Abstract
Introduction
Chronic renal failure (CRF) compromises nutrition, growth, puberty, glycometabolic homeostasis, and adipokine secretion (i.e. adiponectin, resistin, and leptin). Adipokines play a role in the clinical outcome, but data in paediatric patients is scant.
Aim
To evaluate the link between kidney function, adiponectin, resistin, leptin, hormonal status, nutritional state and late outcome of CRF children.
Materials and methods
We studied leptin, adiponectin and resistin levels in 31 CRF patients (19 males, 12 females, aged 12.1 ± 4.47 years) managed conservatively, and 30 healthy age- and gender-matched controls. Clinical, auxological, biochemical, hormonal data, glucose and insulin levels were correlated with adipokine levels.
Results
Six percent of patients had glycaemia T0′ > 126 mg/dl, 23 % glycaemia T60′ > 126, and 23 % glycaemia T120′ ≥ 140. Glycated haemoglobin (HbA1c) measured during follow-up was in the normal range in all patients (4–5.6 %). Insulinaemia was significantly higher in CRF patients than controls. Homeostatic model of assessment-insulin resistance (HOMA-IR) levels were more elevated in patients (32 % had HOMA-IR > 2.5) than controls. Leptin levels were significantly higher in CRF patients than controls and differed significantly between males and females. Leptin correlated significantly with creatinine, body mass index (BMI), BA, pubertal stage, insulin-like growth factor 1, and HOMA-IR in females. Adiponectin levels were significantly higher in patients than controls, higher in patients with BMI < 85th centile and significantly inversely correlated to BMI, BA, haemoglobin, ferritin, proteins, albumin, and creatininuria. Resistin levels showed a direct correlation with C-reactive protein and an inverse correlation with haemoglobin.
Conclusion
Normal resistin levels are an expression of both adequate nutritional state and controlled inflammatory state. Adiponectin could protect against chronic inflammation, atherosclerosis, and cardiovascular diseases. Preventing obesity and ensuring a correct nutritional state are primary goals for physicians following children with CRF. Adipokines could be a useful marker in the follow-up.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic renal failure (CRF) is a possible fearful evolution of severe paediatric congenital and/or acquired kidney diseases. In the most severe cases it could lead to the need for dialysis with further complications for the patients’ clinical outcome [1]. CRF compromises growth [2, 3] and puberty, glycometabolic and lipid homeostasis, nutritional status [4, 5] and the endocrine profile of these children. Growth delay is present in more than 50 % of CRF children and several of these patients have a stature <−2 standard deviation score (SDS) [4]. Adipose tissue is a candidate for secretive disarray, with possible interference in adipocytokine secretion, also mediated by inflammation mediators such as tumor necrosis factor α (TNFα), interleukin (IL)-1 and IL-6 [6]. Several studies have demonstrated the influence of leptin on growth and puberty [7], the role of resistin on glycometabolic state and insulin resistance [8], and the importance of adiponectin as an anti-inflammatory and antiatherogenic factor [9]. However, there are few published reports in the literature evaluating the role of adipokines in CRF children and adolescents [10–14].
Malnutrition is a severe consequence of paediatric CRF with low caloric and protein intake. It depends on several factors, such as anorexia, ageusia linked to Zn deficiency, catabolic state and muscular compromission, endocrine anomalies, dialysis with possible growth failure and pubertal delay [5].
Adipocytes secrete several peptides with endocrine and immunologic properties such as leptin, adiponectin, resistin, TNFα, IL-1 and IL-6. An inflammatory state induces secretion of several peptides by adipocytes, such as adipokines and cytokines: macrophages are the bridge between inflammation and adipocytes activity. In fact the two specific lines of cells show common gene expression of both adipokine and cytokine synthesis [15, 16].
Subjects and methods
We studied 31 paediatric patients (19 males, 12 females), aged 12.1 ± 4.47 years, with CRF defined by the Schwarz formula, managed conservatively. Informed consent was obtained from the patients’ parents prior to subjects’ inclusion in the study. The study was approved by the ethical committee of our hospital and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
We evaluated clinical and biochemical data: blood urea nitrogen (BUN), creatinine, proteins, albumin, haemoglobin (Hb), cholesterol, high-density lipoprotein (HDL), triglycerides, C-reactive protein (CRP), ferritin; auxological parameters: stature, weight, bone age, pubertal stage with testicular volume or ovary echographic diameters, body mass index (BMI); glucose and insulin levels: fasting and post-prandial glucose, insulin, c-peptide, homeostatic model of assessment-insulin resistance (HOMA-IR), homeostatic model of assessment-beta-cell function (HOMA %β); endocrine patterns: follicle-stimulating hormone (FSH), luteinizing hormone (LH), estradiol, testosterone, adrenocorticotropic hormone (ACTH), cortisol, thyroid stimulating hormone (TSH), free thyroid hormones fT3 and fT4, prolactin (PRL), and insulin-like growth factor 1 (IGF-1). The above data were correlated with adipokine (leptin, adiponectin, resistin) levels. We compared all the data with a control group of 30 healthy children matched for gender and age.
All blood samples were collected in the morning after overnight fasting. Adipokines were measured using enzyme-linked immunosorbent assay (ELISA) tests (Linco Research, St Charles, MO, USA). Test sensitivity was 0.5, 0.78, 0.16 ng/ml for leptin, adiponectin and resistin respectively. TSH was assessed by an immunometric assay in chemiluminescence in solid phase; fT3 and fT4 were evaluated by an immunoenzymatic assay in chemiluminescence in solid phase; cortisol, FSH and LH were measured by an immunoenzymatic assay in chemiluminescence.
All variables were tested for normality with the Anderson–Darling normality test. All variables were expressed as mean ± standard deviation (M ± SD). The degree of linear relationship between clinical, microbiological and biochemical parameters was calculated using Pearson’s product moment correlation coefficient; statistical significance was considered at p values <0.05. Calculations were performed using MiniTAB release 13.1 Statistical Software.
Results
Biochemical parameters regarding kidney function, nutritional and inflammatory indexes are reported in Table 1. BMI was 20.53 ± 5.07 with no significant gender difference; 4 patients (13 %, 2 females, 2 males) had a BMI < 10th centile; 20 patients (58 %, 12 males, 8 females) had a BMI 10–85th centile and 7 patients (23 %, 2 females, 5 males) had a BMI > 85th centile.
Blood pressure (BP), evaluated in all patients, showed systolic BP 112 ± 17 mmHg and diastolic BP 71 ± 12 mmHg. There were no significant differences between BP values of males and females. Hypertension was evidenced in eight patients (25.8 %); six of them received anti-hypertensive drugs (angiotensin-converting-enzyme inhibitors). Systolic BP was significantly correlated to BMI (p = 0.004; r = 0.506); diastolic BP was correlated to BMI (p = 0.446; r = 0.142).
Glycaemia T0 was 91.81 ± 18.38 mg/dl; glycaemia T60′: 118.96 ± 26.28 mg/dl; glycaemia T120′: 115.13 ± 35.04 mg/dl; 2 patients (6 %) had glycaemia T0′ > 126 mg/dl; 7 patients (23 %) had glycaemia T60′ > 126 mg/dl; 7 patients (23 %) had glycaemia T120′ ≥ 140 mg/dl. Glycated haemoglobin (HbA1c) detected during follow-up was in the normal range in all patients (4–5.6 %).
Insulinaemia was 13.16 ± 18.05, and significantly higher than in controls (9.1); HOMA-IR was 3 ± 4.14, higher than in controls (1.84). Ten patients (32 %) had HOMA-IR > 2.5.
We found a statistically significant direct correlation between HOMA-IR and BUN (r = 0.671; p < 0.002), between HOMA-IR and creatininemia (r = 0.676; p < 0.002), between insulinaemia and BMI (r = 0.416; p = 0.011) and between IGF-1 and proteins (r = 0.510; p = 0.011). HOMA-IR was directly correlated with BMI (r = 0.32; p = 0.057), but did not reach statistical significance. No correlations were found between either nutritional indexes (albumin, haemoglobin, ferritin) or inflammatory markers (CRP) and insulinaemia or HOMA-IR.
IGF-1 was 346.1 ± 211.5 ng/ml (females 409.58 ± 214.97; males 257.64 ± 182.1). Leptin was 24.72 ± 30.95 ng/ml (males 15.58 ± 21.65; females 39.2 ± 38.4) with a significant difference between males and females (p < 0.001) and was significantly higher (Table 2) in CRF patients than controls (3.97 ± 4.52; p < 0.001). Adipokine levels are reported in Table 2. Leptin showed a significant correlation with: creatinine (r = 0.535; p = 0.001), BMI (r = 0.786; p = 0.000), BMI centiles (r = 0.693; p = 0.000), BA (r = 0.367; p = 0.042), pubertal stage (r = 0.360; p = 0.047), IGF-1 (r = 0.611; p = 0.002), and HOMA-IR in females (r = 0.602; p = 0.038). Leptin was correlated, but not significantly so, with both systolic (p = 0.076; r = 0.323) and diastolic BP (p = 0.573; r = 0.105).
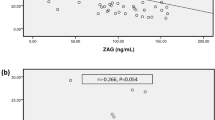
Adiponectin levels were significantly higher in patients than controls (18.89 ± 8.57 vs. 8.63 ± 1.67 μg/ml; p < 0.001), and more elevated in CRF females (males 17.18 ± 7.75; females 21.5 ± 9.51) (Table 2). Adiponectin levels were higher in patients with BMI < 85th centile than BMI > 85th centile (20.33 ± 8.87 vs. 15.18 ± 9.67) and showed a significant inverse correlation with BMI (r = −0.403; p = 0.025), BA (r = −0.392; p = 0.029), haemoglobin (r = −0.368; p = 0.041), ferritin (r = −0.408; p = 0.023), proteins (r = −0.648; p = 0.000), albumin (r = −0.485; p = 0.006), and creatininuria (r = −0.356; p = 0.049). An inverse correlation between adiponectin and BP was observed, but it did not reach statistical significance for either systolic (p = 0.200; r = −0.237) or diastolic BP (p = 0.200; r = −0.101).
Resistin levels were 9.03 ± 1.46 ng/ml (males 8.87 ± 1.72; females 9.26 ± 0.91; see Table 2) with no significant difference vs. controls. Resistin presented a direct correlation with CRP (r = 0.371; p = 0.040) and an inverse correlation with Hb (r = −0.475; p = 0.047) and fT4 (r = −0.394; p = 0.028).
Discussion
CRF is associated with an increased risk of cardiovascular disease. Hyperinsulinaemia and insulin resistance are risk factors for cardiovascular disease, also mediated by inflammation and malnutrition. The high glycaemia, insulinaemia and HOMA-IR observed in our mild-moderate CRF patients are an expression of insulin resistance. The early occurrence in young patients, also in moderate impaired kidney function, is a significant cardiovascular risk factor with a significant prognostic impact.
As reported in the literature in adult patients [17, 18], leptin levels were significantly higher in our patients with CRF than in controls. Only one study evaluated leptin levels in children with CRF, but on haemodialysis treatment [11]. At the other end of the treatment spectrum, we detected leptin in children with CRF conservatively managed. Increased leptin levels in CRF may depend on reduced glomerular filtration velocity, chronic inflammation, and hyperinsulinism. In fact, in our patents a direct correlation with creatinine (p < 0.05), insulin (p = 0.05), and HOMA-IR (p < 0.05) was found. Furthermore we evidenced a direct statistically significant correlation with nutritional parameters (BMI, and IGF-1).
As reported in the literature, serum resistin levels increase with a decline in the glomerular filtration rate (GFR) which is involved in the inflammatory milieu present in CRF [10]; in our patients normal levels of resistin were an expression of an adequate nutritional and metabolic status, maintained insulin sensitivity and controlled inflammatory state: resistin could be a useful marker in the follow-up of these patients.
Adiponectin levels are reported to be elevated in adult patients with CRF [18–23], but only a few studies have published data related to paediatric patients [12–14]. Our study confirmed higher adiponectin levels in paediatric patients with CRF vs. controls; as reported in the literature [14], in our patients adiponectin showed, in children with BMI < 85th centile, levels higher than in children with BMI > 85th centile, with a significant direct correlation with creatinine clearance (p < 0.005).
On the contrary in obese adult patients with CRF, BMI is the factor with the strongest influence on adiponectin secretion [23]: we confirmed these results also in children with CRF. Adiponectin could be a protective factor against chronic inflammation [24], insulin resistance [12], atherosclerosis and cardiovascular diseases in this group of patients. For this reason obese CRF patients could have an enhanced risk of cardiovascular accidents, with respect to CRF lean patients [25] because they do not benefit from the protective advantages of higher adiponectin levels [12].
The strong correlation between leptin or adiponectin and BMI [13, 26] highlights the role of correct nutrition in nephropathies [27]; in fact an adequate control of BMI ensures good adipokine concentrations, helpful to combat anorexia and prevent cardiovascular involvement [12, 14, 28].
For these reasons the prevention of obesity and ensuring a correct nutritional state are primary goals for physicians who follow children with CRF.
The inverse correlation between adiponectin and BP values highlights the role of adiponectin as a protective factor with anti-hypertensive and anti-atherogenic properties [29]. Adiponectin was lower in patients with higher systolic and/or diastolic BP values. Also leptin was directly correlated with BP values, especially with systolic BP. The statistically significant inter-correlation between systolic BP, BMI and leptin emphasizes the role of leptin as a marker of adequate nutritional state with prognostic consequences. Recently, high leptin levels were reported to be correlated to the risk of developing CRF later in life [30]. We propose the role of increased leptin levels as a indicator, linked to overweight, of CRF progression in children.
These findings stress the essential role of an integrated follow-up of children and adolescents with CRF in order to maintain an adequate nutritional state and prevent overweight, reducing cardiovascular risk and ameliorating the long-term prognosis [30].
References
Ardissino G, Daccò V, Testa S et al (2003) Epidemiology of chronic renal failure in children: data from the Italkid Project. Pediatrics 111:e382–e387
Büscher AK, Büscher R, Pridzun L et al (2012) Functional and total IGFBP3 for the assessment of disorders of the GH/IGF1 axis in children with chronic kidney disease, GH deficiency, or short stature after SGA status at birth. Eur J Endocrinol 166(5):923–931
Hodson EM, Willis NS, Craig JC (2012) Growth hormone for children with chronic kidney disease. Cochrane Database Syst Rev 2:CD003264
Kari JA, Gonzalez C, Ledermann SE et al (2000) Outcome and growth of infants with severe chronic renal failure. Kidney Int 57:1681–1687
Wingen AM, Melhs O (2002) Nutrition in children with preterminal chronic renal failure. Myth or important therapeutic aid. Pediatr Nephrol 17:111–120
Chudek J, Wiecek A (2006) Adipose tissue, inflammation and endothelial disfunction. Pharmacol Rep 58(suppl):81–88
Baratta M (2002) Leptin: from a signal of adiposity to a hormone mediator in peripheral tissues. Med Sci Monit 8:RA282–RA292
Shuldiner AR, Yang R, Gong D-W (2001) Resistin, obesity, and insulin resistance—the emerging role of the adipocyte as an endocrine organ. N Engl J Med 345:1345–1346
Matsuda M, Shimomura I, Sata M et al (2002) Role of adiponectin in preventing vascular stenosis. The missing link of adipo-vascular axis. J Biol Chem 277:37487–37491
Nehus E, Furth S, Warady B, Mitsnefes M (2012) Correlates of resistin in children with chronic kidney disease: the chronic kidney disease in children cohort. J Pediatr 161(2):276–280
Szprynger K, Szczepańska M, Kos-Kudła B et al (2005) Serum leptin levels in children with chronic renal failure on continuous ambulatory peritoneal dialysis and on haemodialysis treatment. Wiad Lek 58(Suppl 1):66–70
Kamariski M, Biscardi M, Cestino L et al (2009) Adiponectin in children on peritoneal dialysis: relationship to insulin resistance and nutritional status. Nephron Clin Pract 113(1):c24–c32
Elshamaa MF, Sabry SM, El-Sonbaty MM et al (2012) Adiponectin: an adipocyte-derived hormone, and its gene encoding in children with chronic kidney disease. BMC Res Notes 5:174
Mitsnefes M, Kartal J, Khoury P, Daniels S (2007) Adiponectin in children with chronic kidney disease: role of adiposity and kidney dysfunction. Clin J Am Soc Nephrol 2(1):46–50
Chudek J, Adamczak M, Nieszporek T, Wiecek A (2006) The adipose tissue as an endocrine organ—a nephrologists’ perspective. Contrib Nephrol 151:70–90
Axelsson J, Heimbürger O, Stenvinkel P (2006) Adipose tissue and inflammation in chronic kidney disease. Contrib Nephrol 151:165–174
Pecoits-Filho R, Nordfors L, Heimbürger O et al (2002) Soluble leptin receptors and serum leptin in end-stage renal disease: relationship with inflammation and body composition. Eur J Clin Invest 32(11):811–817
Dervisoglu E, Eraldemir C, Kalender B et al (2008) Adipocytokines leptin and adiponectin, and measures of malnutrition-inflammation in chronic renal failure: is there a relationship? J Ren Nutr 18(4):332–337
Marchlewska A, Stenvinkel P, Lindholm B et al (2004) Reduced gene expression of adiponectin in fat tissue from patients with end-stage renal disease. Kidney Int 66(1):46–50
Zoccali C, Mallamaci F, Tripepi G et al (2002) Adiponectin, metabolic risk factors, and cardiovascular events among patients with end stage renal disease. J Am Soc Nephrol 13:134–141
Stenvinkel P, Marchlewska A, Pecoits-Filho R et al (2004) Adiponectin in renal disease: relationship to phenotype and genetic variation in the gene encoding adiponectin. Kidney Int 65(1):274–281
Yoo DE, Lee MJ, Oh HJ et al (2012) Low circulating adiponectin levels are associated with insulin resistance in non-obese peritoneal dialysis patients. Endocr J 59(8):685–695
Guebre-Egziabher F, Bernhard J, Funahashi T et al (2005) Adiponectin in chronic kidney disease is related more to metabolic disturbance than to decline in renal function. Nephrol Dial Transpl 20(1):129–134
Axelsson J, Heimburger O, Lindhom B, Stenvinkel P (2005) Adipose tissue and its relation to inflammation: the role of adipokines. J Ren Nutr 15:131–136
Samuels J, Ng D, Flynn JT et al Chronic Kidney Disease in Children Study Group. (2012) Ambulatory blood pressure patterns in children with chronic kidney disease. Hypertension 60(1):43–50
Oliveira JC, Machado Neto Fde A, Morcillo AM et al (2005) Chronic renal failure and growth hormone: effects on GH-IGF axis and leptin. Arq Bras Endocrinol Metabol 49(6):964–970
Fontan MP, Garcia Buela J, Cordido F, Rodriguez Carmona A (1999) Hyperleptinemia in uremic patients undergoing conservative management, peritoneal dialysis, and hemodialysis: a comparative analysis. Am J Kidney Dis 34:824–831
Furuhashi M, Ura N, Higashuiura K et al (2003) Blockade of the renin-angiotensin system increases adiponectin concentrations in patient with essential hypertension. Hypertension 42:76–81
Brambilla P, Antolini L, Street ME et al (2013) Adiponectin and hypertension in normal-weight and obese children. Am J Hypertens 26(2):257–264
Gunta SS, Mak RH (2013) Is obesity a risk factor for chronic kidney disease in children? Pediatr Nephrol 28(10):1949–1956
Conflict of interest
The authors have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maggio, M.C., Montaperto, D., Maringhini, S. et al. Adiponectin, resistin and leptin in paediatric chronic renal failure: correlation with auxological and endocrine profiles. J Nephrol 27, 275–279 (2014). https://doi.org/10.1007/s40620-013-0015-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-013-0015-2