Abstract
Background
Tuberculosis is a serious health threat, particularly for people living with human immunodeficiency virus (HIV), and HIV-positive people are more likely than others to contract the disease. Globally, tuberculosis (TB) is one of the leading causes of death among people living with HIV. The purpose of the current study was to identify factors associated with survival rates of TB/HIV co-infected patients using survival models.
Methods
A retrospective study was conducted on TB/HIV co-infected adult patients registered and under follow-up at Tepi General Hospital (TGH) and Mizan-Tepi University Teaching Hospital (MTUTH), southwest Ethiopia. All TB/HIV co-infection patients who were registered and under follow-up from 1st January 2015 through 1st January 2020 were considered. The global Schoenfeld test was used to test the proportional hazard (PH) assumption. Various accelerated failure time (AFT) models were compared to determine the best model for the time to death of TB/HIV co-infected patients’ data set. Among the most commonly used accelerated failure time models (AFT models), the study used exponential, Weibull, log-logistic, and log-lognormal AFT models. AIC and BIC were used to compare the performance of fitted models. The data were analyzed with the statistical software R.
Results
Of 363 TB/HIV co-infected patients followed for 60 months, 79 (21.8%) died, while the remaining 284 (78.2%) were censored. The overall median survival time was 15.6 months. The proportional hazard assumption was checked and it was violated. In comparison to other models, the lognormal AFT model performed better. The results of the multivariable lognormal AFT model showed that age, residence, substance use, educational status, clinical stages of the disease, cluster of differentiation 4 (CD4 count/mm3), functional status, cotrimoxazole prophylactic therapy use (CPT use), and INH were all found to be significant factors, while gender, illness other than TB, and disclosure of status were insignificant variables at 5% level of significance.
Conclusion
Current study results revealed that older age, substance use, advanced WHO clinical stages of the disease (stage IV), bedridden functional status, and CD4 less than 200 count/mm3 were significantly associated with shorter survival time to death of HIV/TB co-infected patients while having advanced educational status, being from urban residence, CPT use, and INH significantly increase the survival time to death of TB/HIV co-infected patients. Patients with TB/HIV co-infection should be given special attention based on these important factors to improve their health and prolong their lives.
Summary
-
HIV-positive patients are more likely than others to contract the TB disease.
-
The risk of death among TB/HIV co-infected patients was found to be high.
-
Out of all patients, 79 (21.8%) died.
-
Accelerated failure time models are good alternatives for scenario Cox proportional hazard assumptions not met.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
HIV/AIDS is one of the world’s most frequent and deadly chronic diseases, responsible for a major part of morbidity and mortality [1]. Most HIV patients are afflicted with tuberculosis (TB), the most frequent infection among HIV patients [2]. It can develop at any stage of HIV infection, and those living with HIV are at a higher risk of contracting the disease than those who do not [3, 4]. Co-infection of HIV with TB hastens the weakening of the host’s immunological capacities when active TB is growing, increasing sensitivity to initial contamination, recontamination, and/or reactivation for sufferers with latent TB [5].
Both TB and HIV are public health issues that have a synergistic influence on one another. People who have HIV and latent TB infection (LTBI) are at a substantially higher risk of developing active TB disease than people who only have latent TB infection [6]. The HIV-negative person has a 5–10% lifetime risk of acquiring clinical TB, in comparison to the HIV-positive person who has a 50% lifetime risk. Even if they are taking their treatment on a regular way, TB kills 90% of co-infected people if they are not treated within 6 months [7, 8].
Even though the burden of these two diseases is reducing, they nevertheless contribute significantly to mortality [9]. Globally, the incidence of TB has decreased over the previous 2 decades, from 172 cases per 100,000 in 2012 to 132 cases per 100,000 in 2018, owing to improved coverage and efficient TB management, HIV awareness, and widespread use of antiretroviral therapy (ART) [10, 11]. Despite this, more than 70 million people have been infected with HIV, with around 35 million dying as a result of the virus. In Ethiopia, 40–70% of HIV patients also have TB [12].
Ethiopia is one of the world’s 20 high-burden countries for HIV/TB co-infection, according to a WHO assessment [7]. As many as two-thirds of TB patients in Sub-Saharan Africa are HIV/AIDS co-infected, and TB is the leading cause of mortality among HIV/AIDS patients globally [13, 14]. Furthermore, the number of people in Ethiopia on antiretroviral therapy (ART) for HIV/TB co-infection is continuously increasing year after year [15]. As a result, co-infections of TB and HIV pose unique diagnostic and treatment issues, putting a huge strain on healthcare systems in extensively affected nations [16]. Co-infection with HIV and TB causes a slew of issues in Ethiopia, including diagnostic and treatment hurdles in healthcare settings, as well as the loss of a person’s life [14, 17, 18].
There have been numerous studies on the factors that influence TB/HIV co-infection mortality. Age, gender, marital status, educational level, religion, occupation, residence, weight, AIDS stage, TB clinical presentation, and calendar year are among them [19, 20]. A high mortality rate has been linked to a low CD4 count at the commencement of treatment, advanced disease at the time of treatment, and not receiving treatment [21]. Furthermore, when a patient’s CD4 cell count drops, their chance of death rises [22, 23].
Understanding the predictors of mortality for TB-HIV co-infected patients in the local context is critical for Ethiopia to improve the co-management of TB-HIV co-infected patients. Although recognizing and comprehending the factors influencing survival time could be critical for any developing country attempting to achieve effective control of tuberculosis and HIV/AIDS, such information is scarce in Ethiopia [24]. Prior studies [25,26,27,28] used a semiparametric Cox regression model to identify factors associated with the survival and mortality of TB/HIV co-infected patients. However, the semiparametric Cox regression model requires that the assumption of PH hold. As a result, the current study incorporated the AFT model to identify factors associated with the survival time to death of TB/HIV co-infected patients using multi-center data. Thus, the goal of this study was to analyze determinants of the time to death of HIV/AIDS and TB co-infected adult patients in Tepi General Hospital (TGH) and Mizan-Tepi University Teaching Hospital (MTUTH), Southwest Ethiopian Peoples Region.
Material and Methods
Data Source, Study Design, and Procedure of Selecting Study Subjects
A retrospective study was conducted on TB/HIV co-infected adult patients registered and under follow-up at Tepi General Hospital (TGH) and Mizan-Tepi University Teaching Hospital (MTUTH), southwest Ethiopia. All TB/HIV co-infection patients who were registered and under follow-up from 1st January 2015 through 1st January 2020 were considered. Each patient was followed retrospectively from the start of TB treatment until death, loss to follow-up, treatment completion, or the end of the study. Those who stopped ART for any reason during TB treatment were excluded since it is the known risk factor that increases death by many folds. Additionally, patients who did not have full recorded information regarding the disease were excluded (Fig. 1). The survival time of these patients after treatment was determined, and patients transfer out, death other than TB, and still alive at the end of study time were considered censored. The data were analyzed with the statistical software R, version 4.1.1.
Data Extraction and Quality Control
A data extraction tool adapted from national ART and anti-TB treatment standard registries was used to extract data. Standard registries of ART and anti-TB treatments, electronic format, patient medical records (cards), and intake forms were used as data sources. The data was extracted by four trained nurses, two from each of the hospital’s ART and TB clinics. All extraction processes are overseen by a trained supervisor. During data extraction, both the supervisor and the principal investigator checked the checklists for completeness and consistency daily. Socio-demographic, clinical, behavioral, and health service–related baseline data were extracted from the sources.
Study Variables
The response variable for this study was survival time to death of TB/HIV co-infected patients from the time of diagnosis to study ends.
The explanatory variables that were expected to be the determinant factors for the time to death of TB/HIV co-infected patients are as follows:
Demographic Variables
Demographic Variables: gender, age (in years), residence, marital status, religion, educational status, employment, and substance use.
Clinical Variables
WHO clinical stage, a cluster of differentiation 4 (CD4 count/ mm3), body mass index (BMI (weight/height2)), illness other than TB (opportunistic infection), disclosure status, functional status, INH (isoniazid or isonicotinic acid hydrazide), and CPT (cotrimoxazole prophylactic therapy) use were considered.
Operational Definition
Substance Use
Substance use is simply defined as a pattern of consuming a substance in larger quantities for a long period of time in order to change one’s mood, for instance, being alcohol user, Khat, and cigarette smoking. People who use such substances daily or often might be under risk of health issue. In the current study, substance users are defined as people who have used any of these substances in the past.
Survival Analysis
Survival analysis is an important statistical technique used to describe and model time-to-event data. It is a collection of statistical procedures for data analysis for which the outcome variable of interest is time until an event occurs [29].
Kaplan–Meier Estimator
The Kaplan–Meier estimator of the survivorship function [30] is also called the product-limit estimator. Suppose we have a sample of independent observations, their survival times denoted by \({t}_{1}, {t}_{2},\dots {t}_{n}\), and indicators of censoring denoted by \({\delta }_{1},\) \({\delta }_{2}\), …,\({\delta }_{n}\).
Thus, the survival data are denoted by \(({t}_{i} , {\delta }_{i});\) \(i = 1, 2, \dots n.\)
Kaplan and Meier (1958) develop an estimator for the survival function as follows:
where \({d}_{i}\) is the number of patients who experienced an event at \({t}_{i}\) and \({n}_{i}\) is the number of patients at risk before \({t}_{i}\).
Log-Rank Test
This was used to compare the death rate between two distinct groups, conditional on the number at risk in the groups. The hypothesis to be tested:
-
H0: No difference between survival curves
-
H1: There is a difference between survival curves
The log-rank test statistic for comparing two groups is given by:
where \({W}_{i}\)=1. The null hypothesis of equality of survival functions will be rejected for large values of Q [31].
Checking Proportional Hazard (PH) Assumption
Before proceeding with further survival analysis, it is mandatory to check PH assumptions. We have checked the assumption of PH using the GLOBAL Schoenfeld test. The null hypothesis of the PH assumption holds rejected for small p-values [32].
Accelerated Failure Time Model
The accelerated failure time (AFT) model describes the relationship between survival probabilities and a set of covariates [33]. For a group with covariates \(({x}_{1},{x}_{2}, {\dots ,x}_{p})\), the AFT model is written mathematically as.
where \(So(t)\) denotes the baseline survival function and \(\phi\) is an acceleration factor, i.e., a ratio of survival times corresponding to any fixed value of \(S(t).\)
The acceleration factor is given according to the formula
It is noted that under an AFT model, the covariate effects are assumed to be constant and multiplicative on the time scale; that is, the covariate impacts on survival by a constant factor (acceleration factor, also called time ratio). The corresponding log-linear form of the AFT model to time is given by:
where \(\mu\) is an intercept, \(\beta\) denotes the vector of the regression coefficient, \(\sigma\) is a scale parameter, and \({\varepsilon }_{i}\) denotes a random error, assumed to have a particular distribution [34].
We used the exponential, Weibull, log-logistic, and log-normal AFT models to model the data. In each case, we first fitted the model in the univariable setting for each covariate, and covariates significant at 25% significance level were selected for multivariable analysis.
Model Selection
AIC and BIC statistics were used to compare different models and/or models with different numbers of parameters. The AIC value of the model is computed as:
where \(p\) denotes the number of covariates in the model without including the constant term and \(s\) is the number of parameters minus 1.
The BIC value for the model can be obtained as:
where L is the maximized value of the likelihood function of the model, n is the number of observations, and k is the number of parameters estimated by the model. According to the criterion, a model with a small AIC or BIC value is considered as it best fits the data [35].
Model Diagnostics Checking
The Cox-Snell residual plot was done to determine whether the AFT model is well fitted. Residual plots can also be used in the graphical assessment of the adequacy of a fitted model. Thus, the plot of the estimated hazard rate of the Cox-Snell residuals should give a straight line with unit slope and zero intercept if the fitted model is good [36].
Results
Demographic Characteristics of TB/HIV Co-infected Patients
Females made up more than half of the patients in this study (55.6%), of which 35.6% died during the follow-up period. Regarding age in years of patients, a large percentage of patients 167 (46.0%) were aged between 25 and 34 years, while only 41 (11.3%) of patients were aged ≥ 45 years of which 61.0% died. Furthermore, in terms of religion, 27%, 25.3%, and 47.7% were Muslim, protestant, and orthodox followers, respectively. Similarly, among 161 (44.4%) married participants, 33 (20.5%) died. Regarding the educational status, more than one-third, 151 (41.6%), had attended primary school and out of them more than a quarter (31.8%) died. Regarding employment, more than half, 201 (55.4%), were full-time workers of which 46 (22.9%) died. About 176 (48.5%) of patients were substance users of which nearly half, 47.2%, died (Table 1).
Summaries of Clinical Features for TB/HIV Co-infected Patients
Out of 363 study subjects included in this study, those with a baseline CD4 count of fewer than 200 cells/mm3 were 187 (51.5%) of which 81 (43.3%) died during the follow-up period. A large percentage of participants, 173 (47.7%), had WHO clinical stage III of which 35.5% died during the follow-up period. Regarding the functional status of participants, 185 (51.0%), 127 (35.0%), and 51 (14.0%) were working, ambulatory, and bedridden, respectively. A significant proportion, of the study participants, 201 (55.4%), had one or more illnesses (opportunistic infections) other than TB, of which 87 (43.3%) had the outcome of death. The proportion of death among CPT user participants was 28.9%. Patients who were treated by INH were 108 (29.8%) of which 11.1% died. On the other hand, among the 96 (26.4%) participants who did not disclose their status to anybody, 46 (47.9%) died (Table 2).
Survival Status of TB/HIV Co-infected Patients
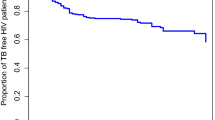
Of 363 TB/HIV co-infected patients followed for 60 months, 79 (21.8%) died, while the remaining 284 (78.2%) were censored. The minimum follow-up period was 2.3 months, and the maximum was 50.6 months. The overall median survival time was 15.6 months.
Kaplan–Meier Curve for Some Covariates
Kaplan–Meier curves for some of the covariates like CD4 count/mm3 and functional status of patients are shown in Fig. 2. By these plots, we can check if the TB/HIV co-infected patient’s survival time differs across various subgroups.
For the patients with covariate CD4 count/mm3, the survival curve for patients with CD4 count/mm3 ≥ 200 lies closely above those patients with CD4 count/mm3 < 200. This implies that the survival time of patients with CD4 count/mm3 ≥ 200 differs significantly from patients with CD4 count/mm3 < 200.
Also, there appear to be differences in the survival time of cotrimoxazole prophylactic therapy (CPT) use and non-use. The survival time of CPT users was higher than non-users, which suggested that these groups are at a lower risk of death than their counterparts.
Evaluation of Proportion Hazard Assumption
We want to check the proportional hazard assumption before moving on to the AFT model. The goodness of fit test gives a significant global p-value (Table 3). Since the global null hypothesis that the proportionality assumption holds is rejected, the PH model is inappropriate in this case. As a result, we have used the AFT model, to analyze the survival time to death for TB/HIV co-infected patients.
Acceleration Failure Time (AFT) Model Fitting
In the univariable AFT model analysis, all the covariates are statistically significantly associated with time to death of TB/HIV co-infection except employment, marital status, and religion which were insignificant at a 25% level of significance. We shall reanalyze the covariates selected at univariable analysis using a multivariable AFT model.
Evaluation of AFT Models
To check the adequacy of the baseline hazard function, the plots have been done for the exponential, Weibull, logistic, and log-normal models (Fig. 3). Nevertheless, all models are almost linear and have the same structure; the plot of the lognormal AFT is found to be more linear than the others. As a result, lognormal assumptions outperform exponential, Weibull, and log-logistic models. Therefore, for the TB/HIV co-infected patient’s data set, lognormal AFT is the more suitable baseline distribution in the model than others (Fig. 3). This is also revealed by model comparison criteria values shown in Table 4.
Comparison of Fitted Models
To compare the performance of all the models, we used the Akaike information criterion (AIC) and the Bayesian information criterion (BIC). The model with the smallest AIC or BIC is considered to provide the best fit. Based on the two criteria, the lognormal model is found to have minimum information criteria values, hence the appropriate AFT model for the TB/HIV co-infection data (Table 4).
Results of Multivariable Lognormal AFT Model
The results of the multivariable lognormal AFT model (final model) fitted are presented in Table 5. Age is a significant predictor of the time to death of TB/HIV co-infected patients, and older age is at a slightly higher risk of death as compared to the younger age group. In other words, the survival time to death of TB/HIV co-infected patients aged ≥ 45 years was decelerated/shortened by a factor of 0.712 [\(\phi\) =0.712, 95%CI: 0.57–0.89]. When compared to rural residents, the survival time to death of patients living in cities was increased by a factor of 3.75 [\(\phi\) =3.75, 95%CI: 2.02–6.96].
Substance use is a significant predictor of time to death of TB/HIV co-infected patients, and users are at a slightly higher risk of death as compared to non-users 0.51 [\(\phi\) =0.712, 95%CI: 0.28–0.92]. As compared to those who have no education at all, patients who have secondary education had a higher survival time to death from TB/HIV co-infection with an acceleration factor of 2.56 [\(\phi\) =2.56, 95%CI: 1.10–5.93].
Regarding WHO clinical stages of the disease, those who have stage IV had a lower survival time to death from TB/HIV co-infection with an acceleration factor of 0.15 [\(\phi\) =0.15, 95%CI: 0.05–0.51] than those with stage I. Patients with a CD4 count of greater than two hundred cells per cubic millimeter was significantly associated with good survival of TB/HIV co-infected patient with a factor of 1.88 [\(\phi\) =1.88, 95%CI: 1.05–3.36]. Survival time to death of a bedridden group of patients was significantly associated with poor survival as compared to working functional status. This is revealed by a time ratio of less than one 0.30 [\(\phi\) =0.30, 95%CI: 0.13–0.67] indicating a shorter survival time and greater risk of death. Patients who used CPT were nearly two times 2.12 [\(\phi\) =2.12, 95%CI: 1.13–3.95] higher survival time to death, and hence, taking CPT was significantly associated with prolonged survival among TB/HIV co-infected patients during TB treatment. INH-treated patients had a prolonged survival time to death from TB/HIV co-infection by a factor of 5.21 [\(\phi\) =5.21, 95%CI: 2.37–11.44].
Overall Goodness of Fit
Cox-Snell residuals are used in survival models to determine how well the model fits the data set. Using the Cox-Snell residuals plot, we evaluated the lognormal AFT model’s goodness of fit. It was calculated by fitting the lognormal model to the data set of a TB/HIV co-infected patient using maximum likelihood estimation (Fig. 4). This plot shows that when the Cox-Snell residuals of the lognormal models were compared to the exponential, lognormal, and log-logistic baselines (not shown here), the line was closest to the line through the origin, indicating that this model accurately characterizes the data set of TB/HIV co-infected patients.
Checking Adequacy of Accelerated Failure Time (AFT) Models
When two populations are used, a Q-Q plot (also known as a quantile–quantile plot) is used to ensure that the accelerated failure time fits the data well. By comparing some significantly different groups, we could check the AFT’s adequacy graphically. We have shown a Q-Q plot for CPT use, CD4 count/mm3, and functional status among significant covariates in the current study (Fig. 5). The figures appear to be nearly linear for all covariates. As a result, an accelerated failure time model with lognormal as the baseline best described the TB/HIV data set.
Discussion
This 5-year retrospective cohort study of TB/HIV co-infected patients on ART provides insight into survival and its determinants in a hospital setting at TGH and MTUTH. The death rate was high in this retrospective study, with 21.8% of TB/HIV co-infected patients dying during TB treatment. This is consistent with the previous study in Northern Ethiopia, 22% [28]. However, the death rate of HIV/TB co-infected patients in the current study is higher than the 13.7% found in Myanmar [37] and 7.7% in Harar town [38]. These discrepancies could be due to differences in follow-up periods, study areas, and/or facility existence. Furthermore, our study revealed that the median time to death was 15.6 months. Age, residence, substance use, educational status, clinical stages of the disease, CD4 count/mm3, functional status, CPT use, and INH were all found to be significant factors in the multivariable lognormal AFT model, whereas gender, illness other than TB, and disclosure of status were insignificant variables.
The effect of a covariate is to accelerate or decelerate the survival time of TB/HIV co-infected patients. To understand this better, an acceleration factor also called time ratio (TR) is estimated. The acceleration factor for a given covariate is the (natural) exponent of the corresponding coefficient (i.e., \(exp(\beta )\)). A positive coefficient means the effect of the covariate is to prolong the survival time while a negative coefficient is to shorten the time to death of TB/HIV co-infected patients. Relatively, a time ratio greater than 1 implies that the effect of the covariate increases the survival time and otherwise decreases (“speeds up”) the time to death of TB/HIV co-infected patients.
The survival time to death of patients in urban was enhanced by a factor of 3.75 when compared to rural people. Previous research supports this result [39]. This could be attributed to a lack of access to TB services to ensure rapid diagnosis and treatment among rural residents in resource-constrained areas. Results of the current study revealed that substance use is a significant predictor of time to death in TB/HIV co-infected individuals, and users are at a slightly higher risk than non-users. This is consistent with the literature [40], which states that substance use harmed HIV-infected drug users’ health, resulting in higher morbidity and death in HIV-infected drug users than in infected people who did not use drugs [40].
Age was found to be a significant predictor of time to death in TB/HIV co-infected individuals in our study. The time it took for TB/HIV co-infected patients of advanced age to die was reduced by a factor of 0.712. This could be attributable to the diminishing immunity level of patients at an older age. According to the findings of a study conducted in Brazil [19], and Southwest Ethiopia [25], study participants over the age of 65 had a nearly threefold increased risk of death compared to younger patients. Patients were aged 41–60 years, and those aged more than 60 years were 7.8 and 21.34 times more likely to die early than patients aged less than 20 years, according to an Indian study [41]. Another study [42] found that for every year of age gained, the death rate increased by 4% [42]. This could be explained by the fact that elderly people are more likely to be diagnosed with HIV and/or TB later. It is widely known that late diagnosis aids poor prognosis and mortality owing to immune insufficiency from rapid advancement to AIDS and extrapulmonary TB [43,44,45,46].
In terms of functional status, bedridden co-infected patients’ survival time to death was substantially connected with a shorter survival time and a higher risk of mortality than those who were working. A study [26, 47,48,49] supported this conclusion. In keeping with this, a prior study at Jimma found that bedridden patients had a threefold higher risk of death than those who were working [25]. Our findings were consistent with those of another study conducted in Bahir Dar, Ethiopia [28], which found that bedridden patients were approximately four times more likely to die than patients who were working. These findings are not shocking because bedridden patients are more likely to be exposed to infections and have a poorer health outcomes due to a vicious cycle of reduced immunity, which can lead to patients falling to opportunistic infections and mortality [50]. As a result, it is reasonable to advocate for mandatory and frequent screening for opportunistic infections and other diseases to implement early and effective management techniques and reduce preventable deaths.
A substantial statistical connection between the WHO clinical stage and time to death of TB-HIV co-infection was discovered in the current investigation. Previous research has shown that those co-infected patients with WHO clinical stage IV have a shorter survival time than co-infected patients with stage I [14]. Previous studies reported that advanced WHO clinical stages enhanced mortality among TB patients with HIV, and the WHO clinical stage is a risk factor for HIV-TB co-infection [27, 38]. Individuals with a WHO clinical stage of IV were 8.6 times more likely to die than those with a WHO clinical stage of III, according to a study conducted at Gondar University Hospital [14]. This could be because individuals with advanced WHO clinical stages were more likely to get additional opportunistic infections.
Several studies looked at the impact of CD4 cell count on survival rates, and the results showed that a low CD4 cell count was related to a greater risk of mortality [26, 47, 51, 52]. In support of this, a current study found that patients with a CD4 count of more than 200 cells per cubic millimeter had a higher chance of surviving. A study conducted in Barcelona, Spain [20], found that patients with CD4 cell counts of less than 200/mm3 had the lowest chance of survival [20, 53]. This is because a low CD4 count makes it difficult for the body to fight against additional infections, making even a simple infection like a cold considerably more severe. This is due to the body’s inability to respond to new infections.
The patient’s educational level was also found to be a significant determinant factor in the longevity of HIV/TB co-infected individuals, according to this study. This result is in line with the previous findings. The co-infected patient’s survival time appears to increase with their level of education. The findings are consistent with this finding [54, 55]. Therapeutic failure and poor adherence to therapy are more common among those with low income and education levels [56, 57]. This could be because people with low education put off seeking healthcare or face obstacles in doing so. As a result, patients have a hard time getting diagnosed and treated, which can lead to mortality [58, 59].
Taking CPT during TB treatment was linked to a higher rate of survival among TB/HIV co-infected individuals. Patients who received CPT had 2.12 times longer survival time to death than those who did not. This is in line with a study conducted in Tehran, Iran, which found that cotrimoxazole preventive therapy had a significant impact on patient survival, with patients who did not receive cotrimoxazole preventive therapy having a 3.68 times higher risk of death than patients who received cotrimoxazole preventive therapy [60]. Furthermore, the current study found that INH-treated TB/HIV co-infected patients had a 5.21-fold longer survival time to death than non-treated TB/HIV co-infected patients.
Limitation of the Study
This study is not without limitations. First, because the study was retrospective, data was taken from the patient’s medical history and registry, which could lead to selection bias. Second, because the studies included all deaths regardless of the cause of death, there is a chance that the cause of death was misclassified. Furthermore, socioeconomic parameters (living situation, social support, distance to the health institution), as well as treatment and disease-related variables (adherence level, virus load, drug resistance), were not documented. Despite the constraints highlighted, this study was undertaken at two major health facilities with a bigger sample size to better understand the issue.
Conclusion
The lognormal accelerated failure time model outperformed the other models in the current study. These study findings showed that older age, residence, substance use, educational status, WHO clinical stages of the disease (stage IV), CD4 less than 200 count/mm3, bedridden functional status, CPT use, and INH use all had a significant impact on TB/HIV co-infected patients’ survival time to death. To improve their health and extend their lives, patients with TB/HIV co-infection should be given special attention based on these important factors.
Data Availability
The data sets used in this study are available from the corresponding author on reasonable request.
Abbreviations
- AIC:
-
Akaike information criterion
- AFT :
-
Accelerated failure time model
- ART:
-
Antiretroviral therapy
- BIC :
-
Bayesian information criterion
- BMI :
-
Body mass index
- CD4:
-
Cluster of differentiation
- CPT:
-
Cotrimoxazole prophylactic therapy
- HIV:
-
Human immunodeficiency virus
- INH:
-
Isonicotinic acid hydrazide
- MTUTH:
-
Mizan-Tepi University Teaching Hospital
- mm3 :
-
Millimeter cube
- QQ plot:
-
Quantile-quantile plot
- TB:
-
Tuberculosis
- TGH:
-
Tepi General Hospital
- WHO:
-
World Health Organization
References
World Health Organization. Global tuberculosis control: epidemiology, strategy, financing: WHO report 2009. World Health Organization. 2009.
World Health Organization. Global Tuberculosis Control: WHO Report. Geneva, Switzerland. World Health Organization. 2011.
Padmapriyadarsini C, Narendran G, Swaminathan S. Diagnosis & treatment of tuberculosis in HIV co-infected patients. Indian J Med Res. 2011;134:850.
Cain KP, Anekthananon T, Burapat C, Akksilp S, Mankhatitham W, Srinak C, Nateniyom S, Sattayawuthipong W, Tasaneeyapan T, Varma JK. Causes of death in HIV-infected persons who have tuberculosis, Thailand. Emerg Infect Dis. 2009;15:258.
Birhan H, Derebe K, Muche S, Melese B. Statistical analysis on determinant factors associated with time to death of HIV/TB co-infected patients under HAART at Debre Tabor Referral Hospital: an application of accelerated failure time-shared frailty models. HIV/AIDS (Auckland, NZ). 2021;13:775.
Kalsdorf B, Skolimowska KH, Scriba TJ, Dawson R, Dheda K, Wood K, Hofmeister J, Hanekom WA, Lange C, Wilkinson RJ. Relationship between chemokine receptor expression, chemokine levels and HIV-1 replication in the lungs of persons exposed to Mycobacterium tuberculosis. Eur J Immunol. 2013;43:540–9.
World Health Organization. Global tuberculosis report 2013. World Health Organization. 2013.
Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, Dye C. the growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–21.
Mollel EW, Todd J, Mahande MJ, Msuya SE. Effect of tuberculosis infection on mortality of HIV-infected patients in Northern Tanzania. Trop Med Health. 2020;48:1–10.
Ji Y-J, Liang P-P, Shen J-Y, Sun J-J, Yang J-Y, Chen J, Qi T-K, Wang Z-Y, Song W, Tang Y. Risk factors affecting the mortality of HIV-infected patients with pulmonary tuberculosis in the cART era: a retrospective cohort study in China. Infect Dis Poverty. 2018;7:1–9.
Kruk ME, Gage AD, Arsenault C, Jordan K, Leslie HH, Roder-DeWan S, Adeyi O, Barker P, Daelmans B, Doubova SV. High-quality health systems in the Sustainable Development Goals era: time for a revolution, Lancet. Glob Heal. 2018;6:e1196–252.
Kassu A, Mengistu G, Ayele B, Diro E, Mekonnen F, Ketema D, Moges F, Mesfin T, Getachew A, Ergicho B. Coinfection and clinical manifestations of tuberculosis in human immunodeficiency virus-infected and-uninfected adults at a teaching hospital, northwest Ethiopia. J Microbiol Immunol Infect. 2007;40:116.
Harries AD, Zachariah R, Lawn SD. Providing HIV care for co-infected tuberculosis patients: a perspective from sub-Saharan Africa [State of the art series. Tuberculosis. Edited by ID Rusen. Number 3 in the series], Int J Tuberc Lung Dis. 2009; 13:6–16.
Wondimeneh Y, Muluye D, Belyhun Y. Prevalence of pulmonary tuberculosis and immunological profile of HIV co-infected patients in Northwest Ethiopia. BMC Res Notes. 2012;5:1–6.
Sterling TR, Pham PA, Chaisson RE. HIV infection—related tuberculosis: clinical manifestations and treatment. Clin Infect Dis. 2010;50:S223–30.
Mitku AA, Dessie ZG, Muluneh EK, Workie DL. Prevalence and associated factors of TB/HIV co-infection among HIV infected patients in Amhara region, Ethiopia. Afr Health Sci. 2016;16:588–95.
Nglazi MD, Bekker L-G, Wood R, Kaplan R. The impact of HIV status and antiretroviral treatment on TB treatment outcomes of new tuberculosis patients attending co-located TB and ART services in South Africa: a retrospective cohort study. BMC Infect Dis. 2015;15:1–8.
Swaminathan S, Rekha B. Pediatric tuberculosis: global overview and challenges. Clin Infect Dis. 2010;50:S184–94.
Domingos MP, Caiaffa WT, Colosimo EA. Mortality, TB/HIV co-infection, and treatment dropout: predictors of tuberculosis prognosis in Recife, Pernambuco State, Brazil. Cad Saude Publica. 2008;24:887–96.
Català L, Orcau A, García de Olalla P, Millet JP, Rodríguez-Mondragón A, Caylà JA, T.-H.W. Group. Survival of a large cohort of HIV-infected tuberculosis patients in the era of highly active antiretroviral treatment. Int J Tuberc Lung Dis. 2011;15:263–9.
Agbor AA, Bigna JJR, Billong SC, Tejiokem MC, Ekali GL, Plottel CS, Noubiap JJN, Abessolo H, Toby R, Koulla-Shiro S. Factors associated with death during tuberculosis treatment of patients co-infected with HIV at the Yaoundé Central Hospital, Cameroon: an 8-year hospital-based retrospective cohort study (2006–2013). PLoS One. 2014;9:e115211.
Demissie MB, Lindtjørn B, Belete T. Human immunodeficiency virus (HIV) infection in tuberculosis patients in Addis Ababa. Ethiopian J Heal Dev. 2000;14:3.
Ahmed Yassin M, Takele L, Gebresenbet S, Girma E, Lera M, Lendebo E, Cuevas LE. HIV and tuberculosis coinfection in the southern region of Ethiopia: a prospective epidemiological study, Scand. J Infect Dis. 2004;36:670–3.
Reda AA, Biadgilign S, Deribew A, Gebre B, Deribe K. Predictors of change in CD4 lymphocyte count and weight among HIV infected patients on anti-retroviral treatment in Ethiopia: a retrospective longitudinal study. PLoS One. 2013;8:e58595.
Gesesew H, Tsehayneh B, Massa D, Gebremedhin A, Kahsay H, Mwanri L. Predictors of mortality in a cohort of tuberculosis/HIV co-infected patients in Southwest Ethiopia. Infect Dis Poverty. 2016;5:1–9.
Refera H, Wencheko E. Survival of HIV-TB co-infected adult patients under ART in Ambo Referral Hospital, Ethiopia. Ethiop J Heal Dev. 2013;27:88–93.
Iliyasu Z, Musa B. Prevalence and predictors of tuberculosis coinfection among HIV seropositive patients attending the Aminu Kano Teaching Hospital, northern Nigeria. J Epidemiol. 2009;0903030070.
Sileshi B, Deyessa N, Girma B, Melese M, Suarez P. Predictors of mortality among TB-HIV co-infected patients being treated for tuberculosis in Northwest Ethiopia: a retrospective cohort study. BMC Infect Dis. 2013;13:1–10.
Klein, JP, Moeschberger ML. Survival analysis: techniques for censored and truncated data. Vol. 2. New York: Springer; 2003.
Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81.
Kleinbaum DG, Klein M. Kaplan-Meier survival curves and the log-rank test, in: Surviv. Anal., Springer;2012. pp. 55–96.
Abeysekera WWM, Sooriyarachchi MR. Use of Schoenfeld’s global test to test the proportional hazards assumption in the Cox proportional hazards model: an application to a clinical study. 2009.
Lawless, JF. Parametric models in survival analysis. Wiley StatsRef: Stat Ref Online; 2014.
Khanal SP, Sreenivas V, Acharya SK. Accelerated failure time models: an application in the survival of acute liver failure patients in India. Int J Sci Res. 2014;3:161–6.
Kuha J. AIC and BIC: Comparisons of assumptions and performance. Sociol Methods Res. 2004;33:188–229.
Coppini DV, Bowtell PA, Weng C, Young PJ, Sönksen PH. Showing neuropathy is related to increased mortality in diabetic patients—a survival analysis using an accelerated failure time model. J Clin Epidemiol. 2000;53:519–23.
Aung ZZ, Saw YM, Saw TN, Oo N, Aye HNN, Aung S, Oo HN, Cho SM, Khaing M, Kariya T. Survival rate and mortality risk factors among TB–HIV co-infected patients at an HIV-specialist hospital in Myanmar: a 12-year retrospective follow-up study. Int J Infect Dis. 2019;80:10–5.
Tola A, Mishore KM, Ayele Y, Mekuria AN, Legese N. Treatment outcome of tuberculosis and associated factors among TB-HIV co-infected patients at public hospitals of Harar town, eastern Ethiopia. A five-year retrospective study. BMC Public Health. 2019;19:1–12.
Nguyen DT, Jenkins HE, Graviss EA. Prognostic score to predict mortality during TB treatment in TB/HIV co-infected patients. PLoS One. 2018;13:e0196022.
Altice FL, Kamarulzaman A, Soriano VV, Schechter M, Friedland GH. Treatment of medical, psychiatric, and substance-use comorbidities in people infected with HIV who use drugs. Lancet. 2010;376:367–87.
Pardeshi G. Survival analysis and risk factors for death in tuberculosis patients on directly observed treatment-short course. 2009.
Mugusi FM, Mehta S, Villamor E, Urassa W, Saathoff E, Bosch RJ, Fawzi WW. Factors associated with mortality in HIV-infected and uninfected patients with pulmonary tuberculosis. BMC Public Health. 2009;9:1–8.
Mojumdar K, Vajpayee M, Chauhan NK, Mendiratta S. Late presenters to HIV care and treatment, identification of associated risk factors in HIV-1 infected Indian population. BMC Public Health. 2010;10:1–8.
Xu X, Liu JH, Cao SY, Zhao Y, Dong XX, Liang Y, Lu ZX. Delays in care seeking, diagnosis and treatment among pulmonary tuberculosis patients in Shenzhen, China. Int J Tuberc Lung Dis. 2013;17:615–20.
Raizada N, Chauhan LS, Babu BS, Thakur R, Khera A, Wares DF, Sahu S, Bachani D, Rewari BB, Dewan PK. Linking HIV-infected TB patients to cotrimoxazole prophylaxis and antiretroviral treatment in India. PLoS One. 2009;4:e5999.
Tarekegn S. The effect of HAART on incidence of tuberculosis among HIV infected patients in Hawassa University Referral Hospital, South Ethiopia. Unpublished dissertation in partial fulfillment of the requirements for the degree of Master of Science, Tanzania. 2011.
Gezie LD. Predictors of CD4 count over time among HIV patients initiated ART in Felege Hiwot Referral Hospital, northwest Ethiopia: multilevel analysis. BMC Res Notes. 2016;9:1–9.
Temesgen A, Gurmesa A, Getchew Y. Joint modeling of longitudinal CD4 count and time-to-death of HIV/TB co-infected patients: a case of Jimma University Specialized Hospital. Ann Data Sci. 2018;5:659–78.
Sinshaw Y, Alemu S, Fekadu A, Gizachew M. Successful TB treatment outcome and its associated factors among TB/HIV co-infected patients attending Gondar University Referral Hospital, Northwest Ethiopia: an institution based cross-sectional study. BMC Infect Dis. 2017;17:1–9.
Alene KA, Nega A, Taye BW. Incidence and predictors of tuberculosis among adult people living with human immunodeficiency virus at the University of Gondar Referral Hospital, Northwest Ethiopia. BMC Infect Dis. 2013;13:1–9.
Mageda K, Germana HL, Mmbaga EJ. High initial HIV/AIDS Related mortality and-its predictors among patients on antiretroviral therapy in the kagera region of Tanzania: a five-year retrospective cohort study. AIDS Res Treat. 2012.
Nansera D, Bajunirwe F, Elyanu P, Asiimwe C, Amanyire G, Graziano FM. Mortality and loss to follow-up among tuberculosis and HIV co-infected patients in rural southwestern Uganda. Int J Tuberc Lung Dis. 2012;16:1371–6.
Maruza M, Albuquerque M, Braga MC, Barbosa MTS, Byington R, Coimbra I, Moura LV, Batista JDL, Diniz GTN, Miranda-Filho DB. Survival of HIV-infected patients after starting tuberculosis treatment: a prospective cohort study. Int J Tuberc Lung Dis. 2012;16:618–24.
Taha M, Deribew A, Tessema F, Assegid S, Duchateau L, Colebunders R. Risk factors of active tuberculosis in people living with HIV/AIDS in southwest Ethiopia: a case control study, Ethiop. J Health Sci. 2011;21:131–40.
Lawrence M. Tuberculosis (TB) treatment outcomes in adult TB patients. 2009.
Gao J, Zheng P, Jia Y, Chen H, Mao Y, Chen S, Wang Y, Fu H, Dai J. Mental health problems and social media exposure during COVID-19 outbreak. PLoS One. 2020;15:e0231924.
Reepalu A, Balcha TT, Skogmar S, Güner N, Sturegård E, Björkman P. Factors associated with early mortality in HIV-positive men and women investigated for tuberculosis at Ethiopian health centers. PLoS One. 2016;11:e0156602.
Naidoo P, Peltzer K, Louw J, Matseke G, Mchunu G, Tutshana B. Predictors of tuberculosis (TB) and antiretroviral (ARV) medication non-adherence in public primary care patients in South Africa: a cross sectional study. BMC Public Health. 2013;13:1–10.
Jappar SB, Low SY. Tuberculosis trends over a five-year period at a tertiary care university-affiliated hospital in Singapore. Singapore Med J. 2015;56:502.
Roshanaei G, Ghannad MS, Poorolajal J, Mohraz M, Molaeipoor L. Survival rates among co-infected patients with human immunodeficiency virus/tuberculosis in Tehran, Iran. Iran J Public Health. 2017;46:1123.
Acknowledgements
We would like to thank Tepi General Hospital and Mizan-Tepi University Teaching Hospital for allowing this data to be collected from patients’ cards. We would also like to thank Mizan-Tepi University for assisting us in collecting data and conducting this crucial study.
Author information
Authors and Affiliations
Contributions
MEL and BMT: study conception and design. BMT and SA: data collection. MEL: data analysis and interpretation. SAT: support interpretation and draft writing. MEL, SAT, and SSH: writing the manuscript draft. SAA and AMM: reviewing the manuscript draft. Finally, all authors read and approved the manuscript before submission.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Ethical approval to conduct the study and human subject research approval for this study was received from Mizan-Tepi University, College of Natural and Computational Sciences Research and Ethics Review Board. The author requested data from Tepi General Hospital and Mizan-Tepi University Teaching Hospital and was granted permission to collect data from patients’ cards. Furthermore, because the study was retrospective, the Research and Ethics Review Board (RERB) of Mizan-Tepi University waived the requirement for written informed consent of study participants, but data were kept anonymous and confidential.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lelisho, M.E., Teshale, B.M., Tareke, S.A. et al. Modeling Survival Time to Death Among TB and HIV Co-infected Adult Patients: an Institution-Based Retrospective Cohort Study. J. Racial and Ethnic Health Disparities 10, 1616–1628 (2023). https://doi.org/10.1007/s40615-022-01348-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40615-022-01348-w