Abstract
Purpose
Polychlorinated biphenyls (PCBs) are persistent and bioaccumulative environmental toxicants acting as endocrine disruptors. Many researches evidenced that PCBs affect the male reproductive system in adult rats and it can transfer from mother to offspring through milk. We investigated whether the lactational exposure to PCBs affects the Sertoli cell function in F1 offspring.
Methods
Dams were orally treated with different doses of PCB—Aroclor 1254 (1, 2 and 5 mg/kg bw/day, respectively) from postpartum day 1–20. Male offspring rats were killed on PND 21 and PND 60. Testes were used both for histological study and to isolate Sertoli cell. Serum and testicular interstitial fluid (TIF) levels of testosterone, ABP and estradiol were analyzed by ELISA method. The mRNA and protein expressions of follicle-stimulating hormone (FSHR), androgen-binding protein (ABP), Inhibinβ, androgen receptor (AR) and estrogen receptor (ERβ) were studied using real-time PCR and immunoblotting, respectively.
Results
The testicular architecture was altered in PCB-treated groups of both prepuberal and puberal rats. Testosterone, estradiol and androgen-binding protein levels were altered in both serum and TIF in PCB treated groups. The gene expression level of FSHR, ABP, ERβ and AR was decreased in a dose-dependent manner, whereas Inhibinβ gene expression level was increased in PCB-treated groups.
Conclusion
Lactational exposure to PCB affects both the histoarchitecture of testis, Sertoli cell maker and functional regulators in both prepuberal and puberal F1 male progeny.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polychlorinated biphenyls (PCBs) are a class of legacy chemicals with high environment persistency, and it remains to be a global environmental problem [1]. Exposure to PCBs was associated with increased risk of myocardial infarction in men [2], defects in the learning and motor coordination in the rat [3], and it alters pubertal timing in animal studies [4]. Testicular cancer risk was increased in occupational and environmental exposure to PCB in humans [5]. A series of studies from our laboratory reported that exposure to PCB alters testicular function by affecting Sertoli, Leydig cells and male accessory sex organs such as prostate, epididymis and thus leads to infertility in adult rats [6–13]. We also found that PCB induces oxidative stress-mediated neurodegeneration and neuronal damage in adult rats [14, 15].
Sertoli cell (SCs) is the only somatic cells, present within seminiferous tubules. SCs provide a physical and nutritional support for germ cells [16]. Dysfunction in Sertoli cells may have adverse effects on spermatogenesis [17]. Accordingly, any chemical compounds that impair the viability and function of Sertoli cells may have a negative impact on the normal growth of germ cell. The testicular toxicity of polychlorinated biphenyls (PCBs) in adult male rats has been extensively studied. However, the PCB transfer could occur from mother to offspring and information concerning the effects of neonatal PCB exposure via milk on the reproductive function of F1 male offspring is still obscure. Hence, the present study was conducted to investigate the effect of lactational exposure to PCB (Aroclor 1254) on Sertoli cells in both prepuberal and puberal F1 male offspring.
Materials and methods
Chemicals
PCBs Sandy loam (Aroclor 1254, No. CRM 913-50G, Lot No. DG913), total RNA isolation reagent (TRIR) and primers were purchased from Sigma-Aldrich Private Limited (USA). iScriptcDNA synthesis kit was purchased from Bio-Rad (USA), and RT-PCR Ready Mix was purchased from Takara Bio Inc (Japan). Primary antibodies for FSHR, ABP, Inhibinβ, AR and ERβ were purchased from Santa Cruz biotechnology, USA. The secondary antibodies, horse radish peroxidase (HRP)-conjugated rabbit-antimouse IgG, donkey-antigoat IgG and goat-antirabbit IgG, were obtained from Genei, Bangalore, India. Testosterone and estradiol ELISA kits were purchased from Wuhan Fine Biological Technology Co., Ltd, China, and Androgen-binding protein ELISA kit was purchased from Elabscience Biotechnology Co., Ltd, China. All other chemicals of analytical grade were purchased from Sisco Research Laboratories Pvt. Ltd. (SRL), Mumbai.
Animal care and maintenance
Healthy adult female Wistar albino rats (Rattus norvegicus) (8–9 weeks old, 180–200 g bw) were used for this study and were supplied by our institution animal house. Breeding was done in specific pathogen-free atmosphere with controlled temperature 27–30.5 °C and regulated humidity (70–90 %) conditions. Animals were housed in clean polypropylene cages and maintained in air-conditioned animal house with constant photoperiod of 12-h light/dark cycle. Animals were fed with standard rat pellet diet (Lipton India, Mumbai, India) and water (purified by UV and reverse osmosis) ad libitum throughout the study.
Experimental design
The healthy adult female Wistar albino rats were mated with normal healthy males. The day when the sperm was detected in the vaginal smear was considered day 0, and the dams were separated from male and maintained in individual cages in all cases. Dams were divided into four groups; each group consists of six animals. Group I: control (corn oil alone—vehicle), Group II: 1 mg Aroclor 1254/kg bw/day, Group III: 2 mg Aroclor 1254/kg bw/day and Group IV: 5 mg Aroclor 1254/kg bw/day. Both Aroclor 1254 and corn oil (as a vehicle) were administered daily through oral gavage to the lactating female rats from postnatal day PND 1 to PND 20. The male offspring from all the four groups were Sacrificed on PND 21 and PND 60. Blood was collected, and serum was separated and stored at −80 °C for the estimation of testosterone, androgen-binding protein and estradiol.
Analysis of hormones
Testosterone, androgen-binding protein and estradiol concentrations were measured in serum and testicular interstitial fluid using enzyme-linked immunosorbent assay (ELISA) kits (Wuhan Fine Biological Technology Co., Ltd, China and Elabscience Biotechnology Co., Ltd, China) according to the manufacturer’s instructions. The ELISA plates were read in Bio-Rad plate reader (California, USA). Samples and standards were analyzed in duplicate. The intra and interassay coefficients of variation were <8 and <10 %, respectively, for testosterone, <9.3 and <10.1 %, respectively, for ABP, and <8 and <10 %, respectively, for estradiol. Testosterone level was expressed as ng/ml, whereas level of ABP and estradiol was expressed as pg/ml.
Collection of testicular interstitial fluid (TIF)
Testicular interstitial fluid of F1 puberal rats was collected from individual testes as described by Sharpe and Cooper [18]. Immediately after the removal of the testis, the caudal end of the testicular capsule was incised carefully and the testis was placed upright in a test tube such that the testis was suspended 1–2 cm above the test tube bottom. Fluid was then allowed to percolate from the testis into the test tube bottom over the next 16–20 h at 4 °C. The testis was then removed, the tubes were centrifuged for 5 min at 1000 g to precipitate any contaminating erythrocytes, and the interstitial fluid volume was measured. The fluid was then diluted with 10 volumes of 0.01 M phosphate-buffered saline (pH 7.5) containing 0.2 % BSA and stored at −20 °C. TIF testosterone, ABP, estradiol levels were measured by ELISA method.
Histology
The testis was separated and fixed by 4 % paraformaldehyde for 24 h. Then, testis was cut transversally to the long axis into two slices, which were placed again into the fixative for additional 24 h. Testes samples were dehydrated in an ethanol series and embedded in paraffin wax. Sections (5 µm) of testes were stained with hematoxylin and eosin for histological examination.
Isolation and purification of Sertoli cells
The testes from prepubertal and puberal F1 rats were collected. The SCs were isolated based on the procedure described by Majumdar et al. [19] with some modification. The testicular tissues were chopped and sequentially digested with collagenase IV, collagenase I and pancreatin with intermittent agitation. After washing with DMEM medium by centrifugation at 800 rpm for 5 min, the final cell suspension was filtered through nylon mesh cell strainers (80 μm pore size). Purity of SCs was checked using positive staining for Oil Red O [20]. The isolated Sertoli cells are used to determine the gene expression and protein expression level.
Total RNA isolation
The total RNA was isolated from SCs (1 × 106) using TRI reagent (Sigma) by following the method of Chomczynski and Sacchi [21]. The concentration and purity of total RNA were determined spectrophotometrically at A 260/280 nm, the ratio of which was in the range of 1.8–2.0. Complementary DNA was synthesized from 2 µg of total RNA using a cDNA synthesis kit (iScript, Bio-Rad, USA).
Real-time reverse transcriptase polymerase chain reaction (qRT-PCR)
qRT-PCR was performed in a Cf × 96 real-time detection system, Bio-Rad, using SYBR Green dye. Gene-specific primers were used to determine the relative expression level of follicle-stimulating hormone receptor (FSHR), androgen-binding protein (ABP), inhibinβ, androgen receptor (AR) and estrogen receptor β (ERβ). Amplification reactions were set up of 25 µl by Takara SYBR Green kit method, and the reaction consisted of initial denaturation for 3 min at 95 °C, followed by 40 cycles of 95 °C for 10 s, primer annealing at 58 °C for 30 s (temperature varies gene to gene) and extension at 72 °C for 1 min. Melt curve analysis was performed for specificity. Forward and reverse primer sequences and amplicon size are listed in Table S1. The reactions were repeated in triplicate, and a non-template control was also included. Beta-actin was used as a housekeeping gene for data analysis. The Ct values were obtained from the amplification, and relative gene expression was calculated by the 2−ΔΔCt method [22].
Immunoblotting
The protein expressions were detected by western blot. The SCs were homogenized with radioimmuneprecipitation assay buffer (RIPA) and protease inhibitor cocktails. Proteins were quantified, and then equal amounts of proteins were subjected to 10–12 % SDS–polyacrylamide gels. Following electrophoresis, separated proteins on SDS-PAGE gels were transferred to PVDF membrane (Millipore USA). To block the nonspecific binding, the membranes were incubated with 5 % skimmed milk for 3 h. Membranes were immunoblotted with primary antibodies FSHR, ABP, Inhibinβ, AR and ERβ (1:500–1:1000). The membranes were washed with TBS and incubated with horseradish peroxidase-labeled antimouse rabbit IgG or antigoat IgG and antirabbit mouse IgG antibody at a dilution of 1:10,000. The bands were developed by using ECL kit (Thermo Scientific, USA) in Chemidoc image scanner from Bio-Rad. The band intensity was quantified by Quantity One software (Bio-Rad, California, USA). The membranes were striped and reprobed for β-actin (1:5000) as an internal control.
Statistical analysis
All data are presented as mean ± standard error of the mean (SEM) and were analyzed by one-way analysis of variance (ANOVA) followed by Student–Newman–Keul’s test. Statistical analysis was performed by using the Graph Pad Prism software (GraphPad Software, San Diego, CA, USA). Differences were considered statistically significant when the P level was less than 0.05.
Results
Effect of lactational exposure to PCB on body weight, relative testis and accessory sex organs weight of prepuberal and puberal offspring rats
There is no significant difference observed between control and PCB-treated dam’s body weight (Fig S1). The body weight of both prepuberal and puberal F1 male offspring was significantly decreased in all PCB-treated groups when compared to control (Fig. 1a). 50 % mortality rate was observed in 5 mg PCB treated group. Relative testis weight of both the prepuberal and puberal F1 progeny exposed to PCBs was drastically decreased (Fig. 1b). Specifically 50 % of testis weight reduction was observed in a high dose of PCBs (5 mg PCB)-treated group. The decreased testicular weight in PCBs-exposed rats may be due to reduced tubular size, alteration of FSH and testicular androgenesis. The male accessory sex organs such as seminal vesicle, ventral prostate and epididymis weight also decreased in a dose-dependent manner (Fig. 2a, b).
Effect of lactational exposure to PCB on body weight (a) and relative testis weight (b) of F1 male prepuberal (PND 21) and puberal (PND 60) Wistar albino rats. Each bar represents the mean ± SEM of six animals. a represents control versus 1 mg, 2 mg, 5 mg PCB treated groups and b represents 1 mg PCB versus 2 mg & 5 mg PCB treated groups, c represents 2 mg PCB versus 5 mg PCB group at p < 0.05 level
Effect of lactational exposure to PCB on relative accessory sex organs weight of F1 male prepuberal (a) and puberal (b) Wistar albino rats. Each bar represents the mean ± SEM of six animals. a represents control versus 1 mg, 2 mg, 5 mg PCB treated groups and b represents 1 mg PCB versus 2 mg & 5 mg PCB treated groups, c represents 2 mg PCB versus 5 mg PCB group at p < 0.05 level
Effect of lactational exposure to PCB on hormone levels in serum and testicular interstitial fluid (TIF) of F1 offsprings
The testosterone levels in the serum and TIF showed a dose-dependent decrease in offspring rats exposed to PCB during lactational period when compared to control group (Fig. 3a, b). The testicular volume per testis is also decreased in 2- and 5-mg-PCB-treated groups (Fig S2). Androgen-binding protein levels were significantly decreased in PCB-treated groups compared with control in both serum and TIF (Fig. 4a, b). The estradiol level also significantly decreased in both serum and TIF of the PCB-treated groups when compared to control group (Fig. 5a, b).
Effect of lactational exposure to PCB on testosterone level in serum (3a) & TIF(3b) of F1 male prepuberal and puberal Wistar albino rats. Each bar represents the mean ± SEM of six animals. a represents control versus 1 mg, 2 mg, 5 mg PCB treated groups and b represents 1 mg PCB versus 2 mg & 5 mg PCB treated groups, c represents 2 mg PCB versus 5 mg PCB group at p < 0.05 level
Effect of lactational exposure to PCB on ABP level in serum (4a) and TIF (4b) of F1 male prepuberal and puberal Wistar albino rats. Each bar represents the mean ± SEM of six animals. a represents control versus 1 mg, 2 mg, 5 mg PCB treated groups and b represents 1 mg PCB versus 2 mg & 5 mg PCB treated groups, c represents 2 mg PCB versus 5 mg PCB group at p < 0.05 level
Effect of lactational exposure to PCB on estradiol level in serum (5a) and TIF (5b) of F1 male prepuberal and puberal Wistar albino rats. Each bar represents the mean ± SEM of six animals. a represents control versus 1 mg, 2 mg, 5 mg PCB treated groups and b represents 1 mg PCB versus 2 mg & 5 mg PCB treated groups, c represents 2 mg PCB versus 5 mg PCB group at p < 0.05 level
Effect of lactational exposure to PCB on histoarchitecture of the testis of prepuberal and puberal offspring rats
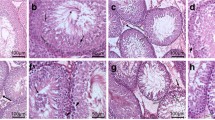
Disorganized tubules, poor tubular content and tubules were hyalinized. Foci containing malformed tubules were observed. Disorganized epithelial lining, wide lumen and interstitial spaces, and reduced interstitial cell populations were also observed in PCB-treated groups of prepuberal rats (Fig. 6a–d). In puberal rats, sections of the testes from control rats (Fig. 6e) showed the normal structure of the testis. Seminiferous tubules had rounded or oval contour with regular basement membrane and were lined with stratified germinal epithelium showing two types of cells, germ and Sertoli cell. Sperms were seen in the lumen of the tubules. Examination of sections obtained from testes of 1 mg and 2 mg PCB treated rats revealed the seminiferous tubules with multiple distortions and loss of germ cells (Fig. 6f, g). Seminiferous tubular lumens were wide with no sperm and some immature tubules also observed in 5 mg PCB treated group of puberal rats (Fig. 6h).
Effect of lactational exposure to PCB on testicular architecture of both F1 prepuberal (PND 21) and puberal (PND 60) Wistar albino rats. Photomicrograph of testicular sections of rat offspring of dams exposed to 1 mg PCB (b, f), 2 mg PCB (c, g), 5 mg PCB (d, h). a, e Seminiferous epithelium presenting normal morphology. b Seminiferous tubules are not intact, c some of the foci containing malformed tubules (asterisk) were seen, d small tubules, poor tubular content were observed, and most of the seminiferous tubules were malformed, f sloughing of germ cell layer (arrow) in seminiferous tubules, g sloughing of germ cell layers (arrow) in seminiferous tubules and sperm depleted (asterisk) in the lumen, h immature seminiferous tubules (×) (hematoxylin and eosin staining, ×40 magnification)
Effect of lactational exposure to PCB on FSHR, AR, ERβ in Sertoli cells of prepuberal and puberal F1 offspring rats
The mRNA and protein expression of FSHR was decreased in a dose-dependent manner on both prepuberal and puberal offspring rats (Fig. 7a, b). In prepuberal rats, AR mRNA and protein expression was decreased gradually in PCB-treated groups, but in the case of puberal rats, AR gene expression was significantly decreased in 2 mg and 5 mg PCB treated groups (Fig. 8a, b). The ERβ mRNA expression level was drastically decreased in 5 mg PCB treated groups of both prepuberal and puberal offspring rats (Fig. 9a). The protein expression of ERβ was decreased in all the PCB-treated groups of prepuberal, but the protein expression of ERβ was increased in 2 mg PCB than in the 1 mg PCB treated groups of puberal rats (Fig. 9b).
Effect of lactational exposure to PCB on FSHR mRNA (a) and protein (b) expression in Sertoli cells of F1 prepuberal (PND 21) and puberal (PND 60) Wistar albino rats. Each bar represents the mean ± SEM of three observations from pooled samples of six animals. a represents control versus 1 mg, 2 mg, 5 mg PCB treated and b represents 1 mg PCB treated versus 2 mg, 5 mg PCB-treated, c represents 2 mg versus 5 mg PCB-treated at p < 0.05 level
Effect of lactational exposure to PCB on AR mRNA (a) and protein (b) expression in Sertoli cells of F1 prepuberal (PND 21) and puberal (PND 60) Wistar albino rats. Each bar represents the mean ± SEM of three observations from pooled samples of six animals. a represents control versus 1 mg, 2 mg, 5 mg PCB-treated; b represents 1 mg PCB-treated versus 2 mg, 5 mg PCB-treated and c represents 2 mg PCB-treated versus 5 mg PCB-treated at p < 0.05 level
Effect of lactational exposure to PCB on ERβ mRNA (a) and protein (b) expression in Sertoli cells of F1 prepuberal (PND 21) and puberal (PND 60) Wistar albino rats. Each bar represents the mean ± SEM of three observations from pooled samples of six animals. a represents Control versus 1 mg, 2 mg, 5 mg PCB-treated and b represents 1 mg PCB-treated versus 2 mg, 5 mg PCB-treated, c represents 2 mg versus 5 mg PCB-treated at p < 0.05 level
Effect of lactational exposure to PCB on ABP and inhibinβ in Sertoli cells of prepuberal and puberal F1 offspring rats
The gene expression of ABP was significantly decreased in a dose-dependent manner on Sertoli cell of both prepuberal and puberal (Fig. 10a, b) F1 offspring rats. The mRNA and protein expression of inhibinβ was significantly increased in all the PCB-treated groups of prepuberal and puberal F1 offspring (Fig. 11a, b).
Effect of lactational exposure to PCB on ABP mRNA (a) and protein (b) expression in Sertoli cells of F1 prepuberal (PND 21) and puberal (PND 60) Wistar albino rats. Each bar represents the mean ± SEM of three observations from pooled samples of six animals. a represents Control versus 1 mg, 2 mg, 5 mg PCB-treated; b represents 1 mg PCB-treated versus 2 mg, 5 mg PCB-treated and c represents 2 mg PCB-treated versus 5 mg PCB-treated at p < 0.05 level
Effect of lactational exposure to PCB on inhibinβ mRNA (a) and protein (b) expression in Sertoli cells of F1 prepuberal (PND 21) and puberal (PND 60) Wistar albino rats. Each bar represents the mean ± SEM of three observations from pooled samples of six animals. a represents Control versus 1 mg, 2 mg, 5 mg PCB-treated, b represents 1 mg PCB-treated versus 2 mg, 5 mg PCB-treated and c represents 2 mg versus 5 mg PCB-treated at p < 0.05 level
Discussion
The “fetal origins of adult disease” hypothesis proposes that the maternal nutritional status during pregnancy and lactation plays a critical role in the postnatal growth and development of the offspring, often leading to permanent changes with lifelong health consequences [23]. PCB is able to pass into breast milk, and therefore, exposure during lactational period is of particular concern. So, the present study deals with the lactation exposure effect of PCB on SCs of F1 male offspring. The body weight and relative testis weight were decreased in a dose-dependent manner in PCB-exposed F1 progeny. Hany et al. [24] also observed that the exposure to PCB during lactation period significantly reduced the growth in offspring and 5–10 % reduction in body weight of adult animals.
The histopathological study has veraciously depicted the adverse, dose-dependent changes in testicular architecture (Fig. 2). Germ cell and interstitial cell population was decreased in PCB-treated groups, suggesting that PCB affects the spermatogenesis in F1 progeny. Seminiferous tubules size was also reduced in PCB-exposed rats, and this may be the reason for the testicular weight reduction. Our recent study also stated that lactational exposure to DEHP causes dose-dependent changes in testicular architecture and perturbation of the tight junctional proteins in F1 rats [25]. PCB affects the testicular histoarchitecture, and this may be due to the alteration of testosterone, ABP and estradiol levels in both serum and TIF, which is very much important for the germ cell development. The testicular interstitial fluid volume was also decreased in the PCB-treated groups; this is evident that the PCB could affect the germ cell development.
SCs normally stop proliferation at early puberty in rodents and numbers remain static thereafter, but in mice lacking FSHR there is a postpubertal decline in SCs numbers [26]. In this study, FSHR expression level was decreased in PCB-treated group, suggesting that this may reduce the SC number in PCB-treated group. Inhibinβ controls FSH secretion via a negative feedback mechanism [27]. The overexpression of inhibinβ in the present study may inhibit the FSH secretion, and therefore it inhibits the SCs proliferation and it may lead to subfertility. Krishnamoorthy et al. [8] found that PCB disrupts Sertoli cellular metabolic functions such as decreased ABP, lactate secretions and activity of antioxidant enzymes in adult rats. In the present study also, the gene expression level of ABP was significantly decreased in the PCB-treated group of both prepuberal and puberal F1 offspring, suggesting that this may be the reason for the reduction of testosterone level in both serum and TIF in PCB-treated groups.
The decreased expressions of androgen receptor in PCB-treated group of both the prepuberal and puberal F1 offspring suggest that the lactational exposure to PCB may adversely affect the progression of spermatocytes, survival of round spermatids and release of elongated spermatids into the lumen of seminiferous tubules. The ERβ gene expression level was decreased in Sertoli cell of all the PCB-treated groups in both prepuberal and puberal F1 rats. The serum and TIF estradiol level also decreased in PCB-treated groups of F1 offspring. Delbes et al. [28] also found that estrogen receptors are the pivotal player which is required for spermatogenesis. The decreased expression of estrogen receptor may affect the testicular development and spermatogenesis.
Conclusion
To conclude, lactational exposure to PCB (Aroclor 1254) affects the SC function in both prepuberal and puberal F1 rats. It may lead to infertility in F1 male offspring by deteriorating SCs function. Further studies are needed to prove the relentlessness of PCB toxicity in F1 progeny.
References
Cai JL, Sun LB, Guo ZZ, Jiang XM, Zheng GC, Qiu HL, Sha AG, Wang CG, Ren JZ, Zuo ZH (2015) Decrease in prosaposin in spermatozoon is associated with polychlorinated biphenyl exposure. Int J Clin Exp Pathol 8(3):2436–2448
Bergkvist C, Berglund M, Glynn A, Wolk A, Akesson A (2015) Dietary exposure to polychlorinated biphenyls and risk of myocardial infarction—a population-based prospective cohort study. Int J Cardiol 183:242–248. doi:10.1016/j.ijcard.2015.01.055
Bavithra S, Priya ES, Selvakumar K, Krishnamoorthy G, Arunakaran J (2015) Effect of melatonin on glutamate: BDNF signaling in the cerebral cortex of polychlorinated biphenyls (PCBs)—exposed adult male rats. Neurochem Res 40(9):858–1869. doi:10.1007/s11064-015-1677-z
Parent AS, Franssen D, Fudvoye J, Gérard A, Bourguignon JP (2015) Developmental variations in environmental influences including endocrine disruptors on pubertal timing and neuroendocrine control: revision of human observations and mechanistic insight from rodents. Front Neuroendocrinol 38:12–36. doi:10.1016/j.yfrne.2014.12.004
Paoli D, Giannandrea F, Gallo M, Turci R, Cattaruzza MS, Lombardo F, Lenzi A, Gandini L (2015) Exposure to polychlorinated biphenyls and hexachlorobenzene, semen quality and testicular cancer risk. J Endocrinol Invest 38(7):745–752. doi:10.1007/s40618-015-0251-5
Anbalagan J, Kanagaraj P, Srinivasan N, Aruldhas M, Arunakaran J (2003) Effect of polychlorinated biphenyl, Aroclor 1254 on rat epididymis. Indian J Med Res 118:236–242
Venkataraman P, Sridhar M, Dhanamma S, Vijayababu M, Arunkumar A, Srinivasan N, Arunakaran J (2004) Effects of vitamin supplementation on PCB (Aroclor 1254)-induced changes in ventral prostatic androgen and estrogen receptors. Endocr Res 30(3):469–480
Krishnamoorthy G, Murugesan P, Muthuvel R, Gunadharini DN, Vijayababu MR, Arunkumar A, Venkataraman P, Aruldhas MM, Arunakaran J (2005) Effect of Aroclor 1254 on Sertoli cellular antioxidant system, androgen binding protein and lactate in adult rat in vitro. Toxicology 212(2–3):195–205
Krishnamoorthy G, Venkataraman P, Arunkumar A, Vignesh R, Aruldhas M, Arunakaran J (2007) Ameliorative effect of vitamins (α-tocopherol and ascorbic acid) on PCB (Aroclor 1254) induced oxidative stress in rat epididymal sperm. Reprod Toxicol 23(2):239–245
Murugesan P, Muthusamy T, Balasubramanian K, Arunakaran J (2007) Effects of vitamins C and E on steroidogenic enzymes mRNA expression in polychlorinated biphenyl (Aroclor 1254) exposed adult rat Leydig cells. Toxicology 232(3):170–182
Murugesan P, Muthusamy T, Balasubramanian K, Arunakaran J (2008) Polychlorinated biphenyl (Aroclor 1254) inhibits testosterone biosynthesis and antioxidant enzymes in cultured rat Leydig cells. Reprod Toxicol 25(4):447–454. doi:10.1016/j.reprotox.2008.04.003
Elumalai P, Krishnamoorthy G, Selvakumar K, Arunkumar R, Venkataraman P, Arunakaran J (2009) Studies on the protective role of lycopene against polychlorinated biphenyls (Aroclor 1254)-induced changes in StAR protein and cytochrome P450 scc enzyme expression on Leydig cells of adult rats. Reprod Toxicol 27(1):41–45. doi:10.1016/j.reprotox.2008.11.053
Selvakumar K, Banu LS, Krishnamoorthy G, Venkataraman P, Elumalai P, Arunakaran J (2011) Differential expression of androgen and estrogen receptors in PCB (Aroclor 1254)-exposed rat ventral prostate: impact of alpha-tocopherol. Exp Toxicol Pathol 63(1–2):105–112. doi:10.1016/j.etp.2009.10.003
Venkataraman P, Selvakumar K, Krishnamoorthy G, Muthusami S, Rameshkumar R, Prakash S, Arunakaran J (2010) Effect of melatonin on PCB (Aroclor 1254) induced neuronal damage and changes in Cu/Zn superoxide dismutase and glutathione peroxidase-4 mRNA expression in cerebral cortex, cerebellum and hippocampus of adult rats. Neurosci Res 66(2):189–197. doi:10.1016/j.neures.2009.10.015
Selvakumar K, Bavithra S, Ganesh L, Krishnamoorthy G, Venkataraman P, Arunakaran J (2013) Polychlorinated biphenyls induced oxidative stress mediated neurodegeneration in hippocampus and behavioral changes of adult rats: anxiolytic-like effects of quercetin. Toxicol Lett 222(1):45–54. doi:10.1016/j.toxlet.2013.06.237
Mruk DD, Cheng CY (2004) Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev 25(5):747–806
Choi MS, Park HJ, Oh JH, Lee EH, Park SM, Yoon S (2014) Nonylphenol-induced apoptotic cell death in mouse TM4 Sertoli cells via the generation of reactive oxygen species and activation of the ERK signaling pathway. J Appl Toxicol 34(6):628–636. doi:10.1002/jat.2886
Sharpe RM, Cooper I (1983) Testicular interstitial fluid as a monitor for changes in the intratesticular environment in the rat. J Reprod Fertil 69(1):125–135
Majumdar SS, Tsuruta J, Griswold MD, Bartke A (1995) Isolation and culture of Sertoli cells from the testes of adult Siberian hamsters: analysis of proteins synthesized and secreted by Sertoli cells cultured from hamsters raised in a long or a short photoperiod. Biol Reprod 52(3):658–666
Raychoudhury S, Thompson EW, Blackshaw A, Irving M (1993) Sertoli cells as paracrine modulators of DNA synthesis in rat peritubular myoid cells in culture. J Reprod Fertil 99(2):513–518
Chomczynski P, Sacchi N (2006) The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: twenty-something years on. Nat Protoc 1(2):581–585
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25(4):402–408
Edwards LJ, Coulter CL, Symonds ME, McMillen IC (2001) Prenatal undernutrition, glucocorticoids and the programming of adult hypertension. Clin Exp Pharmacol Phys 28(11):938–941
Hany J, Lilienthal H, Sarasin A, Roth-Härer A, Fastabend A, Dunemann L, Lichtensteiger W, Winneke G (1999) Developmental exposure of rats to a reconstituted PCB mixture or Aroclor 1254: effects on organ weights, aromatase activity, sex hormone levels, and sweet preference behavior. Toxicol Appl Pharmacol 158(3):231–243
Sekaran S, Balaganapathy P, Parsanathan R, Elangovan S, Gunashekar J, Bhat FA, Jagadeesan A (2015) Lactational exposure of phthalate causes long-term disruption in testicular architecture by altering tight junctional and apoptotic protein expression in Sertoli cells of first filial generation pubertal Wistar rats. Hum Exp Toxicol 34:575–590. doi:10.1177/0960327114555926
O’shaughnessy PJ, Monteiro A, Abel M (2012) Testicular development in mice lacking receptors for follicle stimulating hormone and androgen. PLoS ONE 7:e35136. doi:10.1371/journal.pone.0035136
Meachem SJ, Nieschlag E, Simoni M (2001) Inhibin B in male reproduction pathophysiology and clinical relevance. Eur J Endocrinol 145(5):561–571
Delbes G, Levacher C, Habert R (2006) Estrogen effects on fetal and neonatal testicular development. Reproduction 132(4):527–538
Acknowledgments
Support from the Department of Science and Technology (Grant award No: SR/SO/AS-43/2012 dated 27/5/2013), New Delhi, is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the “Institutional Animal Ethical Committee guidelines” (IAEC) and were approved with Ref No: 01/04/13.
Informed consent
For this type of study, informed consent is not required.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sugantha Priya, E., Sathish Kumar, T., Balaji, S. et al. Lactational exposure effect of polychlorinated biphenyl on rat Sertoli cell markers and functional regulators in prepuberal and puberal F1 offspring. J Endocrinol Invest 40, 91–100 (2017). https://doi.org/10.1007/s40618-016-0539-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-016-0539-0