzade et al. [23] demonstrated the significantly higher serum levels of IL-2 in HD patients with UP compared to those without UP. Impairment of T helper (TH) cells balanced with TH1 predominance seems to be a main provider for systemic inflammation in UP [13]. TH1 cells produce inflammatory cytokines such as interferon (IFN) that engage and trigger leukocytes; consequently, the over-activity of TH1 cells results in an inflammatory response. Alternatively, TH2 cells produce anti-inflammatory cytokines such as IL-4 and are correlated with allergic responses [30].
As a result, any prescribed medication, by decreasing the inflammatory cascade in ESRD patients, might be effective in the modulation of UP. Previous research indicates curcumin may have potential as a therapeutic agent in inflammatory diseases such as arthritis, pancreatitis, inflammatory bowel disease and chronic anterior uveitis [31]. Curcumin (diferuloylmethane), a component of turmeric (Curcuma longa) that is inexpensive, orally bioavailable, and very safe in humans, has been shown to be able to block TNF-α action and production in in vitro and in vivo studies [32].
Hanai et al. reported the efficacy of curcumin in suppression of induced inflammatory bowel disease and promoting changes in cytokine profiles from the pro-inflammatory Th1 to the anti-inflammatory Th2 type. Curcumin has inhibitory effects on major inflammatory mechanisms like COX-2, TNF-α, IFN-γ, and nuclear factor (NF)-κB [33]. Curcumin-mediated inhibition of IL-12 production led to the inhibition of Th1 and a possible enhancement of Th2 cytokine synthesis in CD4+ T cells. The mechanism by which curcumin inhibits IL-12 production appears to be in the course of the down-regulation of NF-κB-mediated activation. NF-kB activation is considered to play a major role in the regulation of proinflammatory gene transcription; consequently, curcumin may inhibit early steps of inflammation and modulate up-regulation of multiple proinflammatory genes [34].
Although in our study there was no statistical association between hs-CRP and pruritus severity, this trial supports the idea that turmeric can be used to reduce pruritus and hs-CRP as a major inflammatory marker in patients with ESRD. No adverse reactions were seen on general health and biochemical marker tests. Turmeric is an inexpensive drug and has very few side effects [35]. In contrast to gabapentin that has been established to be useful for the treatment of CKD-associated pruritus, turmeric has less adverse effects [36].
Thus, it seems that turmeric is a safe medication in this population. However, the safety of turmeric therapy in ESRD patients should be further evaluated in larger trials with longer duration of therapy. The main limitations of our study and of the interpretation of its results are the small sample size and short duration of the treatment phase.
In conclusion, to the best of our knowledge, this is the first randomized, double-blind, clinical trial that demonstrates the possible efficacy and safety of turmeric in attenuating UP and hs-CRP in ESRD patients. Future multicenter randomized trials with larger sample size and longer treatment duration are necessary to further confirm the long-term efficacy and safety of adding turmeric in the HD population.
References
Manenti L, Tansinda P, Vaglio A (2009) Uraemic pruritus: clinical characteristics, pathophysiology and treatment. Drugs 69(3):251–263
Narita I, Iguchi S, Omori K, Gejyo F (2008) Uremic pruritus in chronic hemodialysis patients. J Nephrol 21(2):161–165
Grob JJ, Revuz J, Ortonne JP, Auquier P, Lorette G (2005) Comparative study of the impact of chronic urticaria, psoriasis and atopic dermatitis on the quality of life. Br J Dermatol 152:289–295
Marquez D, Ramonda C, Lauxmann JE, Romero CA, Vukelic VL, Martinatto C, Barron B, Novoa PA, Peixoto AJ, Orías M (2012) Uremic pruritus in hemodialysis patients: treatment with desloratidine versus gabapentin. J Bras Nefrol 34(2):148–152
Vila T, Gommer J, Scates AC (2008) Role of gabapentin in the treatment of uremic pruritus. Ann Pharmacother 42(7):1080–1084
Murphy M, Reaich D, Pai P, Finn P, Carmichael AJ (2003) A randomized, placebo-controlled, double-blind trial of ondansetron in renal itch. Br J Dermatol 148(2):314–317
Silva SR, Viana PC, Lugon NV, Hoette M, Ruzany F, Lugon JR (1994) Thalidomide for the treatment of uremic pruritus: a crossover randomized double-blind trial. Nephron 67(3):270–273
Wikstrom B, Gellert R, Ladefoged SD, Danda Y, Akai M, Ide K, Ogasawara M (2005) Kappa-opioid system in uremic pruritus: multicenter, randomized, double-blind, placebo-controlled clinical studies. J Am Soc Nephrol 16(12):3742–3747
Ko MJ, Yang JY, Wu HY, Hu FC, Chen SI, Tsai PJ, Jee SH, Chiu HC (2011) Narrowband ultraviolet B phototherapy for patients with refractory uraemic pruritus: a randomized controlled trial. Br J Dermatol 165(3):633–639
Kuypers DR, Claes K, Evenepoel P, Maes B, Vanrenterghem Y (2004) A prospective proof of concept study of the efficacy of tacrolimus ointment on uraemic pruritus (UP) in patients on chronic dialysis therapy. Nephrol Dial Transplant 19(7):1895–1901
Chiu YL, Chen HY, Chuang YF, Hsu SP, Lai CF, Pai MF, Yang SY, Peng Y (2008) Association of uraemic pruritus with inflammation and hepatitis infection in haemodialysis patients. Nephrol Dial Transplant 23(11):3685–3689
Chen HY, Chiu YL, Hsu SP, Pai MF, Lai CF, Yang JY, Peng YS, Tsai TJ, Wu KD (2010) Elevated C-reactive protein level in hemodialysis patients with moderate/severe uremic pruritus: a potential mediator of high overall mortality. QJM 103(11):837–846
Kimmel M, Alscher DM, Dunst R, Braun N, Machleidt C, Kiefer T, Stulten C, van der Kuip H, Pauli-Magnus C, Raub U, Kuhlmann U, Mettang T (2006) The role of micro-inflammation in the pathogenesis of uraemic pruritus in haemodialysis patients. Nephrol Dial Transplant 21(3):749–755
Baliga MS, Katiyar SK (2006) Chemoprevention of photocarcinogenesis by selected dietary botanicals. Photochem Photobiol Sci 5(2):243–253
Aggarwal BB, Sundaram C, Malani N, Ichikawa H (2007) Curcumin: the Indian solid gold. Adv Exp Med Biol 595:1–75
Roberto M, Roberta F, Rekha B, Colin JG (2000) Curcumin, an antioxidant and anti-inflammatory agent induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic Biol Med 28:1303–1312
Goel A, Kunnumakkara AB, Aggarwal BB (2008) Curcumin as “Curecumin”: from kitchen to clinic. Biochem Pharmacol 75(4):787–809
Hatcher H, Planalp R, Cho J, Torti FM, Torti SV (2008) Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci 65:1631–1652
Duo LJ (1987) Electrical needle therapy of uremic pruritus. Nephron 47(3):179–183
He X-G, Lin L-Z, Lian L-Z, Lindenmaier M (1998) Liquid chromatography- electrospray mass spectrometric analysis of curcuminoids and sesquiterpenoids in turmeric (Curcuma longa). J Chromatogr A 818:127–132
Wikström B (2007) Itchy skin—a clinical problem for haemodialysis patients. Nephrol Dial Transplant 22(Suppl 5):3–7
Duque MI, Thevarajah S, Chan YH, Tuttle AB, Freedman BI, Yosipovitch G (2006) Uremic pruritus is associated with higher kt/V and serum calcium concentration. Clin Nephrol 66(3):184–191
Fallahzadeh MK, Roozbeh J, Geramizadeh B, Namazi MR (2011) Interleukin-2 serum levels are elevated in patients with uremic pruritus: a novel finding with practical implications. Nephrol Dial Transplant 26(10):3338–3344
Narita I, Alchi B, Omori K, Sato F, Ajiro J, Saga D, Kondo D, Skatsume M, Maruyama S, Kazama JJ, Akazawa K, Gejyo F (2006) Etiology and prognostic significance of severe uremic pruritus in chronic hemodialysis patients. Kidney Int 69(9):1626–1632
Floege J, Schäffer J, Koch KM, Shaldon S (1992) Dialysis related amyloidosis: a disease of chronic retention and inflammation? Kidney Int Suppl 38:78–85
Yeun JY, Levine RA, Mantadilok V, Kaysen GA (2000) C-Reactive protein predicts all-cause and cardiovascular mortality in hemodialysis patients. Am J Kidney Dis 35(3):469–476
Tripepi G, Mallamaci F, Zoccali C (2005) Inflammation markers, adhesion molecules, and all-cause and cardiovascular mortality in patients with ESRD: searching for the best risk marker by multivariate modeling. J Am Soc Nephrol 16(Suppl 1):83–88
Lugon JR (2005) Uremic pruritus: a review. Hemodial Int 9(2):180–188
Aoki R, Kawamura T, Goshima F, Ogawa Y, Nakae S, Nakao A, Moriishi K, Nishiyama Y, Shimada S (2013) Mast cells play a key role in host defense against herpes simplex virus infection through TNF-α and IL-6 production. J Invest Dermatol 133:2170–2179
Abbas AK, Murphy KM, Sher A (1996) Functional diversity of helper T lymphocytes. Nature 383:787–793
Jurenka JS (2009) Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev 14(2):141–153
Aggarwal BB, Gupta SC, Sung B (2013) Curcumin: an orally bioavailable blocker of TNF and other pro-inflammatory biomarkers. Br J Pharmacol 169:1672–1692
Hanai H, Iida T, Takeuchi K, Watanabe F, Maruyama Y, Andoh A, Tsujikawa T (2006) Curcumin maintenance therapy for ulcerative colitis: randomized, multicenter, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol 4(12):1502–1506
Ukil A, Maity S, Karmakar S, Datta N, Vedasiromoni JR, Das PK (2003) Curcumin, the major component of food flavour turmeric, reduces mucosal injury in trinitrobenzene sulphonic acid-induced colitis. Br J Pharmacol 139(2):209–218
Fan X, Zhang C, Liu DB, Yan J, Liang HP (2013) The clinical applications of curcumin: current state and the future. Curr Pharm Des 19(11):2011–2031
Gunal AI, Ozalp G, Yoldas TK, Gunal SY, Kirciman E, Celiker H (2004) Gabapentin therapy for pruritus in haemodialysis patients: a randomized, placebo-controlled, double-blind trial. Nephrol Dial Transplant 19(12):3137–3139
Acknowledgments
The Shiraz Nephro-Urology Research Center of Shiraz University of Medical Science funded this study, which is derived from the student’s thesis (Dr. Fatemeh Basiri). The authors would like to thank Dr. Nasrin Shokrpour at the Center for Development of Clinical Research of Nemazee Hospital for editorial assistance.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pakfetrat, M., Basiri, F., Malekmakan, L. et al. Effects of turmeric on uremic pruritus in end stage renal disease patients: a double-blind randomized clinical trial. J Nephrol 27, 203–207 (2014). https://doi.org/10.1007/s40620-014-0039-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-014-0039-2