Abstract
Background
Previous observational studies have revealed a potentially robust bidirectional relationship between frailty and low back pain (LBP). However, the precise causal relationship remains unclear.
Methods
To examine the potential causal association between frailty and LBP, we conducted bidirectional two-sample Mendelian randomization analysis (MR) study. Genetic data on frailty index (FI) and LBP were acquired from publicly available genome-wide association studies (GWAS). Various MR methodologies were utilized, such as inverse variance weighting (IVW), weighted median, and MR-Egger, to evaluate causality. Additionally, sensitivity analyses were conducted to evaluate the robustness of the findings.
Results
Genetically predicted higher FI (IVW, odds ratio [OR] = 1.66, 95% CI 1.17–2.36, p = 4.92E-03) was associated with a higher risk of LBP. As for the reverse direction, genetic liability to LBP showed consistent associations with a higher FI (IVW, OR = 1.13, 95% CI 1.07–1.19, p = 2.67E-05). The outcomes from various MR techniques and sensitivity analyses indicate the robustness of our findings.
Conclusion
Our research findings provide additional evidence bolstering the bidirectional causal relationship between frailty and LBP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Low back pain (LBP) is characterized by pain and discomfort in the area beneath the ribcage, above the buttock crease, and between the mid-axillary lines, with or without associated leg pain [1]. According to a comprehensive systematic review of 165 studies conducted across 54 countries, the estimated point prevalence of LBP ranged from 11.9 to 13.9% [2]. LBP has emerged as one of the primary causes of disability and work absences worldwide [3]. It represents a significant public health concern and imposes a considerable economic burden on society [4, 5]. In the United States, the annual financial burden attributed to LBP surpasses $100 billion, encompassing expenses related to medical treatments, lost wages, and reduced productivity [6]. An increasing number of medical practice guidelines recommend multiple treatments to alleviate pain and mitigate its consequences in the management of LBP [7, 8]. Considering the substantial global prevalence and significant burden associated with LBP, there is an urgent imperative to elucidate potential causal risk factors for LBP.
Frailty denotes a multifaceted clinical syndrome characterized by diminished physiological capacity in multiple organs or systems, coupled with heightened vulnerability to stress [9]. With the aging of populations, frailty is progressively increasing on a global scale. It is associated with adverse health outcomes including multimorbidity, disability, and increased mortality rates [10]. The Frailty Index (FI) is acknowledged as a reliable and effective instrument for identifying individuals who are at risk of developing frailty [11]. It is a continuous metric that quantifies frailty based on the proportion of health deficits attributable to the ageing process as a proportion of all deficits. These deficits may manifest as symptoms, signs, diseases, disabilities or abnormalities, which can be identified through laboratory tests, radiological imaging or even social factors [12]. Previous studies have shown a significant bidirectional correlation between frailty and LBP. Over a 2-year period, a significant correlation was observed in an Asian population between frailty and the prevalence of LBP. The prevalence of LBP was significantly higher in both pre-frail and frail groups compared to healthy individuals [13]. Leopoldino et al. showed that in older adults with LBP, frailty led to more disability and lower physical status scores for quality of life [14]. Coyle et al. showed that older adults with LBP were more likely to be frail than those without LBP [15]. However, whether there is a causal relationship between frailty and LBP remains unclear, and there is an urgent need for large-sample randomized controlled trials (RCTs).
However, due to methodological challenges and ethical constraints, conducting RCTs might not be feasible. In such circumstances, Mendelian randomization (MR), an epidemiological research strategy, can be employed to assess the causal relationship between exposure and outcome [16]. This approach emulates the methodological design of RCT studies, providing high-level evidence when direct RCTs are difficult to conduct. In MR, single nucleotide polymorphisms (SNPs) are utilized as instrumental variables (IVs) to assess causal effects between exposure and outcome [17]. Since the genotypes are established during conception, MR is generally not susceptible to reverse causation or confounding factors [18, 19]. This advantage has led to wide utilization of MR methodology for inferring causality, particularly using publicly available data from genome-wide association studies (GWAS). Recent investigations into the connection between frailty and various diseases using MR [20, 21], no study has yet reported a causal relationship between frailty and LBP. Therefore, this study used a two-sample MR approach to assess the potential bidirectional causality between frailty and LBP by obtaining GWAS data on large-scale FI and LBP.
Methods
Study design
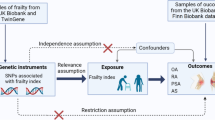
Figure 1 presents an overview of the study design. This study utilized non-overlapping GWAS summary data within a standard two-sample MR framework to investigate the bidirectional causal relationship between frailty and LBP. All the data used in this study is publicly available, and the original study received ethical clearance and informed consent. MR is a data analysis technique utilized to assess etiological inferences in epidemiological studies. It employs genetic variants that demonstrate a strong correlation with exposure factors as IVs. The analysis relies on three fundamental assumptions: (1) The assumption of association: A robust association exists between SNPs and exposure factors. (2) The assumption of independence: Independence is present between SNPs and confounders. (3) The assumption of exclusivity: SNPs exclusively affect outcomes through exposure factors. These assumptions play a vital role in the accurate interpretation of causal relationships in MR studies.
Data sources and genetic instrument selection
Detailed information is shown in Table 1. Summary statistics of frailty, assessed by the FI phenotype, were obtained through a comprehensive meta-analysis of GWAS conducted in the United Kingdom Biobank and Swedish TwinGene cohorts, which included 175,226 individuals of European ancestry [22]. The FI served as the proxy indicator of overall health; it is based on the accumulation of age-related deficits [23]. The FI was calculated on the basis of 49 and 44 self-reported items according to the cumulative error theory of the UK Biobank and TwinGene, respectively [22, 24]. The summary data for GWAS on LBP were obtained from the FinnGen dataset, which comprised 300,293 individuals of European ancestry. The identification of LBP was based on the International Classification of Diseases (ICD) codes obtained from nationwide registries in Finland (ICD10 - M54.5).
In conducting the MR analysis, we meticulously assessed the IVs used. To address the three main hypotheses of MR described earlier, we conducted a screening for SNPs that demonstrated a strong association with exposure using a stringent threshold (p < 5E-08) [25]. Additionally, we excluded weak IVs to prevent potential bias, including only IVs with an F statistic greater than 10. Furthermore, we performed clustering (r2 = 0.001, cluster distance = 10,000 kb) to address the potential bias caused by apparent linkage disequilibrium (LD) among the selected SNPs [26]. This step aimed to eliminate LD among the included IVs.
Statistical analysis
Before conducting the MR analysis, we initially conducted the MR-Platform for Robust Estimation of Errors in Causality Testing (MR-PRESSO) test to identify any outliers. Following the identification and removal of these outliers, we proceeded with the MR analysis. The MR-PRESSO procedure was performed with a cycle number of 10,000 and P < 0.05 was used as a threshold to detect and remove outliers.
Given that inverse variance weighting (IVW) is known to offer accurate and stable results, we employed IVW as the principal analytical approach [27]. IVW is an extension of the Wald ratio estimator, founded on the principles of meta-analysis. Additionally, we employ the MR Egger and weighted median methods as supplementary approaches to MR. The variation in assumptions between these tests leads to a higher level of robustness when consistent effects are observed across multiple methods. The significance threshold was set at p < 0.05. A series of sensitivity analyses was subsequently conducted. The MR-Egger intercept test and Cochran’s Q statistic were employed to assess the presence of horizontal pleiotropy and heterogeneity, respectively [28, 29]. Additionally, a leave-one-out analysis was employed to assess the influence of individual SNPs on the overall estimates. The analyses in this study were conducted using R software (version 4.2.1). We utilized the “Two Sample MR” R package for our MR study [30].
Results
Instrumental variables for mendelian randomization
This study investigated the bidirectional causal relationship between frailty and the risk of LBP through two-sample MR. To assess the impact of frailty on the risk of LBP, we initially incorporated a set of 15 SNPs as IVs strongly linked to a FI. Furthermore, in the reverse MR analysis, we screened 11 SNPs as IVs specifically for LBP. All individual SNPs exhibited an F-statistic exceeding 10, signifying adequate instrumental strength. One SNP was lost when the outcome variable was merged. When investigating the effect of FI on the risk of developing LBP, we eliminated 2 SNPs by identifying outliers using MR-PRESSO, whereas no SNPs were eliminated at this step in reverse MR, and we finally included 12 and 10 SNPs, respectively, as IVs in the investigation. Tables S1 and S2 provide comprehensive details on the IVs, and Table S3 provides information on the outliers.
The effect of frailty on the risk of low back pain
The results of the MR analysis indicate a causal relationship between FI and LBP. According to the primary method of MR, the IVW results showed a significant association between genetically predicted higher FI.
and an increased risk of LBP (IVW, OR = 1.66, 95% CI 1.17–2.36, p = 4.92E-03; Table 2, Figure S1). Additionally, even though MR Egger and Weighted median did not yield consistent results compared to IVW, the beta values remained consistent across all methods (Fig. 2). Given the precision and robustness of IVW, we maintain a positive interpretation of the MR results. While Cochran’s Q statistic detected heterogeneity (Q = 20.60, p < 0.05, Table 3), the MR-Egger intercept suggested that horizontal pleiotropy did not influence the outcome in any analysis (intercept p value = -8.72E-04, P > 0.05, Table 3). Furthermore, the funnel plot (Figure S3) is symmetric, and the leave-one-out (Figure S2) results indicated that the MR results were not influenced by a single SNP.
Results of reverse mendelian randomization analysis
In the reverse direction, there were significant associations between genetic liability to LBP and a higher FI (IVW, OR = 1.13, 95% CI 1.07–1.19, p = 2.67E-05; Table 2, Figure S1). The weighted median method yielded similar results. Although the results of MR Egger did not support the above hypothesis, we still conclude that LBP increases the risk of elevated FI. This conclusion is based on the lower precision of the MR-Egger method compared to other methods and the consistent direction of the beta value across all methods (Fig. 2). Cochran’s Q statistical test reveal significant heterogeneity in causality estimates among the IVs (Q = 16.96, p < 0.05, Table 3). However, the MR Egger intercept analysis found no evidence of directed pleiotropy (intercept p value = -0.005, p > 0.05, Table 3). Additionally, Additionally, The results of “leave one out” indicate that there is no single SNP that has a large role in driving the outcome (Figure S2). Additionally, the funnel plot provides further evidence that the study is unbiased (Figure S3).
Discussion
A two-sample MR study was conducted utilizing the publicly available GWAS summary dataset to investigate the bidirectional causal relationship between the frailty and LBP. The MR analysis revealed a bidirectional causal relationship, where FI increased the risk of developing LBP, which in turn led to an increase in FI. This study is the first to assess the causal relationship between frailty and LBP using MR. These findings provide a theoretical basis for the development of management strategies targeting frailty and LBP in elderly patients.
Frailty and LBP are common issues in older adults that can severely impact their quality of life and overall health. Numerous epidemiological studies have examined the association between these two conditions. For instance, a 12-month longitudinal study involving 165 older adults suffering from LBP revealed that over two-thirds of the participants were classified as being either pre-frail or frail. Furthermore, the researchers observed that frailty was significantly linked with increased disability in older adults affected by LBP [31]. A longitudinal observational study of older adults in Brazil revealed a significant correlation between the degree of LBP and frailty [32]. Additionally, a prospective cohort study involving 602 individuals revealed that physical frailty was associated with increased pain intensity, lower scores in both physical and psychological aspects of quality of life, and higher disability scores among individuals with LBP [14]. It is important to acknowledge that prior research has been limited in its ability to determine causation of the relationship between frailty and LBP due to the susceptibility of observational studies to reverse causation and confounding variables. Our current study provides additional support of a bidirectional causal effect between FI and LBP, using a MR approach which is less susceptible to confounding bias than traditional observational designs. The discovery of this bidirectional relationship has important implications for public health and clinical practice. Frailty and LBP are reversible conditions with many modifiable factors. By understanding the relationship between frailty and LBP, risk factors can be proactively identified and appropriate interventions can be implemented. For example, older adults with LBP can receive regular assessments and treatment to reduce the likelihood of frailty. In addition to addressing LBP, it is important to focus on frailty management, including nutritional support, exercise rehabilitation, and psychological support. Ongoing early screening and treatment of frailty and LBP in older adults, along with the development of interventions to address common risk factors, can effectively reduce the adverse outcomes associated with frailty and improve the quality of life of older adults, which can play an important role in reducing the burden on society and families.
Several potential factors may explain the bidirectional causal relationship between frailty and LBP. First, frailty may lead to undesirable consequences such as falls, reduced endurance and altered morphology of the lumbar paravertebral muscles, and ultimately LBP [33, 34]. Additionally, frailty can lead to factors such as inadequate nutrition, sleep and mood disorders, increased healthcare expenses, and reduced social interaction, which may also contribute significantly to the development of LBP [35,36,37]. Conversely, mood and sleep disorders related to LBP may also increase the risk of frailty [38]. LBP has also been linked to cognitive impairment, which may further contribute to the development of frailty [39, 40]. Moreover, treatments that are effective for frailty and LBP can have synergistic benefits. For instance, physical activity not only enhances physical function in older and vulnerable populations, but also reduces pain and disability while improving quality of life in individuals with LBP [41, 42]. Therefore, the bidirectional relationship between frailty and LBP is not a random occurrence, and all of these findings provide support for this hypothesis. The current etiological model of the bidirectional causal relationship between frailty and LBP is too intricate to attribute to one or a few factors. Hence, additional research is imperative to investigate the specific mechanisms that underlie the bidirectional causality between frailty and LBP.
The present study aimed to elucidate the bidirectional causal relationship between frailty and LBP, and it highlights the following key aspects. Firstly, this study is the first of its kind to investigate the causal relationship between frailty and LBP utilizing a comprehensive GWAS pooled dataset. Secondly, our study employed multiple sensitivity analyses to test the hypotheses, thus enhancing the reliability of our results to a certain extent. Lastly, we employed MR analysis methods to minimize the impact of confounding factors, resulting in more accurate estimates in this study. However, there are limitations associated with our findings. Specifically, our analysis exclusively focused on a European population, which limits the generalizability of our results to other populations. Second, our utilization of summary datasets impeded our ability to stratify the data by gender, age, or other relevant factors. Third, it must be recognised that MR analyses are inherently less reliable than RCTs in providing evidence of causality. To address these limitations, future large-scale GWAS studies should be conducted across ethnic groups and differentiated by gender. There is also an urgent need for more high-quality RCT studies to confirm and strengthen these findings. However, investigating the causal relationship between frailty and LBP through RCTs is ethically challenging; therefore, extended prospective cohort studies may be a viable alternative. In addition, the effectiveness of interventions tailored to address common risk factors for frailty and LBP in improving patient care management warrants further investigation in future trials.
Conclusion
This study provides support for a bidirectional causal relationship between frailty and LBP. Our findings suggest the importance of promoting frailty screening among patients with LBP. Furthermore, effective management of LBP is also crucial in mitigating the risk of frailty.
Data availability
No datasets were generated or analysed during the current study.
References
Koes BW, van Tulder MW, Thomas S (2006) Diagnosis and treatment of low back pain. BMJ 332:1430–1434. https://doi.org/10.1136/bmj.332.7555.1430
Hoy D, Bain C, Williams G et al (2012) A systematic review of the global prevalence of low back pain. Arthritis Rheum 64:2028–2037. https://doi.org/10.1002/art.34347
(2018) Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of Disease Study 2017. Lancet (London England), 392, 1789–1858. https://doi.org/10.1016/s0140-6736(18)32279-7
Daly C, Ghosh P, Jenkin G et al (2016) A review of animal models of intervertebral disc degeneration: pathophysiology, regeneration, and translation to the clinic. Biomed Res Int 2016:5952165. https://doi.org/10.1155/2016/5952165
Risbud MV, Shapiro IM (2014) Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol 10:44–56. https://doi.org/10.1038/nrrheum.2013.160
Katz JN (2006) Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am 88(Suppl 2):21–24. https://doi.org/10.2106/jbjs.E.01273
Bunzli S, Watkins R, Smith A et al (2013) Lives on hold: a qualitative synthesis exploring the experience of chronic low-back pain. Clin J Pain 29:907–916. https://doi.org/10.1097/AJP.0b013e31827a6dd8
Waterschoot FPC, Dijkstra PU, Hollak N et al (2014) Dose or content? Effectiveness of pain rehabilitation programs for patients with chronic low back pain: a systematic review. Pain 155:179–189. https://doi.org/10.1016/j.pain.2013.10.006
Dent E, Martin FC, Bergman H et al (2019) Management of frailty: opportunities, challenges, and future directions. Lancet (London England) 394:1376–1386. https://doi.org/10.1016/s0140-6736(19)31785-4
Clegg A, Young J, Iliffe S et al (2013) Frailty in elderly people. Lancet (London England) 381:752–762. https://doi.org/10.1016/s0140-6736(12)62167-9
Martin FC, O’Halloran AM (2020) Tools for assessing Frailty in Older people: General concepts. Adv exp med biol 1216:9–19
Ma L, Liu Z, Fu L et al (2024) Bidirectional causal relational between frailty and mental illness: a two-sample mendelian randomization study. Front Psychiatry 15:1397813
Tsuji S, Shinmura K, Nagai K et al (2021) Low back pain is closely associated with frailty but not with sarcopenia: cross-sectional study of rural Japanese community-dwelling older adults. Geriatr Gerontol Int 21:54–59. https://doi.org/10.1111/ggi.14100
Leopoldino AAO, Megale RZ, Diz JBM et al (2021) Influence of Frailty Status on Pain, disability, and quality of life in older adults with Acute Low Back Pain: results from the Back complaints in the elders (BACE-Brazil) Study. Can J Aging = La Revue Canadienne Du Vieillissement 40:367–375. https://doi.org/10.1017/s0714980820000288
Coyle PC, Sions JM, Velasco T et al (2015) Older adults with chronic low back Pain: a clinical Population Vulnerable to Frailty? J Frailty Aging 4:188–190. https://doi.org/10.14283/jfa.2015.75
Haycock PC, Burgess S, Wade KH et al (2016) Best (but oft-forgotten) practices: the design, analysis, and interpretation of mendelian randomization studies. Am j clin nutr 103:965–978
Tomata Y, Wang Y, Hägg S et al (2022) Protein Nutritional Status and Frailty: a mendelian randomization study. J nutr 152:269–275
Smith GD, Ebrahim S (2004) Mendelian randomization: prospects, potentials, and limitations. Int j Epidemiol 33:30–42
Bahls M, Leitzmann MF, Karch A et al (2021) Physical activity, sedentary behavior and risk of coronary artery disease, myocardial infarction and ischemic stroke: a two-sample mendelian randomization study. Clin res Cardiol 110:1564–1573
Cui G, Li S, Ye H et al (2023) Gut microbiome and frailty: insight from genetic correlation and mendelian randomization. Gut Microbes 15:2282795
Deng MG, Liu F, Liang Y et al (2023) Association between frailty and depression: a bidirectional mendelian randomization study. Sci Adv 9:eadi3902
Atkins JL, Jylhävä J, Pedersen NL et al (2021) A genome-wide association study of the frailty index highlights brain pathways in ageing. Aging Cell 20:e13459
Mitnitski AB, Mogilner AJ, Rockwood K (2001) Accumulation of deficits as a proxy measure of aging. TheScientificWorldJournal 1:323–336. https://doi.org/10.1100/tsw.2001.58
Ma T, Chen M, Cheng X et al (2024) Assessment of Bidirectional relationships between Frailty and Mental disorders: a bidirectional mendelian randomization study. J am med dir Assoc 25:506–513e529
Papadimitriou N, Dimou N, Tsilidis KK et al (2020) Physical activity and risks of breast and colorectal cancer: a mendelian randomisation analysis. Nat Commun 11:597. https://doi.org/10.1038/s41467-020-14389-8
Zheng J, Baird D, Borges MC et al (2017) Recent developments in mendelian randomization studies. Curr Epidemiol Rep 4:330–345. https://doi.org/10.1007/s40471-017-0128-6
Pagoni P, Dimou NL, Murphy N et al (2019) Using mendelian randomisation to assess causality in observational studies. Evid Based Ment Health 22:67–71. https://doi.org/10.1136/ebmental-2019-300085
Li W, Lu Q, Qian J et al (2023) Assessing the causal relationship between genetically determined inflammatory biomarkers and low back pain risk: a bidirectional two-sample mendelian randomization study. Front Immunol 14:1174656. https://doi.org/10.3389/fimmu.2023.1174656
Zhu J, Zhou D, Nie Y et al (2023) Assessment of the bidirectional causal association between frailty and depression: a mendelian randomization study. J cachexia Sarcopenia Muscle. https://doi.org/10.1002/jcsm.13319
Hemani G, Zheng J, Elsworth B et al (2018) The MR-Base platform supports systematic causal inference across the human phenome. Elife 7. https://doi.org/10.7554/eLife.34408
Thonprasertvat B, Roopsawang I, Aree-Ue S (2023) Assessing the Predictive Power of Frailty and Life-Space mobility on patient-reported outcomes of disability in older adults with low back Pain. Healthc (Basel Switzerland) 11. https://doi.org/10.3390/healthcare11071012
Rocha VTM, Leopoldino AAO, de Queiroz BZ et al (2023) The impact of low back pain and disability on frailty levels in older women: longitudinal data from the BACE-Brazil cohort. Eur Geriatr Med 14:181–189. https://doi.org/10.1007/s41999-022-00733-2
Han G, Wang W, Yue L et al (2022) Age-dependent differences of Paraspinal Muscle Endurance and morphology in Chinese Community Population without Chronic Low Back Pain. Global Spine J 21925682221103507. https://doi.org/10.1177/21925682221103507
Knox PJ, Coyle PC, Pugliese JM et al (2021) Hip osteoarthritis signs and symptoms are associated with increased fall risk among community-dwelling older adults with chronic low back pain: a prospective study. Arthritis Res Therapy 23:71. https://doi.org/10.1186/s13075-021-02455-5
Sribastav SS, Peiheng H, Jun L et al (2017) Interplay among pain intensity, sleep disturbance and emotion in patients with non-specific low back pain. PeerJ 5:e3282. https://doi.org/10.7717/peerj.3282
Huang Z, Guo W, Martin JT (2023) Socioeconomic status, mental health, and nutrition are the principal traits for low back pain phenotyping: data from the osteoarthritis initiative. JOR Spine 6:e1248. https://doi.org/10.1002/jsp2.1248
Noguchi T, Ikeda T, Kanai T et al (2023) Association of social isolation and loneliness with chronic low back pain among older adults: a cross-sectional study from Japan gerontological evaluation study (JAGES). J Epidemiol. https://doi.org/10.2188/jea.JE20230127
Zhang N, Wang C, Li Y et al (2023) Hypothetical interventions on risk factors for depression among middle-aged and older community-dwellers in China: an application of the parametric g-formula in a longitudinal study. J Affect Disord 327:355–361. https://doi.org/10.1016/j.jad.2023.01.113
Pereira Nery ECH, Rocha NP, Cruz VT et al (2023) Systematic review and meta-analysis on the association between chronic low back pain and cognitive function. Pain Pract 23:399–408. https://doi.org/10.1111/papr.13194
De Roeck EE, van der Vorst A, Engelborghs S et al (2020) Exploring cognitive Frailty: Prevalence and associations with other Frailty domains in older people with different degrees of cognitive impairment. Gerontology 66:55–64. https://doi.org/10.1159/000501168
Vanti C, Andreatta S, Borghi S et al (2019) The effectiveness of walking versus exercise on pain and function in chronic low back pain: a systematic review and meta-analysis of randomized trials. Disabil Rehabil 41:622–632. https://doi.org/10.1080/09638288.2017.1410730
Angulo J, El Assar M, Álvarez-Bustos A et al (2020) Physical activity and exercise: strategies to manage frailty. Redox Biol 35:101513. https://doi.org/10.1016/j.redox.2020.101513
Acknowledgements
This research has been conducted using IEU Open GWAS project and FinnGen Consortium. The authors thank the participants and coordinators for this unique dataset. The Fig. 1 in this article were drawn by Figdraw.
Funding
This work was supported by National Key Research and Development Program of China (2020YFC2008400); Medical Education Research Program of Henan Province (WJLX2023017); The Medical Science and Technology Program of Henan Province (Provincial-Ministry Joint Project) (SBGJ202302052); the National Natural Science Foundation of China (Grant No. 82371235); Henan Province Science and Technology Research Program (242102311242).
Author information
Authors and Affiliations
Contributions
XCF designed the study. ZYLa, LJF and JMF collected the data. QYL, CL, LTM and CLK analyzed the data. ZYLa, HLB and ZYLb wrote the manuscript. XXL, JW and JJY revised the manuscript.
Corresponding authors
Ethics declarations
Ethics approval
All data analyzed in this study were obtained from publicly available databases in which ethical approval was obtained for each cohort, and informed consent was obtained from all participants prior to participation.
Consent for publication
All authors have read and approved the submission of the manuscript.
Conflict of interest
The authors declare no conflicts of interests.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
40520_2024_2843_MOESM4_ESM.tif
Supplementary Fig. 1. Forest plots of causal effects of FI on LBP (A). Forest plots of causal effects of LBP on FI (B). The bars indicate the confidence interval of MR estimates
40520_2024_2843_MOESM5_ESM.tif
Supplementary Fig. 2. Leave-one-out plots of two-sample MR analysis for genetically predicted FI and LBP (A) outcomes. Leave-one-out plots of two-sample MR analysis for genetically predicted LBP and FI (B) outcomes.The dots indicate MR estimates for using IVW method when the SNP was removed. The bars indicate the confidence interval of MR estimates
40520_2024_2843_MOESM6_ESM.tif
Supplementary Fig. 3. Funnel plots assess the presence of potential heterogeneity across genetic instruments for FI on LBP (A), which exhibited symmetry, indicating that the results were unbiased. Funnel plots assess the presence of potential heterogeneity across genetic instruments for LBP on FI (B), which exhibited symmetry, indicating that the results were unbiased. The causal effect of each genetic instrument was presented by dots, and combined causal effect by IVW and MR Egger were depicted by lines
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, Z., Fan, J., Bu, H. et al. Causal associations between frailty and low back pain: a bidirectional two-sample mendelian randomization study. Aging Clin Exp Res 36, 191 (2024). https://doi.org/10.1007/s40520-024-02843-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40520-024-02843-2