Abstract
Purpose
To evaluate differences between patients with unilateral and bilateral adrenal incidentalomas (AIs) in the prevalence of autonomous cortisol secretion (ACS) and related comorbidities.
Methods
In this multicentre retrospective study, AIs ≥ 1 cm without overt hormonal excess were included in the study. ACS was defined by a post-dexamethasone suppression test (DST) serum cortisol ≥ 5.0 µg/dl, in the absence of signs of hypercortisolism. For the association of ACS with the prevalence of comorbidities, post-DST serum cortisol was also analysed as a continuous variable.
Results
Inclusion criteria were met by 823 patients, 66.3% had unilateral and 33.7% bilateral AIs. ACS was demonstrated in 5.7% of patients. No differences in the prevalence of ACS and related comorbidities were found between bilateral and unilateral AIs (P > 0.05). However, we found that tumour size was a good predictor of ACS (OR = 1.1 for each mm, P < 0.001), and the cut-off of 25 mm presented a good diagnostic accuracy to predict ACS (sensitivity of 69.4%, specificity of 74.1%).
During a median follow-up time of 31.2 (IQR = 14.4–56.5) months, the risk of developing dyslipidaemia was increased in bilateral compared with unilateral AIs (HR = 1.8, 95% CI = 1.1–3.0 but, this association depended on the tumour size observed at the end of follow-up (HR adjusted by last visit-tumour size = 0.9, 95% CI = 0.1–16.2).
Conclusions
Tumour size, not bilaterality, is associated with a higher prevalence of ACS. During follow-up, neither tumour size nor bilaterality were associated with the development of new comorbidities, yet a larger tumour size after follow-up explained the association of bilateral AIs with the risk of dyslipidaemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adrenal incidentalomas (AIs) are asymptomatic adrenal lesions that are identified unexpectedly on imaging tests requested by reasons other than adrenal disease [1]. Most AIs are classified as non-functioning adrenocortical adenomas. However, it has been suggested that a significant percentage of these presumably non-functioning AIs (NFAI) actually secrete adrenal steroids in excess. In fact, autonomous cortisol secretion (ACS)—defined as biochemical evidence of cortisol hypersecretion in the absence of signs of Cushing’s syndrome [2]—has been detected in up to 50% of AIs [3]. However, the best test to diagnose ACS remains unclear at present. Even though the overnight 1 mg dexamethasone suppression test (DST) is the most extended screening test, currently there is no consensus on which is the best threshold of post-DST serum cortisol to define ACS [3,4,5].
Regardless of the test or threshold used to diagnosed ACS, there is growing evidence linking this condition with increased metabolic and cardiovascular morbidity and mortality [6,7,8]. Several clinical, biochemical and imaging characteristics of AIs have been associated with ACS [6, 9,10,11]. In this regard, bilaterality of AIs might be associated with higher rates of ACS and related comorbidities [9, 10] although such an association has not been supported by all studies [10, 12,13,14].
The aim of this study was to evaluate the differences in the prevalence of ACS and potential related metabolic comorbidities among patients with unilateral and bilateral AIs. Moreover, we analysed the impact of bilaterality of AIs on the risk of tumour growth and development of ACS and related comorbidities during follow-up.
Methods
Study population
In this retrospective study, we included 977 patients presenting with one or more AIs of at least 1 cm in larger diameter at seven Spanish Hospitals between 2001 and 2020. Bilaterality of AIs was defined by the presence of at least one AI in each of the adrenal glands.
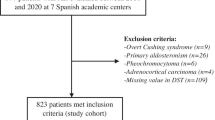
Patients who met one or more of the following criteria were directly excluded to enter in the Spanish Adrenal Incidentaloma Register (i) under age 18 or above age 90 years; (ii) suffering with hereditary syndromes associated with adrenal tumours [4]; (iii) those on chronic treatment with glucocorticoids (GC) or who had received GC treatment with a dose ≥ 10 mg/day of prednisone or equivalent for 3 months during the 3 months preceding hormonal evaluation, (iv) patients under treatment with oral hormonal contraceptives (treatment should be suspended at least 6 weeks before performing the functionality study), (v) patients in whom the imaging test was performed in the context of the study of extension of an extra-adrenal primary cancer and vi) patients with ACTH-dependent Cushing syndrome [15,16,17,18]. For this study, exclusion criteria were: i) overt adrenal hormone excess such as Cushing’s syndrome (n = 9), primary hyperaldosteronism (n = 26), pheochromocytoma (n = 6) or sexual steroid producing adrenal lesions (n = 0); ii) diagnosis of adrenocortical carcinoma or radiological features suggestive of malignancy (irregular and heterogeneous lesions) (n = 4) and iii) patients with missing values in the initial DST (n = 109). A total of 823 (84.6%) patients were finally included in the study (Fig. 1). We analysed patients’ data recorded at entry into the study and at the last follow-up visit after median 31.2 (IQR = 14.4–56.5) months. The study was approved by the local ethical Committee of the Hospital Universitario Ramón y Cajal.
Clinical evaluation
Medical records were reviewed retrospectively to extract demographic information such as age, and sex, medical history of ACS-related comorbidities including hypertension, type 2 diabetes mellitus, obesity, dyslipidaemia, cerebrovascular, and cardiovascular disease, and physical examination variables including body mass index (BMI) and clinical systolic and diastolic blood pressure. These parameters were assessed at baseline and at the last follow-up visit.
Hypertension was diagnosed when blood pressure was ≥ 140/90 mmHg or patients were receiving antihypertensive drugs. Diagnoses of type 2 diabetes and dyslipidaemia were based on established criteria [19, 19]. Obesity was defined by a BMI ≥ 30 kg/m2. Cardiovascular disease was defined as ischemic heart disease or heart failure, and cerebrovascular disease as a transient ischemic attack or acute stroke.
Hormonal and biochemical evaluation
At study entry, all AI patients underwent a DST and measurement of urinary normetanephrine and metanephrine. Other hormones such as serum cortisol, adrenocorticotropic hormone (ACTH), dehydroepiandrosterone sulphate (DHEA-S), and 24-urinary free cortisol (UFC) were also evaluated in some patients at the discretion of the attending physician. Aldosterone/renin ratio was also evaluated in hypertensive or hypokalemic patients; and 17-hydroxyprogesterone and serum basal cortisol concentrations served to rule out non-classic congenital adrenal hyperplasia in patients presenting with bilateral AIs. The DST was repeated at the follow-up visit in 437 patients. Other hormones were also determined at the discretion of the treating physician.
ACS was defined as a serum cortisol concentration ≥ 5.0 µg/dl at 8 am following a single 1 mg dexamethasone oral dose taken at 11 pm the night before extraction, provided that specific signs of cortisol excess such as myopathy, ecchymosis and/or cutaneous atrophy were absent. We used a 5.0 µg/dl post-DST serum cortisol threshold for the definition of ACS based on the last European guidelines about the management of AIs [21]. However, considering the current uncertainties about the optimal cut-off to define ACS, we also analysed post-DST serum cortisol as a continuous variable. AIs were considered as non-functioning tumours when the hormonal evaluation ruled out hormone excess (i.e. cortisol was < 1.8 µg/dl on the DST and the aldosterone/renin ratio and urinary metanephrines concentrations were within the reference ranges of the local laboratories of each Hospital). Patients with post-DST serum cortisol between 1.8 and 5.0 µg/dL were classified as possible ACS [21].
All patients underwent routine biochemical profiles after an overnight fast, at the initial evaluation and at the last follow-up visit. Fasting glucose, total, LDL and HDL cholesterol levels, and triglyceride concentrations were also evaluated. HbA1c was measured in some patients at the discretion of the treating physician depending on glucose concentrations and on whether or not the patient had been diagnosed with diabetes.
Radiological study
Abdominal computed tomography (CT) or MRI were performed in all patients at diagnosis. The maximum diameter informed in the radiological report of the CT or MRI was taken as the adenoma size, and uni- or bilaterality and lipid content were recorded. For bilateral AIs, the size of the largest adenoma was included in the analyses. Moreover, we calculated the total adenomatous mass as the sum of the largest diameters of both adrenal incidentalomas for bilateral AIs. During follow-up, CT was repeated in 386 patients and MRI was repeated in 259 patients.
Statistical analysis
We used STATA version 15 for statistical analyses. Categorical variables were expressed as counts and percentages and continuous variables were expressed as mean ± standard deviation (SD) or median and interquartile range (IQR) depending on if the assumption of normality was fullfilled. The normality assumption was studied with Shapiro–Wilk test and the variance homogeneity assumption with the Levene test. Odds ratios (with 95% confidence intervals) and mean differences were calculated as measures of association. Unpaired t tests and Mann–Whitney U tests lineal were performed as appropriate to compare differences in continuous parameters between unilateral and bilateral AIs. The comparisons of baseline values with those obtained at the end of follow-up used paired t test or McNemar test as appropriate. Cox regression analysis was used for the estimation of hazard ratios during follow-up. Pearson’s or Spearman’s correlation analysis was used to evaluate correlations between continuous variables. Fisher’s exact tests and chi-square tests were performed for the comparison of categorical variables between the groups. A multivariant logistic regression test was performed to analyse the influence of post-DST serum cortisol on AI uni- or bilaterality or in the presence of absence of cardiometabolic comorbidities, while adjusting for confusion variables such as tumour size, age and BMI. Collinearity between continuous variables was discarded confirming a Variance inflation factor (VIF) values higher than 0.1 and lower than 10. ROC curves were performed to calculate the best predictive tumor size for ACS diagnosis. In all cases, a two-tailed P value < 0.05 was considered as statistically significant.
Results
Baseline characteristics
A total of 823 patients were included in the final analysis of whom 472 (58.0%) were women. Mean age was 63.1 ± 11.0 years-old and mean BMI was 28.9 ± 7.3 kg/m2. The mean size of the AIs in the cohort was 20.7 ± 10.5 mm, and only 7 patients with typical radiological benign features presented AIs > 50 mm. 112 patients (17.1%) were classified as tumours poor in lipidic content. AIs were unilateral in 546 patients (66.3%) and bilateral in 277 (33.7%). Forty-seven patients (5.7%) met criteria for ACS as defined by post-DST serum cortisol ≥ 5.0 µg/dl; 522 (63.4%) were classified as non-functioning AIs (NFAI) and the remaining 254 patients as with possible ACS. Among patients with ACS, 35% (n = 14/40) presented plasma ACTH levels below 10 pg/mL; 50% (n = 10/20) high late-night salivary cortisol and only 2 out of 28 patients high UFC. This supposed that 84.6% of the patients with ACS with available information in these tests presented at least one of these associated hormonal alterations.
Regarding comorbidities, globally 83.7% of patients presented one or more comorbidities; 54.7% had hypertension; 51.5% had dyslipidaemia; 32.1% had obesity; 26.0% had type 2 diabetes; 2.3% had cerebrovascular disease and 12.2% had cardiovascular disease. We found that patients with ACS presented a worse cardiometabolic profile than NFAI patients and those patients with possible ACS (Table 1).
The comparisons of patients with unilateral and bilateral AIs at study entry are summarized in Table 2. Tumour size was larger in patients with bilateral AIs compared with those presenting with unilateral AIs, especially when we considered total adenomatous mass (42.0 ± 17.3 vs 19.9 ± 10.3, P < 0.001). We found no other significant differences in baseline clinical or biochemical characteristics or the prevalence of comorbidities, with the exception of the prevalence of male sex that appeared to be more frequent in bilateral compared with unilateral AIs (OR = 1.4, 95% CI = 1.0–1.9).
No differences in the prevalence of ACS were observed between unilateral and bilateral AIs. However, if we considered a threshold of 3.0 µg/dL for the definition of ACS, the prevalence of ACS was increased in patients with bilateral than unilateral AIs (OR = 2.1, 95% CI = 1.4–3.1), yet this difference lost statistical significance after adjusting by tumour size (adjusted OR = 4.7, 95% CI = 0.5–43.1) (Fig. 2).
Differences in tumor size according to laterality and functionality. a Differences between all unilateral and bilateral adrenal incidentalomas (AIs). b Differences between unilateral and bilateral tumors with autonomous cortisol secretion (ACS). c Differences between non-functioning unilateral and bilateral AIs. d Differences between non-functioning AIs and AIs with ACS.
When we analysed data considering post-DST serum cortisol values as a continuous variable, regardless of the diagnosis of ACS, no association was found between DST results and AIs uni- or bilaterality (OR = 1.06 for each µg/dl, 95% CI = 1.0–1.1); nor between the DST results and cardiometabolic comorbidities (Supplementary Material Table 1). However, post-DST serum cortisol concentrations were associated with the size of the adenoma (β = 0.61 mm for each µg/dl in serum cortisol, P = 0.000) and the total adenomatous mass (β = 1.0 mm for each µg/dl in serum cortisol, P < 0.001). Moreover, we found that tumour size was a good predictor of ACS (OR = 1.1 for each mm, P < 0.001) and correlated directly with post-DST serum cortisol concentrations, although the correlation was mildly (r = 0.18, P < 0.001). The best predictive tumour size for ACS was 25 mm, with a sensitivity of 69.4% and specificity of 74.1% (AUC = 0.758, 95% CI = 0.720–0.792). Considering, total adenomatous mass for bilateral AIs, similar results were found [OR = 1.2, P < 0.001 for the prediction of ACS, and a middle correlation with post-DST serum cortisol concentrations (r = 0.22, P < 0.001)].
Differences between unilateral and bilateral adrenal incidentalomas depending on the presence or absence of ACS at study entry
In the subset of patients meeting criteria for ACS, no differences were found between unilateral and bilateral AIs (Table 3). When the same comparisons were analysed in the subset of patients with NFAI, no differences in clinical and biochemical characteristics were found. However, post-DST serum cortisol concentrations were slightly higher in patients with bilateral AIs (Fig. 2, Supplementary Material Table 2).
Follow-up study
Follow-up information, including clinical, hormone and/or radiological information, was available in 673 patients (DST was repeated in 437 patients and abdominal CT/MRI in 621). After a median follow-up of 31.2 (IQR = 14.4–56.5) months, only 13 patients experienced clinically relevant growth defined by an increase > 10 mm in larger diameter. We found no differences in the initial tumour size (19.5 ± 3.5 vs 21.1 ± 0.5 mm, P = 0.569) or post-DST serum cortisol concentrations (2.6 ± 0.2 vs 2.3 ± 0.2 µg/dl, P = 0.716) among AIs that grew and those that remained stable during follow-up. There were 15 patients with NFAI who developed ACS (1.9%), no changes in hormonal status were observed in the rest of the patients.
Bilaterality of AIs was not associated with tumour growth (HR = 1.2, 95% CI = 0.4–3.8) or progression to ACS (HR 2.1, 95% CI = 0.7–6.5). However, bilaterality was associated with an increased risk of developing dyslipidaemia during follow-up (HR 1.8; 95% CI = 1.1–3.0) even though such an association actually depended on the larger tumour size of bilateral AIs (HR adjusted by last visit tumour size = 0.9, 95% CI = 0.1–16.2). Bilaterality of AIs was not associated with other cardiometabolic comorbidities nor in the proportion of patients that need initiation of antihypertensive or antidiabetic medications. However, those patients with bilateral AIs presented a higher risk of need to start lipid-lowering treatment (Supplementary material, Table 3).
Discussion
Our present results suggested that maximum adenoma diameter, and not bilaterality itself, is a risk factor for ACS in patients with AIs. The prevalence of bilateral AIs in our cohort (33.7%) was similar to that described in earlier reports [13, 21]. And even though we found a higher prevalence of ACS in bilateral AIs when we considered the 3.0 µg/dl 1 mg DST threshold for the ACS definition, such an increase did not retain statistical significance after the analysis was adjusted for tumour size.
To date, studies addressing the association of bilaterality of AIs and ACS yielded conflicting results. Three out of 6 previous studies found an association [9, 14, 22]. However, unilateral AIs were smaller than bilateral AIs in two of these studies (23 ± 11 vs 38 ± 12 mm, P < 0.001 [9] and 19 vs 26 mm, P = 0.006 [22], respectively) and, in the other study reporting an association between ACS and the presence of bilateral AIs, the results were not adjusted for tumour size even when size was also found to be a risk factor for ACS (OR 2.6; 95% CI 1.3–5.3) [14]. Furthermore, different biochemical criteria were used for the diagnosis of ACS in previous publications [14], making difficult direct comparisons with our findings. Our hypothesis is that the larger tumour size in bilateral than unilateral AIs could be related to a longer duration of the disease in bilateral tumours, that it may also explain the higher risk of ACS as larger the tumour is.
According to our data analysis, tumour size was a good predictor of ACS with an OR of 1.1 for each mm of increased size and was positively correlated with the results of the DST. Tumour size has been recognised as a risk factor for malignancy [23] and hypercortisolism in adrenal lesions [6, 22, 24, 25]. One study also found that the probability for inadequate suppression of serum cortisol on the DST increased in parallel to tumour size (OR 1.93, P < 0.001) [22]. In agreement with this and our previous work [6] and other authors [26, 27], our present results indicate that tumour size is also associated with the magnitude of ACS. Besides, a recent study found a tumor size > 28 mm was associated with the risk of developing ACS during follow-up (HR 12.4; P = 0.003) [25]. This last finding is supported by the Elhassan meta-analysis [28], that described a higher likelihood of tumour growth in patients with ACS (2.4%) than in non-functioning AIs (1.2%).
Similarly, our finding that uni- or bilaterality of AIs did not influence cardiovascular or metabolic comorbidities is also in agreement with previous publications [14, 25]. In fact, this lack of association of bilateral AIs with the prevalence of comorbidities was observed even in studies reporting an association between the prevalence of ACS and bilaterality of AIs [14, 29]. This apparent disagreement between the prevalence of ACS and that of ACS-related comorbidities might be related to a more subtle or even intermittent cortisol secretory pattern, or to a lower sensitivity to cortisol excess, in patients with bilateral AIs. In fact, certain polymorphisms in the gene encoding the glucocorticoid receptor were found in patients with bilateral AIs [30,31,32].
Follow-up studies of bilateral AIs are scarce, but most of them found that the rates of tumour growth, and of hormonal or metabolic impairment over time, were relatively small [28, 33, 34]. Some studies suggested that tumour size at diagnosis and duration of follow-up could be associated with the development of ACS in patients with AIs [24, 33]. Other studies, on the contrary, suggested that the cumulative risk of tumour growth and of development of metabolic or cardiovascular abnormalities over time were independent of the tumour size at diagnosis [34]. In our cohort, neither bilaterality nor tumour size at diagnosis was associated with tumour growth or progression to ACS. However, bilateral AIs presented a higher risk of developing dyslipidaemia, but this association actually depended on the lager tumour size attained by bilateral AIs during follow-up. This finding highlights the importance of tumour size, which seems to have a stronger association with cardiometabolic risk than the results of the DST itself. The poorer performance of the later to this regard might be explained, at least in part, to its low reproducibility, because the DST may be altered by several factors that lead to both false-positive and false-negative results. On the other hand, it is also possible that larger tumour size was not only associated with increased secretion of cortisol, but also of other bioactive metabolites that are not detected with the usual diagnostic tests [35]. Moreover, treatment interventions to control ACS-related comorbidities should be taken into account when interpreting these results.
Anyhow, we have to acknowledge several limitations of our present study, starting with its observational retrospective design that precludes addressing causal relationships. Albeit sample size analysis indicated that our study had > 95% power to detect differences between unilateral and bilateral AIs, we cannot exclude that relatively small differences in some variables might have been missed for this reason. Moreover, we used a ≥ 5.0 ug/dl post-DST serum cortisol as the cut-off value to define ACS and, had we used a different threshold, we might have obtained different results. To mitigate this limitation, however, we also analysed the post-DST serum cortisol concentrations as a continuous variable and using the 3.0 µg/dL threshold for the definition of ACS. Finally, due to the multicentric nature of our study and the non-centralized lecture of radiological reports, it may be some variability in the reported tumour size among centres and different radiologists. In our humble opinion, these limitations did not invalidate the conclusions of the study, which were in agreement with previous publications, and were strengthen by the large number of cases included and its multicentre design. As best of our knowledge, this is the largest series studying the association of bilaterality and ACS and related comorbidities.
Conclusion
Tumour size, not bilaterality, is associated with a higher prevalence of ACS. During follow-up, neither tumour size nor bilaterality were associated with the tumour growth rate. However, the development of dyslipidaemia was higher for bilateral AIs, but this was explained by the larger tumour size of bilateral lesions at the end of follow-up. Hence, tumour size, and not bilaterality, should be taken into account for the evaluation and follow-up strategies of AIs. Larger prospective studies should be conducted to validate these observations.
References
Wagner J, Aron DC (2012) Incidentalomas—a “disease” of modern imaging technology. Best Pract Res Clin Endocrinol Metab 26(1):3–8. https://doi.org/10.1016/j.beem.2011.08.006
Araujo-Castro M, Sampedro Núñez MA, Marazuela M (2019) Autonomous cortisol secretion in adrenal incidentalomas. Endocrine 64(1):1–13. https://doi.org/10.1007/s12020-019-01888-y
Terzolo M, Stigliano A, Chiodini I, Loli P, Furlani L, Arnaldi G et al (2011) AME position statement on adrenal incidentaloma. Eur J Endocrinol 164(6):851–870. https://doi.org/10.1530/EJE-10-1147
Fassnacht M, Arlt W, Bancos I, Dralle H, Newell-Price J, Sahdev A et al (2016) Management of adrenal incidentalomas: European society of endocrinology clinical practice guideline in collaboration with the European network for the study of adrenal tumours. Eur J Endocrinol 175(2):G1–G34. https://doi.org/10.1530/EJE-16-0467
Chiodini I (2011) Diagnosis and treatment of subclinical hypercortisolism. J Clin Endocrinol Metab 96(5):1223–1236
Araujo-Castro M, Robles Lázaro C, Parra Ramírez P, Cuesta Hernández M, Sampedro Núñez MA, Marazuela M (2019) Cardiometabolic profile of non-functioning and autonomous cortisol-secreting adrenal incidentalomas. Is the cardiometabolic risk similar or are there differences? Endocrine 66(3):650–659. https://doi.org/10.1007/s12020-019-02066-w
Rossi R, Tauchmanova L, Luciano A, Di Martino M, Battista C, Del Viscovo L et al (2000) Subclinical cushing’s syndrome in patients with adrenal incidentaloma: clinical and biochemical features. J Clin Endocrinol Metab 85(4):1440–1448
Terzolo M, Pia A, Ali A, Osella G, Reimondo G, Bovio S et al (2002) Adrenal incidentaloma: a new cause of the metabolic syndrome? J Clin Endocrinol Metab 87(3):998–1003
Vassiliadi DA, Ntali G, Vicha E, Tsagarakis S (2011) High prevalence of subclinical hypercortisolism in patients with bilateral adrenal incidentalomas: a challenge to management. Clin Endocrinol (Oxf) 74(4):438–444. https://doi.org/10.1111/j.1365-2265.2010.03963.x
Ognjanović S, Macut D, Petakov M, Elezović Kovačević V, Isailović T, Bozić Antić I, Ilić DS et al (2016) The occurrence of subclinical hypercortisolism and osteoporosis in patients with incidentally discovered unilateral and bilateral adrenal tumours. J Med Biochem 35(4):401–409. https://doi.org/10.1515/jomb-2016-0020.0020
Yener S, Ertilav S, Secil M, Akinci B, Demir T, Kebapcilar L et al (2012) Increased risk of unfavorable metabolic outcome during short-term follow-up in subjects with nonfunctioning adrenal adenomas. Med Princ Pract 21(5):429–434. https://doi.org/10.1159/000336589
Masserini B, Morelli V, Bergamaschi S, Ermetici F, Eller-Vainicher C, Barbieri AM et al (2009) The limited role of midnight salivary cortisol levels in the diagnosis of subclinical hypercortisolism in patients with adrenal incidentaloma. Eur J Endocrinol 160(1):87–92. https://doi.org/10.1530/EJE-08-0485
Androulakis A II, Kaltsas GA, Markou A, Tseniklidi E, Kafritsa P, Pappa T et al (2011) The functional status of incidentally discovered bilateral adrenal lesions. Clin Endocrinol (Oxf) 75(1):44–49. https://doi.org/10.1111/j.1365-2265.2011.04013.x
Vassilatou E, Vryonidou A, Ioannidis D, Paschou SA, Panagou M, Tzavara I (2014) Bilateral adrenal incidentalomas differ from unilateral adrenal incidentalomas in subclinical cortisol hypersecretion but not in potential clinical implications. Eur J Endocrinol 171(1):37–45. https://doi.org/10.1530/EJE-13-0848
Favia G, Lumachi F, Basso S, D’Amico DF, D’Amico DF (2000) Management of incidentally discovered adrenal masses and risk of malignancy. Surgery 128(6):918–924
Barzon L, Sonino N, Fallo F, Palù G, Boscaro M (2003) Prevalence and natural history of adrenal incidentalomas. Eur J Endocrinol 149(4):273–285
Grumbach MM, Biller BMK, Braunstein GD, Campbell KK, Aidan Carney J, Godley PA et al (2003) Management of the clinically inapparent adrenal mass (“incidentaloma”). Ann Intern Med 138(5):424–429
Anagnostis P, Karagiannis A, Tziomalos K, Kakafika AI, Athyros VG, Mikhailidis DP (2009) Adrenal incidentaloma: a diagnostic challenge. Hormones 8:163–184
Association American Diabetes group (2018) Updates to the Standards of Medical Care in Diabetes-2018. Diabetes Care 41(9):2045–2047. https://doi.org/10.2337/dc18-su09
National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) (2002) Third report of the national cholesterol education program (NCEP) Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation 106(25):3143–3421
Morelli V, Masserini B, Salcuni AS et al (2010) Subclinical hypercortisolism: correlation between biochemical diagnostic criteria and clinical aspects. Clin Endocrinol (Oxf) 73(2):161–166. https://doi.org/10.1111/j.1365-2265.2010.03794.x
Olsen H, Nordenström E, Bergenfelz A, Nyman U, Valdemarsson S, Palmqvist E (2012) Subclinical hypercortisolism and CT appearance in adrenal incidentalomas: a multicenter study from Southern Sweden. Endocrine 42(1):164–173. https://doi.org/10.1007/s12020-012-9622-2
Ilias I, Sahdev A, Reznek RH, Grossman AB, Pacak K (2007) The optimal imaging of adrenal tumours: a comparison of different methods. Endocr Relat Cancer 14(3):587–599
Vassilatou E, Vryonidou A, Michalopoulou S, Manolis J, Caratzas J, Phenekos C et al (2009) Hormonal activity of adrenal incidentalomas: results from a long-term follow-up study. Clin Endocrinol (Oxf) 70(5):674–679. https://doi.org/10.1111/j.1365-2265.2008.03492.x
Falcetta P, Orsolini F, Benelli E, Agretti P, Vitti P, Di Cosmo C et al (2020) Clinical features, risk of mass enlargement, and development of endocrine hyperfunction in patients with adrenal incidentalomas: a long-term follow-up study. Endocrine. https://doi.org/10.1007/s12020-020-02476-1
Huayllas MKP, Sirineni GK, Smith LM, Christopher Gallagher J, Singh RJ, Netzel BC et al (2020) Correlation between size and function of unilateral and bilateral adrenocortical nodules: an observational study. Am J Roentgenol 214(4):800–807. https://doi.org/10.2214/AJR.19.21753
Mosconi C, Vicennati V, Papadopoulos D, Di Dalmazi G, Morselli-Labate AM, Golfieri R, Pasquali R (2017) Can imaging predict subclinical cortisol secretion in patients with adrenal adenomas? A CT predictive score. Am J Roentgenol 209(1):122–129. https://doi.org/10.2214/AJR.16.16965
Elhassan YS, Alahdab F, Prete A, Delivanis DA, Khanna A, Prokop L et al (2019) Natural history of adrenal incidentalomas with and without mild autonomous cortisol excess. Ann Intern Med 171(2):107–116. https://doi.org/10.7326/m18-3630
Morelli V, Palmieri S, Salcuni AS, Eller-Vainicher C, Cairoli E, Zhukouskaya V et al (2013) Bilateral and unilateral adrenal incidentalomas: biochemical and clinical characteristics. Eur J Endocrinol 168(2):235–241. https://doi.org/10.1530/EJE-12-0777
Majnik J, Patocs A, Balogh K, Toth M, Gergics P, Szappanos A et al (2013) Brief report: overrepresentation of the N363S variant of the glucocorticoid receptor gene in patients with bilateral adrenal incidentalomas. Eur J Endocrinol 168(2):235–241. https://doi.org/10.1530/EJE-12-0777
Di Blasio AM, Van Rossum EFC, Maestrini S, Berselli ME, Tagliaferri M, Podestà F et al (2003) The relation between two polymorphisms in the glucocorticoid receptor gene and body mass index, blood pressure and cholesterol in obese patients. Clin Endocrinol (Oxf) 59(1):68–74
Morelli V, Donadio F, Eller-Vainicher C, Cirello V, Olgiati L, Savoca C et al (2010) Role of glucocorticoid receptor polymorphism in adrenal incidentalomas. Eur J Clin Invest 40(9):803–811. https://doi.org/10.1111/j.1365-2362.2010.02330.x
Yener S, Ertilav S, Secil M, Demir T, Akinci B, Kebapcilar L et al (2010) Prospective evaluation of tumour size and hormonal status in adrenal incidentalomas. J Endocrinol Invest 33(1):32–36. https://doi.org/10.3275/6377
Giordano R, Marinazzo E, Berardelli R, Picu A, Maccario M, Ghigo E et al (2010) Long-term morphological, hormonal, and clinical follow-up in a single unit on 118 patients with adrenal incidentalomas. Eur J Endocrinol 162(4):779–785. https://doi.org/10.1530/EJE-09-0957
Brossaud J, Ducint D, Corcuff JB (2016) Urinary glucocorticoid metabolites: biomarkers to classify adrenal incidentalomas. Clin Endocrinol (Oxf) 84(2):236–243. https://doi.org/10.1111/cen.12717
Acknowledgements
SENDIMAD: BECA SENDIMAD de Ayuda a la Investigación en Endocrinología, Nutrición y Diabetes 2019. IRYCIS: Convocatoria intramural de ayudas a proyectos de investigación de investigadores noveles, investigadores clínicos asociados y/o grupos emergentes del Hospital Universitario Ramón y Cajal.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Ethical approval
All procedures performed in the participants of the study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study, no informed consent is required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Araujo-Castro, M., Robles Lázaro, C., Parra Ramírez, P. et al. Maximum adenoma diameter, regardless of uni- or bilaterality, is a risk factor for autonomous cortisol secretion in adrenal incidentalomas. J Endocrinol Invest 44, 2349–2357 (2021). https://doi.org/10.1007/s40618-021-01539-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-021-01539-y