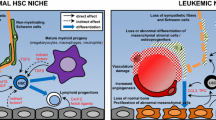
Abstract
Purpose of Review
This review focuses on evidence highlighting the bidirectional crosstalk between the hematopoietic stem cell (HSC) and their surrounding stromal cells, with a particular emphasis on cells of the osteoblast lineage. The role and molecular functions of osteoblasts in normal hematopoiesis and in myeloid hematological malignancies is discussed.
Recent Findings
Cells of the osteoblast lineage have emerged as potent regulators of HSC expansion that regulate their recruitment and, depending on their stage of differentiation, their activity, proliferation, and differentiation along the lymphoid, myeloid, and erythroid lineages. In addition, mutations in mature osteoblasts or their progenitors induce myeloid malignancies. Conversely, signals from myelodysplastic cells can remodel the osteoblastic niche to favor self-perpetuation.
Summary
Understanding cellular crosstalk between osteoblastic cells and HSCs in the bone marrow microenvironment is of fundamental importance for developing therapies against benign and malignant hematological diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Hematopoietic Stem Cell
In addition to its well-established role in providing a mechanical frame for the body and protecting vital organs, the bone is the home of hematopoiesis. Indeed, the skeleton has recently emerged as an important regulator of multiple hematopoietic functions in health as well as in the development and progression of hematological myeloid malignancies. There is ample opportunity for the bone cells and blood cells to influence each other’s fate since an intimate physical interaction between them is established early in life, when blood precursors migrate and colonize the embryonic bone and cartilage spaces, establishing the medullar hematopoiesis and creating the bone marrow cavity. This interaction occurs within the bone marrow (BM), an intricate organ, composed by many different hematopoietic and stromal cells and surrounded by highly vascularized and innervated bone. Approximately one trillion blood cells arise daily in adult human BM, and all of them are derived from a small fraction of cells—0.05 to 0.1% of total cells in the marrow—the hematopoietic stem cells (HSC).
The study of HSCs began right after the bombing of Hiroshima and Nagasaki in 1945. People in the outskirts of affected areas that were exposed to lower radiation doses during a prolonged period of time, showed compromised hematopoiesis associated with lethality. Shortly after, studies designed to replicate such conditions demonstrated that mice exposed to whole-body gamma irradiation, developed the same radiation syndromes and that at the minimal lethal-dose, mice died from hematopoietic failure within 2 weeks of exposure. We now know that exposure to radiation has a devastating effect in mitotically active cells, with the hematopoietic system being the most sensitive vital organ affected. It was in the 1950s, when it was demonstrated that clonal HSCs in the BM could not only self-renew but also reconstitute blood cell production by multi-lineage reconstitution in irradiated (aka myeloablated) mice [1, 2]. Since these initial discoveries, the definition of the HSC has evolved, and nowadays an HSC is defined as a cell that has the ability to both generate the entire hematopoietic system, including the myeloid and lymphoid lineages, and to replace itself throughout the lifetime of an adult [3]. Essentially, all HSC activity has been shown to be contained within the lineage−/lo (Lin−/lo) Sca1+ c-kithi (LSK) HSC compartment [4]. However, this compartment contains a functionally heterogeneous cell population in terms of self-renewal, life span, and differentiation. The prevailing model of hematopoiesis relies on the existence of two functionally different HSC subpopulations, the short- and the long-term population (LT-HSCs and ST-HSCs, respectively), the behavior of which differs following transplantation into lethally irradiated hosts. LT-HSCs have life-long self-renewing potential, while the ST-HSCs—that show more restricted self-renewing capacity—can differentiate into all types of blood cells following transplantation [5].
Bone and the Hematopoietic Stem Cell Niche
Self-renewal occurs in a cell-autonomous manner but it is greatly influenced by the so-called stem cell niche. R. Schofield proposed the concept of the BM HSC niche in 1978 [6] suggesting for the first time that HSCs reside in a specialized BM microenvironment (niche). Schofield’s groundbreaking concept implied that the process of hematopoiesis requires a supportive BM structure surrounding the HSCs. Indeed, in vitro culture of HSCs without a supportive stromal cell layer leads to loss of their long-term engraftment capacity which eventually occurs even if HSCs are cultured in the presence of cytokines and growth factors promoting their stemness [7], such as stem cell factor (SCF), thrombopoietin (TPO), Flt3 ligand, interleukin-3, −6, and −11 [8,9,10,11,12], pleiothropin, insulin-like growth factor 2 (IGF-2), fibroblast growth factor (FGF), angiopoietin-like proteins [13,14,15,16], and/or their combinations. In fact, robust ex vivo maintenance of repopulating cells has not yet been achieved or is limited to extremely short culture periods. It is nowadays clear that in adults, HSCs are localized in highly specialized microanatomical environments within the BM that provide the signals necessary for their maintenance and regulation. This dynamic microenvironment that tightly regulates HSC homeostasis consists of a combination of stromal cell types, as well as a plethora of secreted factors, surface receptors, and extracellular matrix molecules with different functional roles, all of which support HSCs (see Fig. 1). These stromal niches are comprised by osteoblastic, endothelial, and mesenchymal stromal cells [17]. Interestingly, several studies have shown that alterations of the BM microenvironment finally lead to hematopoiesis disruption and/or malfunction, ultimately resulting in hematopoietic malignancies [18, 19, 20••, 21•].
Cellular and molecular structure of the bone marrow niche. Hematopoietic stem cells (HSCs) reside inside specialized microenvironments or niches within the bone marrow. Different cells constitute the niches and contribute to the maintenance and differentiation of HSCs. The endosteal niche is comprised by osteocytes, osteoblasts, and osteoclasts, with osteoblasts being the main cells supporting myelopoiesis throughout the release of soluble factors such as granulocyte colony-stimulating factor (G-CSF), Osteopontin (OPN), Annexin-2 (Anxa2), Thrombopoietin (TPO), and Angiopoietin-1 (Ang-1). Osteoblasts in the endosteal niche as well as endothelial cells, leptin-receptor expressing perivascular cells (LepR+), CXCL12-abundant reticular (CAR) cells, and Nestin+ mesenchymal stem cells (MSC) in the perivascular niche, secrete the CXC-chemokine ligand 12 (CXCL12) which controls HSC homing, retention, and repopulation of HSCs. Osteoblasts, Nestin+ MSC and mainly perivascular cells release Stem cell factor (SCF), a key niche component that maintains HSCs homing, retention/and repopulation. Opposite gradients of oxygen (O2) and calcium (Ca2+) characterize the niche and influences HSC maintenance, self-renewal, and differentiation
This review will focus on evidence highlighting the bidirectional crosstalk between the HSC and their surrounding stromal cells, with a particular emphasis on cells of the osteoblastic lineage. The role of these cells in normal hematopoiesis and in hematological malignancies will be discussed. The impact of these observations lies with the fact that understanding the molecular interactions between osteoblasts and HSCs may allow to target these pathways to promote hematopoiesis. Alternatively, interrupting interacting signals may help to create a niche hostile to dysplastic cells in hematological malignancies.
Hematopoiesis and the Endosteal Niche
Osteoblasts, the main contributors to the endosteal niche, are organized in teams of cells layering the endosteal bone surface, acting as the first interface between calcified bone and the BM (see Fig. 1). Cells of the osteoblast lineage have been early pointed as key players in the control of hematopoiesis [22,23,24,25]. In contrast, osteoclasts, the bone resorbing cells, appear to not be involved in the maintenance and mobilization of HSCs [26].
In 2003, two studies identified cells of the osteoblast lineage, as critical components of the HSC-niche that regulate hematopoiesis by affecting HSC-renewal and expansion [27, 28]. In these studies, genetic or pharmacological manipulation of osteoblasts provided the first evidence that specific cells of the osteoblast lineage regulate HSC fate in vivo (although whether they do it directly or indirectly) was unclear. Alterations in the number of cells of the osteoblast lineage have also shown opposite effects, with some studies showing limited HSC expansion [29, 30]. Indeed, LT-HSCs from osteoblast-ablated mice demonstrate loss of quiescence and reduced long-term engraftment and self-renewal capacity [31] and develop a hematological phenotype that favors myeloid but suppresses lymphoid and erythroid expansion [21•]. Interestingly, this shift to myeloid bias at the expense of lymphopoiesis may precondition the BM to easier engraftment and development of acute myeloid leukemia (AML) [21•].
Interestingly, osteoblasts appear to regulate homing of HSCs [32]. Studies in mice have shown that upon transplantation, HSCs preferentially colonize the endosteal region in close association with the bone lining cells [33,34,35,36,37]. Moreover, HSCs isolated from the endosteal region show higher proliferative potential as well as greater long-term hematopoietic reconstitution potential [38, 39], whereas more differentiated hematopoietic progenitors are found mainly in the central BM region, around the perivascular niche. Two recent studies in HSC-transgenic reporter mice revealed that the majority of the quiescent HSC are located in the perivascular niche and localized all along the marrow [40•, 41••].
In addition to HSCs, B lymphopoiesis is regulated by microenvironmental factors in the BM. B cell precursors (pre-pro-B cells and plasma cells) require CXCL12 to differentiate and are localized in direct contact with stromal cells in the marrow space that express this chemokine [42]. Among them, osteoblasts have been shown to play a role in the regulation of B lymphopoiesis. A common regulation of B cell and osteoblast formation or function was suggested by the fact that several factors that regulate B-cell homeostasis exert direct effects on osteoblasts; conversely, factors that regulate the bone remodeling influence B-cell maturation. Osteoblasts support the differentiation of primitive HSC through lymphoid commitment and subsequent differentiation to all stages of B-cell precursors and mature B cells [43] whereas depletion of osteoblasts leads to loss of B lymphocytes prior to the decrease in HSC numbers [44]. Similarly, cells of the osteoblast lineage extrinsically regulate B lymphopoiesis by a mechanism involving Gsα signaling in an at least in part, IL-7-dependent manner [45]. More recently, the inflammatory cytokine granulocyte colony-stimulating factor (G-CSF) was proposed to reprogram BM stromal cells (including CXCL12-abundant reticular CAR cells and osteoblasts) resulting in suppression of B lymphopoiesis in mice [46]. Remarkably, in the BM stroma osteoblasts are the main cells with a myelopoietic supportive capacity, which is mediated by sustained G-CSF release [23, 47]. Therefore, BM stromal cells, and in particular osteoblasts, support B lymphopoiesis and inhibiting this capacity could favor myelopoiesis.
Lastly, and as will be discussed below, recent studies have refined the intricate functions of the osteoblastic niche on regulation of hematopoiesis, suggesting that those may depend on their differentiation stage [45, 48,49,50,51,52•].
Mechanisms of Crosstalk between Osteoblasts and HSCs
In the endosteal niche, several mechanisms are involved in the crosstalk between osteoblasts and HSCs. Osteoblasts produce numerous soluble growth factors and cytokines that regulate HSC homing/mobilization and quiescence such as CXC-chemokine ligand 12 (CXCL12), Stem-Cell Factor, Osteopontin, granulocyte colony-stimulating factor, Annexin 2, Angiopoietin-1 or Thrombopoietin [23, 27, 30, 53,54,55,56] (see Fig. 1). Moreover, several signaling pathways such as Notch or parathyroid hormone (PTH) pathways have been involved in this crosstalk. Activation of PTH-receptor on osteoblasts induces not only an increase in osteoblast numbers (bone volume) but also increases the expression of the Notch-ligand Jagged-1 [27, 57], and at the same time stimulates self-renewal of HSC. Self-renewing HSCs show Notch-1 activation in vivo and inhibition of Jagged-1/Notch-1 signaling abrogates the PTH-dependent increase in HSCs [27]. Interestingly, the increase in HSC numbers occurs despite these cells are lacking the PTH receptor PTH1R, suggesting a microenvironmentally mediated effect and proving that osteoblastic cells are a regulatory component of the HSC niche in vivo that influences stem cell function through Notch activation [27]. Along with other pathways, Notch signaling induced by ligands expressed by stromal cells of the BM niche, is indeed known as a mediator of HSC self-renewal [58,59,60]. Subsequent studies have further highlighted the role of Notch signaling between osteoblasts and HSC in maintaining repopulating potential [61], and in the context of β-catenin activation in osteoblasts, in inducing myeloid malignancies [20••, 60]. These studies examined the results of enhanced Notch signals in the regulation of self-renewal or differentiation of HSCs in vitro and in vivo, but they did not address the contribution of Notch signaling under physiological conditions. Another study using Notch-deficient HSCs showed normal LT-HSC activity as well as low expression of Notch target genes in primitive HSCs, suggesting that cell-autonomous canonical Notch signals are not essential for HSC maintenance in vivo [62]. However, this study does not exclude the possibility that the role of canonical Notch signaling in HSCs might occur through induction of Notch ligand expression by the BM microenvironment, indicating the need for further assessing the role of Notch signaling in the context of the HSC niche, especially in pathological conditions such as hematological malignancies (discussed later).
CXCL12 or stromal-derived factor-1 (SDF-1) is produced mainly by immature osteoblasts as well as by endothelial cells and controls HSC homing, retention, and repopulation [56]. Stem cell factor (SCF) is a key niche component that maintains HSCs and is secreted by osteoblasts although its main sources are perivascular cells [63]. In the adult BM, the expression of Osteopontin (OPN) is restricted to the endosteal surface where HSCs that show higher proliferative potential and homing efficiency reside [38]. OPN is a phosphorylated matrix glycoprotein secreted by osteoblasts (and many other different cell types) that has been demonstrated to be critical for the retention, migration, and negative regulation of HSC proliferation and differentiation within the endosteal region [29, 30, 64]. Interestingly, in the BM stroma, osteoblasts are the main cells with a myelopoietic supportive capacity which is mediated by sustained release of granulocyte colony-stimulating factor (G-CSF) [23, 47]. Both osteoblasts and endothelial cells express Annexin 2 (Anxa2) at high levels, and Anxa2-deficient animals show impaired adhesion of HSC to osteoblasts. Moreover, treatment of mice with Anxa2 inhibitors impaired HSC homing and engraftment [55]. Angiopoietin-1 (Ang-1) is expressed by osteoblasts and, through its interaction with the receptor tyrosine kinase Tie2, promotes stronger adhesion and quiescence in HSCs [53]. Genetic deletion of both Tie1 and Tie2 in mice results in a loss of HSC maintenance in the marrow, again suggesting a role of Ang-1 in the regulation of hematopoiesis [65]. Finally, Thrombopoietin (TPO), is expressed by osteoblastic cells and plays a critical role in the regulation of LT-HSC quiescence [54].
Not only soluble growth factors and cytokines secreted by osteoblasts determine HSC fate choices but also, short-lived biological active compounds can act as potential osteoblastic-dependent HSC regulators. This is the case for prostaglandin E2 (PGE2), known to affect both stromal and hematopoietic components of the BM. Specifically, in vivo treatment with PGE2 preferentially increases ST-HSC without affecting LT-HSC [66]. Ex vivo treatment of HSCs with dimethyl-PGE2 increases HSC repopulating potential both in mouse BM and in human cord blood samples [67,68,69]. Moreover, in an effort to clinically exploit this effect to accelerate hematopoietic recovery after BM transplantation, it has been shown that exogenous, short-term exposure to PGE2 leads to a transient increase in HSC homing and engraftment potential in mice, suggesting that in vivo manipulation of the HSC pool could be used to improve hematopoietic recovery [69,70,71].
In addition to its specific cellular composition, the endosteal niche has two characteristics that make it unique. First, there is a low oxygen tension or hypoxia state (less than 2% O2), despite the dense vascularity of the endosteum [72]. Hypoxia has recently been identified as an important regulator of HSC quiescence in the niche [73, 74]. Second, the endosteal niche is exposed to elevated levels of Ca2+ due to the intense bone remodeling activity of the bone (see Fig. 1). During this continuous remodeling, soluble Ca2+ ions are being released by both the removal of mineralized bone by osteoclasts and by the following formation of the bone by osteoblasts. HSCs respond to extracellular ionic Ca2+ gradients through the calcium-sensing receptor (CaR) [75]. Mice deficiency in this receptor show HSC in the blood and spleen but not in the endosteal niche, indicating that Ca2+ sensing through the CaR is required for retaining HSCs in close proximity to the endosteal surface.
Role of Osteoblast Differentiation Stage in Hematopoietic Functions
The role of the differentiation state of the osteolineage cells in maintaining HSCs has been investigated. Self-renewing skeletal progenitors in BM sinusoids can form supportive HSC niches [48], supporting the idea that the immature osteoblastic cells are critical for HSC regulation. Interestingly, in several situations in which bone remodeling is compromised, hematopoiesis is also deregulated. Mouse embryos lacking the transcription factor Cbfa1/RunX2—indispensable for osteoblast differentiation—show complete absence of osteoblastic maturation as well as abnormal definitive hematopoiesis (these mice showed extra-medullar hematopoiesis up until E18.5), indicating that osteoblasts are required to initiate BM hematopoiesis [76, 77]. Similarly, osteoblast ablation in Col2.3-TK transgenic mice induces not only a progressive bone loss that is consistent with the ablation of osteoblasts, but also dramatic loss of BM cellularity, suggesting that osteoblasts are important for the development of active hematopoiesis [44].
A unique role for osteoprogenitor cells (skeletal stem cells or mesenchymal stem cells, MSC) that express Nestin has emerged as an essential HSC niche component. Nestin+ MSCs colocalize with HSC and express high levels of HSC-maintenance genes. Depletion of Nestin+ MSCs results in a reduction of HSC content in the BM [50]. On the other hand, activation of the PTHR1 in mature osteocytes (terminally differentiated osteoblasts), despite expanding the osteoblastic pool, does not expand HSCs numbers neither their function [51]. While some studies have shown that quiescent HSCs specifically associate with small arterioles preferentially found in the endosteal surface [52•], two recent ones have indicated, that those cells are more closely associated with sinusoids—rather than with arterioles—and that Leptin-receptor (LepR) cells play an important role in maintaining HSC quiescence [40•]. Moreover, 94% of LT-HSCs (identified as homeobox B5, Hoxb5+ expressing cells) are located in the perivascular space [41••], suggesting that mature osteoblasts do not affect BM-HSC maintenance directly. In fact, depletion of CXCL12 or SCF in osteoblasts using the Osterix-Cre or Col2.3-cre mice, does not affect HSC numbers but instead, reduced the numbers of B lymphoid progenitors [63, 78••, 79••], ruling out a role for osteoblasts in BM-HSC maintenance. Another recent study revealed how high-fat diet alters gut microbiota, altering the HSC niche by shifting MSC differentiation into adipocytes via activation of PPAR-γ and decreasing the population of osteoblasts, finally altering hematopoiesis [80•]. Nevertheless, one of the main problems still pending to be solved in the study of the endosteal niche is the lack of specificity of the genetic promoters that define each mesenchymal lineage subset [81].
Taken together these observations may suggest that differentiating osteoblasts or osteolineage cells play a dual role in HSC regulation: the most immature subset would influence HSC proliferation, while the mature osteoblast subset would favor HSC differentiation along the lymphoid, myeloid, and erythroid lineages. As of today, the prevalent idea is that there are several specialized niches for different types of HSC and that multiple cell types might control each of those niches. There is indeed an overlap between perivascular and endosteal niches that impacts HSC function, involving also endothelial and mesenchymal cells. The discrete BM region occupied by the HSC provides a unique “cocktail” of signals that finally determine its behavior and its different biological properties.
Hematological Malignancies and the Osteoblastic Niche
Deregulation of hematopoiesis is a hallmark of malignant diseases such as leukemia, or MDS. In general, those arise from stem cells or more committed progenitors that undergo several stages of evolution during which they accumulate genetic and/or epigenetic alterations [82,83,84,85]. These events contribute to tumorigenic transformation (involving hyperproliferation, deregulation of apoptosis, immune evasion, telomere retention, and/or upregulation/downregulation of either oncogenes or tumor suppressor genes, respectively), clonal expansion, and establishment of malignancy. In leukemia, these cells also termed leukemia stem cells (LSCs), similar to HSCs, reside in specific niches that help them maintain their properties, escape the immune system, preserve their phenotypic plasticity, and facilitate their metastatic potential. The niche once again, plays a crucial role in tumor initiation and progression. Importantly, since LSCs are able to survive many commonly employed cancer therapies, targeting the niche components involved in MDS or AML establishment and/or transformation may provide an additional therapeutic approach.
AML is the most common adult leukemia, characterized by the clonal expansion of immature myeloblasts initiating from rare LSCs. MDS is a heterogeneous group of hematological disorders associated with defective hematopoiesis. MDS represent a diverse cluster of clonal disorders with aberrant myeloid differentiation and dysplasia, showing an increased risk of transformation into AML. The genetics events that ultimately lead to the MDS/AML development are among the ones most widely studied for human disease. However, despite decades of research, MDS/AML remains considered as a difficult-to-treat cancer, mainly due to its refractory nature to targeted therapy.
The crucial role played by the stromal cells of the niche was shown by the fact that genetic and functional alterations in those cells are able to both affect and induce myeloid malignancies. Ablation of osteoblastic cells leads to accelerated leukemia progression in several murine models of leukemia that include AML, chronic myeloid leukemia (CML), myelomonocytic leukemia, and lymphoblastic leukemia [21•]. There is evidence that leukemic cells actively inhibit osteoblastic cells (or induce the differentiation of functionally altered osteoblasts), likely through inflammatory mediators such as TPO or the chemokine CCL3 (also known as MIP-1α) to compromise their ability to maintain normal HSCs, but effectively support LSCs [86, 87••]. Similarly, AML and MDS decrease osteoblast numbers both in patients and in mouse models of AML [21•]. The reinstatement of osteoblasts numbers and function by pharmacological approach—with inhibitors of gut-derived serotonin—reduces tumor burden in all sites and prolongs survival [21•].
Furthermore, under certain conditions, osteolineage cells have been demonstrated to trigger the formation of leukemia initiating cells in the hematopoietic lineage. Primary stromal dysfunction can result in secondary neoplastic disease, supporting the concept of niche-induced oncogenesis [18, 20••, 60]. Osteoblasts were directly implicated in the development of myeloid malignancies when it was shown that global disruption of gene expression in osteoblast progenitors by deletion of Dicer1 leads to MDS in mice [18], and constitutive activation of β-catenin signaling in osteoblasts disrupts hematopoiesis, altering the differentiation potential of myeloid and lymphoid progenitors, and initiating the development of MDS, rapidly progressing to AML [20••]. In the case of overexpression of constitutively active β-catenin, the development of AML-like hematopoietic malignancy requires overexpression of the Notch ligand Jagged-1 on osteoblastic cells and leads to clonal expansion characterized by recurrent chromosomal aberrations and somatic mutations [20••, 60]. As a result, AML can be transferred to healthy irradiated recipients. These findings are relevant to human disease, since 38% of AML and MDS/AML patient, show activation of the β-catenin/Jagged-1 leukemogenic signaling in osteoblasts. Manipulating the osteoblast or its progenitor at different stages of the lineage can differentially affect the functions and influences of these cells in whole body physiology. Indeed, activation β-catenin in osteocytes enhances components of the Notch signaling pathway, but does not alter hematopoiesis or survival [88]. In addition to the fact that disparate outcomes of β-catenin activation depend on the stage of the osteolineage at which activation occurs, these observations may also reveal another important component in the mode of osteoblastic β-catenin-induced AML. They may indicate that cell-to-cell interaction between osteoblast and HSC is required for AML to develop. Further supporting the role of the stromal niche in hematological myeloid malignancies, in another study, genetic manipulation of mesenchymal stem/progenitor cells and osteoprogenitors using the Nestin promoter, showed that induction of activating mutations of the protein tyrosine phosphatase SHP2 promotes the development and progression to a myeloproliferative neoplasm (MPN) [89••]. Interestingly, administration of CCL3 receptor antagonists effectively reverses MPN development [89••]. In addition, a Schwachman-Diamond syndrome mutation in stromal cells drives MDS through TLR signaling that predicts AML progression in patients [90].
These studies highlight both the importance of osteolineage cells for the maintenance of a benign HSC population, and the potential that the primary defect in many hematologic malignancies may lay in the supportive BM microenvironment. Together, these observations suggest that functional changes in osteoblasts and/or osteoprogenitors might eventually impact on their ability to regulate hematopoiesis. Conversely, leukemic myeloid cells were shown to stimulate osteoblast expansion into myeloproliferative cells that effectively support expansion of LSCs [87••]. Therefore, a crosstalk exists between the stromal niche and the pre-malignant HSC or the LSC. Deregulation of specific signaling pathways in stromal cells including osteoblasts, can trigger transformation of healthy HSCs to malignant ones; and, signals from LSCs can remodel the stromal niche to favor self-perpetuation.
Remodeling of the BM niche has been reported in blood malignancies. AML and acute lymphoblastic leukemia (ALL) cells target the HSC niche by directly competing with HSCs for occupancy of the benign HSC niche [91, 92]. Moreover, increasing the niche size promotes leukemia engraftment, while decreasing it compromises dissemination. Once in the niche, LSCs reduce HSC numbers by driving their terminal differentiation. Studies in xenograft and syngeneic models of AML and ALL have suggested that growth of leukemic cells disrupts the BM niches of normal HSC and normal interactions between HSCs and their microenvironment, making this niche hospitable to them [91, 92]. Moreover, LSCs are able to communicate with BM stromal cells through cytokines, chemokines, and intracellular signals, and those interactions can protect them from chemotherapy-induced apoptosis, leading to relapse of the disease [93, 94]. In AML models, this protection can be abrogated by inhibition of the CXCL12/CXCR4 axis [95, 96]. In models of ALL, it was demonstrated that following chemotherapy, residual leukemic cells reside in a specialized niche that is dependent on leukemic production of CCL3 and TGFβ-1 [97••]. These observations suggest that disruption of the interactions between LSCs and their microenvironment may represent a promising strategy of compromising LSCs by targeting their BM microenvironment to restore normal hematopoiesis.
Conclusions
The importance of the endosteal microenvironment in the regulation of hematopoiesis—and thus in hematological malignancies—is starting to be elucidated, setting the groundwork for direct translation into the treatment of MDS, refractory leukemia, metastasis, and hematopoietic recovery after stress or injury. Several studies indicate that multiple cell populations of the BM microenvironment intersect to regulate the complex of HSC fate. There is also evidence that the malignant hematopoietic cells alter the number and function of specific stromal cell populations in the BM in an effort to expand and resist treatment.
With multiple anatomically and developmentally distinct specialized niches providing support for different and separate populations of HSCs or different HSC fates, as well as a plethora of secreted molecules, the BM niche, comprised of many cell types, may also provide several potential therapeutic targets. Moreover, since hematological malignancies “hijack” the healthy HSC niche, the identification of the different niche components and their roles could also be exploited in the treatment of hematological malignancies or prevention of relapse with niche-targeted therapies.
Characterization of the different cells and their roles in the HSC niche will likely continue to rapidly progress over the next few years to increase our understanding of the complex cellular relationships within the BM. Although the interplay between the hematopoietic and non-hematopoietic compartments is likely more complex than we imagine, common principles will guide the design of new approaches to tackle hematologic diseases. Additionally, it is important to delineate the extrinsic influences from the supportive niche that change under different physiological conditions, such as aging. Increased understanding of the aging process in the BM niche may also uncover critical information for the treatment of age-related BM illnesses like MDS/AML. Likewise, understanding how the niche controls hematopoiesis during stress in the acute settings of trauma, surgery, sepsis, radio- and chemotherapy, and/or stem cell transplantation may prove crucial for improving blood cell production and survival.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Jacobson LO, Simmons EL, Marks EK, Eldredge JH. Recovery from Radiation Injury. Science. American Association for the Advancement of Science; 1951;113:510–1.
McCulloch EA, Till JE. The radiation sensitivity of normal mouse bone marrow cells, determined by quantitative marrow transplantation into irradiated mice. Radiat Res. 1960;13:115–25.
Yamamoto R, Morita Y, Ooehara J, Hamanaka S, Onodera M, Rudolph KL, et al. Clonal analysis unveils self-renewing lineage-restricted progenitors generated directly from hematopoietic stem cells. Cell. 2013;154:1112–26.
Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62.
Yang L. Identification of Lin-Sca1+kit+CD34+Flt3- short-term hematopoietic stem cells capable of rapidly reconstituting and rescuing myeloablated transplant recipients. Blood. 2005;105:2717–23.
Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25.
Sauvageau G, Iscove NN, Humphries RK. In vitro and in vivo expansion of hematopoietic stem cells. Oncogene. 2004;23:7223–32.
Bodine DM, Karlsson S, Nienhuis AW. Combination of interleukins 3 and 6 preserves stem cell function in culture and enhances retrovirus-mediated gene transfer into hematopoietic stem cells. Proc Natl Acad Sci. 1989;86:8897–901.
Ku H, Yonemura Y, Kaushansky K, Ogawa M. Thrombopoietin, the ligand for the Mpl receptor, synergizes with steel factor and other early acting cytokines in supporting proliferation of primitive hematopoietic progenitors of mice. Blood. 1996;87:4544–51.
Matsunaga T, Kato T, Miyazaki H, Ogawa M. Thrombopoietin promotes the survival of murine hematopoietic long-term reconstituting cells: comparison with the effects of FLT3/FLK-2 ligand and interleukin-6. Blood. 1998;92:452–61.
Miller CL, Eaves CJ. Expansion in vitro of adult murine hematopoietic stem cells with transplantable lympho-myeloid reconstituting ability. Proc Natl Acad Sci. 1997;94:13648–53.
Ema H, Takano H, Sudo K, Nakauchi H. In vitro self-renewal division of hematopoietic stem cells. J Exp Med. 2000;192:1281–8.
Himburg HA, Muramoto GG, Daher P, Meadows SK, Russell JL, Doan P, et al. Pleiotrophin regulates the expansion and regeneration of hematopoietic stem cells. Nat Med. 2010;16:475–82.
Zhang CC, Kaba M, Ge G, Xie K, Tong W, Hug C, et al. Angiopoietin-like proteins stimulate ex vivo expansion of hematopoietic stem cells. Nat Med. 2006;12:240–5.
Zhang CC, Lodish HF. Insulin-like growth factor 2 expressed in a novel fetal liver cell population is a growth factor for hematopoietic stem cells. Blood. 2004;103:2513–21.
de Haan G, Weersing E, Dontje B, van Os R, Bystrykh LV, Vellenga E, et al. In vitro generation of long-term repopulating hematopoietic stem cells by fibroblast growth factor-1. Dev Cell. 2003;4:241–51.
Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–34.
Raaijmakers MHGP, Mukherjee S, Guo S, Zhang S, Kobayashi T, Schoonmaker JA, et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. 2010;464:852–7.
Walkley CR, Olsen GH, Dworkin S, Fabb SA, Swann J, McArthur GA, et al. A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor γ deficiency. Cell. 2007;129:1097–110.
•• Kode A, Manavalan JS, Mosialou I, Bhagat G, Rathinam CV, Luo N, et al. Leukaemogenesis induced by an activating β-catenin mutation in osteoblasts. Nature. 2014;506:240–4. This study showed for the first time that a mutation in osteoblasts leads to MDS rapidly progressing to AML with clonal cytogenetic abnormalities. The leukemogenic pathway is active in a subpopulation of MDS and AML patients .
• Krevvata M, Silva BC, Manavalan JS, Galán-Díez M, Kode A, Matthews BG, et al. Inhibition of leukemia cell engraftment and disease progression in mice by osteoblasts. Blood. 2014;124:2834–46. This paper indicates that osteoblast numbers are compromised in patients with MDS and AML and osteoblasts are protective against AML engraftment and progression in mice .
Gong JK. Endosteal marrow: a rich source of hematopoietic stem cells. Science. 1978;199:1443–5.
Taichman RS, Emerson SG. Human osteoblasts support hematopoiesis through the production of granulocyte colony-stimulating factor. The Journal of Experimental Medicine. Rockefeller University Press; 1994;179:1677–82.
Lord BI, Testa NG, Hendry JH. The relative spatial distributions of CFUs and CFUc in the normal mouse femur. Blood. 1975;46:65–72.
El-Badri N, Wang BY, Cherry, Good RA. Osteoblasts promote engraftment of allogeneic hematopoietic stem cells. Exp Hematol. 1998;26:110–6.
Miyamoto K, Yoshida S, Kawasumi M, Hashimoto K, Kimura T, Sato Y, et al. Osteoclasts are dispensable for hematopoietic stem cell maintenance and mobilization. J Exp Med. 2011;208:2175–81.
Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:836–41.
Zhang J, Niu C, Ye L, Huang H, He X, Tong W-G, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:832–6.
Nilsson SK. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood. 2005;106:1232–9.
Stier S, Ko Y, Forkert R, Lutz C, Neuhaus T, Grünewald E, et al. Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. J Exp Med. 2005;201:1781–91.
Bowers M, Zhang B, Ho Y, Agarwal P, Chen C-C, Bhatia R. Osteoblast ablation reduces normal long-term hematopoietic stem cell self-renewal but accelerates leukemia development. Blood American Society of Hematology. 2015;125:2678–88.
Beate Heissig, Koichi Hattori, Dias S, Friedrich M, Barbara Ferris, Hackett NR, et al. Recruitment of Stem and Progenitor Cells from the Bone Marrow Niche Requires MMP-9 Mediated Release of Kit-Ligand. Cell. 2002:1–22.
Nilsson SK, Johnston HM, Coverdale JA. Spatial localization of transplanted hemopoietic stem cells: inferences for the localization of stem cell niches. Blood. 2001;97:2293–9.
Celso Lo C, Fleming HE, Wu JW, Zhao CX, Miake-Lye S, Fujisaki J, et al. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2009;457:92–6.
Xie Y, Yin T, Wiegraebe W, He XC, Miller D, Stark D, et al. Detection of functional haematopoietic stem cell niche using real-time imaging. Nature. 2009;457:97–101.
Zhang J, Niu C, Ye L, Huang H, He X, Tong W-G, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–41.
Kim S, Lin L, Brown GAJ, Hosaka K, Scott EW. Extended time-lapse in vivo imaging of tibia bone marrow to visualize dynamic hematopoietic stem cell engraftment. Leukemia. 2017.
Grassinger J, Haylock DN, Williams B, Olsen GH, Nilsson SK. Phenotypically identical hemopoietic stem cells isolated from different regions of bone marrow have different biologic potential. Blood. 2010;116:3185–96.
Haylock DN, Williams B, Johnston HM, Liu MCP, Rutherford KE, Whitty GA, et al. Hemopoietic stem cells with higher hemopoietic potential reside at the bone marrow endosteum. Stem Cells. 2007;25:1062–9.
• Acar M, Kocherlakota KS, Murphy MM, Peyer JG, Oguro H, Inra CN, et al. Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal. Nature. 2015;526:126–30. This study shows that quiescent HSC are more associated with BM sinusoids rather than arterioles and that, Leptin receptor expressing cells play and important role in HSC quiescence maintenance.
•• Chen JY, Miyanishi M, Wang SK, Yamazaki S, Sinha R, Kao KS, et al. Hoxb5 marks long-term haematopoietic stem cells and reveals a homogenous perivascular niche. Nature. 2016;530:223–7. This paper identifies a unique marker to mark LT-HSCs, the HSCs that replicate indefinitely and are critical to lifelong blood production.
Tokoyoda K, Egawa T, Sugiyama T, Choi B-I, Nagasawa T. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity. 2004;20:707–18.
Zhu J, Garrett R, Jung Y, Zhang Y, Kim N, Wang J, et al. Osteoblasts support B-lymphocyte commitment and differentiation from hematopoietic stem cells. Blood. 2007;109:3706–12.
Visnjic D, Kalajzic Z, Rowe DW, Katavic V, Lorenzo J, Aguila HL. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004;103:3258–64.
Wu JY, Purton LE, Rodda SJ, Chen M, Weinstein LS, McMahon AP, et al. Osteoblastic regulation of B lymphopoiesis is mediated by Gsa-dependent signaling pathways. Proceedings of the National Academy of Sciences; 2008;105:16976–81.
Day RB, Bhattacharya D, Nagasawa T, Link DC. Granulocyte colony-stimulating factor reprograms bone marrow stromal cells to actively suppress B lymphopoiesis in mice. Blood; 2015;125:3114–7.
Morad V, Pevsner-Fischer M, Barnees S, Samokovlisky A, Rousso-Noori L, Rosenfeld R, et al. The myelopoietic supportive capacity of mesenchymal stromal cells is uncoupled from multipotency and is influenced by lineage determination and interference with glycosylation. Stem Cells. 2008;26:2275–86.
Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–36.
He Q, Scott Swindle C, Wan C, Flynn RJ, Oster RA, Chen D, et al. Enhanced hematopoietic stem cell self-renewal-promoting ability of clonal primary mesenchymal stromal/stem cells versus their osteogenic progeny. Stem Cells. 2017;35:473–84.
Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–34.
Calvi LM, Bromberg O, Rhee Y, Weber JM, Smith JNP, Basil MJ, et al. Osteoblastic expansion induced by parathyroid hormone receptor signaling in murine osteocytes is not sufficient to increase hematopoietic stem cells. Blood. 2012;119:2489–99.
• Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D, et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502:637–43. This paper characterizes histologically the different vascular structure subtypes and show that the peri-arteriolar mesenchymal cells control HSC maintenance.
Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–61.
Yoshihara H, Arai F, Hosokawa K, Hagiwara T, Takubo K, Nakamura Y, et al. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell. 2007;1:685–97.
Jung Y, Wang J, Song J, Shiozawa Y, Wang J, Wang J, et al. Annexin II expressed by osteoblasts and endothelial cells regulates stem cell adhesion, homing, and engraftment following transplantation. Blood. 2007;110:82–90.
Ponomaryov T, Peled A, Petit I, Taichman RS, Habler L, Sandbank J, et al. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J Clin Invest. 2000;106:1331–9.
Weber JM, Forsythe SR, Christianson CA, Frisch BJ, Gigliotti BJ, Jordan CT, et al. Parathyroid hormone stimulates expression of the notch ligand Jagged1 in osteoblastic cells. Bone. 2006;39:485–93.
Milner LA, Bigas A. Notch as a mediator of cell fate determination in hematopoiesis: evidence and speculation. Blood. 1999;93:2431–48.
Karanu FN, Murdoch B, Gallacher L, Wu DM, Koremoto M, Sakano S, et al. The notch ligand jagged-1 represents a novel growth factor of human hematopoietic stem cells. J Exp Med. 2000;192:1365–72.
Kode A, Mosialou I, Manavalan SJ, Rathinam CV, Friedman RA, Teruya-Feldstein J, et al. FoxO1-dependent induction of acute myeloid leukemia by osteoblasts in mice. Leukemia. 2016;30:1–13.
Chitteti BR, Cheng Y-H, Poteat B, Rodriguez-Rodriguez S, Goebel WS, Carlesso N, et al. Impact of interactions of cellular components of the bone marrow microenvironment on hematopoietic stem and progenitor cell function. Blood. American Society of Hematology; 2010;115:3239–48.
Maillard I, Koch U, Dumortier A, Shestova O, Xu L, Sai H, et al. Canonical notch signaling is dispensable for the maintenance of adult hematopoietic stem cells. Cell Stem Cell. 2008;2:356–66.
Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–62.
Grassinger J, Haylock DN, Storan MJ, Haines GO, Williams B, Whitty GA, et al. Thrombin-cleaved osteopontin regulates hemopoietic stem and progenitor cell functions through interactions with alpha9beta1 and alpha4beta1 integrins. Blood. 2009;114:49–59.
Puri MC, Bernstein A. Requirement for the TIE family of receptor tyrosine kinases in adult but not fetal hematopoiesis. Proceedings of the National Academy of Sciences. 2003;100:12753–8.
Frisch BJ, Porter RL, Gigliotti BJ, Olm-Shipman AJ, Weber JM, O'Keefe RJ, et al. In vivo prostaglandin E2 treatment alters the bone marrow microenvironment and preferentially expands short-term hematopoietic stem cells. Blood. 2009;114:4054–63.
North TE, Goessling W, Walkley CR, Lengerke C, Kopani KR, Lord AM, et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–11.
Goessling W, Allen RS, Guan X, Jin P, Uchida N, Dovey M, et al. Prostaglandin E2 enhances human cord blood stem cell xenotransplants and shows long-term safety in preclinical nonhuman primate transplant models. Cell Stem Cell. 2011;8:445–58.
Hoggatt J, Singh P, Sampath J, Pelus LM. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood. 2009;113:5444–55.
Hoggatt J, Mohammad KS, Singh P, Hoggatt AF, Chitteti BR, Speth JM, et al. Differential stem- and progenitor-cell trafficking by prostaglandin E2. Nature. 2013;495:365–9.
Porter RL, Georger MA, Bromberg O, McGrath KE, Frisch BJ, Becker MW, et al. Prostaglandin E2 increases hematopoietic stem cell survival and accelerates hematopoietic recovery after radiation injury. Stem Cells. 2013;31:372–83.
Spencer JA, Ferraro F, Roussakis E, Klein A, Wu J, Runnels JM, et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature. 2014;508:269–73.
Takubo K, Goda N, Yamada W, Iriuchishima H, Ikeda E, Kubota Y, et al. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell. 2010;7:391–402.
Pollard PJ, Kranc KR. Hypoxia signaling in hematopoietic stem cells: a double-edged sword. Cell Stem Cell. 2010;7:276–8.
Adams GB, Chabner KT, Alley IR, Olson DP, Szczepiorkowski ZM, Poznansky MC, et al. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439:599–603.
Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–54.
Lee B, Thirunavukkarasu K, Zhou L, Pastore L, Baldini A, Hecht J, et al. Missense mutations abolishing DNA binding of the osteoblast-specific transcription factor OSF2/CBFA1 in cleidocranial dysplasia. Nat Genet. 1997;16:307–10.
•• Greenbaum A, Hsu Y-MS, Day RB, Schuettpelz LG, Christopher MJ, Borgerding JN, et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495:227–30. This study performed a systematic analysis of the BM-CXCL12-expressing, demonstrating the specific role of MSCs and endothelial cells in regulating HSC maintenance .
•• Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495:231–5. This paper is another systematic analysis of CXCL12-expressing cells in the BM and demonstrated that HSC and restricted progenitors depend on distinct cellular niches .
• Luo Y, Chen G-L, Hannemann N, Ipseiz N, Krönke G, Bäuerle T, et al. Microbiota from obese mice regulate hematopoietic stem cell differentiation by altering the bone niche. Cell Metab. 2015;22:886–94. This paper shows that altering gut microbiota by feeding the mice a high fat diet can disrupt HSC quiescence by influencing the HSC niche through altering stromal cell populations, increasing MSC differentiation towards adipocytes rather than osteoblasts .
Joseph C, Quach JM, Walkley CR, Lane SW, Celso Lo C, Purton LE. Deciphering hematopoietic stem cells in their niches: a critical appraisal of genetic models, lineage tracing, and imaging strategies. Cell Stem Cell. 2013;13:520–33.
Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11.
Hope KJ, Jin L, Dick JE. Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nat Immunol. 2004;5:738–43.
Scadden DT. Cancer stem cells refined. Nat Immunol. 2004;5:701–3.
Passegué E, Jamieson CHM, Ailles LE, Weissman IL. Normal and leukemic hematopoiesis: are leukemias a stem cell disorder or a reacquisition of stem cell characteristics? Proceedings of the National Academy of Sciences. 2003;100 Suppl 1:11842–9.
Frisch BJ, Ashton JM, Xing L, Becker MW, Jordan CT, Calvi LM. Functional inhibition of osteoblastic cells in an in vivo mouse model of myeloid leukemia. Blood. 2012;119:540–50.
•• Schepers K, Pietras EM, Reynaud D, Flach J, Binnewies M, Garg T, et al. Myeloproliferative Neoplasia Remodels the Endosteal Bone Marrow Niche into a Self-Reinforcing Leukemic Niche. Stem Cell. Elsevier Inc; 2013;13:285–99. This study demonstrated that dysplastic myeloproliferative cells can reprogram osteoblast in the endosteal niche to favor self-perpetuation .
Tu X, Delgado-Calle J, Condon KW, Maycas M, Zhang H, Carlesso N, et al. Osteocytes mediate the anabolic actions of canonical Wnt/β-catenin signaling in bone. Proc Natl Acad Sci. 2015;112:E478–86.
•• Dong L, Yu W-M, Zheng H, Loh ML, Bunting ST, Pauly M, et al. Leukaemogenic effects of Ptpn11 activating mutations in the stem cell microenvironment. Nature. 2016;539:304–8. This study identifies a mutation in the Nestin-expressing stromal niche that can lead to leukemia.
Zambetti NA, Ping Z, Chen S, Kenswil KJG, Mylona MA, Sanders MA, et al. Mesenchymal inflammation drives genotoxic stress in hematopoietic stem cells and predicts disease evolution in human pre-leukemia. Cell Stem Cell. 2016;19:613–27.
Colmone A, Amorim M, Pontier AL, Wang S, Jablonski E, Sipkins DA. Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science. 2008;322:1861–5.
Glait-Santar C, Desmond R, Feng X, Bat T, Chen J, Heuston E, et al. Functional niche competition between normal hematopoietic stem and progenitor cells and myeloid leukemia cells. Stem Cells. 2015;33:3635–42.
Lane SW, Wang YJ, Celso Lo C, Ragu C, Bullinger L, Sykes SM, et al. Differential niche and Wnt requirements during acute myeloid leukemia progression. Blood. 2011;118:2849–56.
Ishikawa F, Yoshida S, Saito Y, Hijikata A, Kitamura H, Tanaka S, et al. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol. 2007;25:1315–21.
Nervi B, Ramirez P, Rettig MP, Uy GL, Holt MS, Ritchey JK, et al. Chemosensitization of acute myeloid leukemia (AML) following mobilization by the CXCR4 antagonist AMD3100. Blood. 2009;113:6206–14.
Zeng Z, Shi YX, Samudio IJ, Wang R-Y, Ling X, Frolova O, et al. Targeting the leukemia microenvironment by CXCR4 inhibition overcomes resistance to kinase inhibitors and chemotherapy in AML. Blood. 2009;113:6215–24.
•• Duan C-W, Shi J, Chen J, Wang B, Yu Y-H, Qin X, et al. Leukemia propagating cells rebuild an evolving niche in response to therapy. Cancer Cell. 2014;25:778–93. This study highlights the role of the leukemia stromal niche in resistance to therapy and its regulation by leukemic cells .
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Marta Galán-Díez and Stavroula Kousteni declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Molecular Biology of Skeletal Development
Rights and permissions
About this article
Cite this article
Galán-Díez, M., Kousteni, S. The Osteoblastic Niche in Hematopoiesis and Hematological Myeloid Malignancies. Curr Mol Bio Rep 3, 53–62 (2017). https://doi.org/10.1007/s40610-017-0055-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40610-017-0055-9