Abstract
Background
The change in the composition of gut microbiota has been reported in the elderly and in the frail individuals; however, studies on gut microbiota in frail elderly are limited.
Aims
This study aimed to investigate the gut microbiota of the frail elderly.
Methods
From September 2017 to February 2018, 27 elderly patients hospitalized in the Department of Geriatrics of our hospital were enrolled and divided into the frailty group (n = 15) and the control group (n = 12) based on the cutoff of 0.25 for the frailty index. The fecal samples were collected for 16S rRNA-amplicon sequencing to analyze the composition and richness of gut microbiota. Operational taxonomic unit (OTU) clustering was performed using Usearch software. Intra-sample diversity (alpha-diversity) analysis and inter-sample diversity (beta-diversity) analyses were performed. The community richness was compared between the two groups at family and genus levels.
Results
There were 1903 and 1880 OTUs identified in the control and frailty groups, respectively, with 1282 OTUs overlap between the two groups. The alpha diversity of microbiota community was similar between the two groups, whereas the frailty group had larger beta diversity than the control group. The top-10 taxonomy categories and abundances of gut microbiota between the two groups were similar. As for the gut microbiota composition, 4 families and 17 genera were significantly different between the two groups (p < 0.05).
Conclusion
These results suggested that frailty can affect gut microbiota diversity and compositions in late elderly hospitalized patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Population aging is a global issue, and the speed of population aging is much faster than in the past. According to the United Nations, the number of people aged over 60 is estimated at 1.2 billion by 2025 [1]. In China, the population over 60 years old is estimated at 300 million by 2025 [2]. This demographic shift brings major challenges to the health and social systems of all countries. Frailty is an aging-associated syndrome characterized by significant vulnerability to stressors due to physiological decline across multiple organ systems, elevating the risk of adverse outcomes, such as falls, disability, hospitalization and even death [3]. Frailty is associated with aging, malnutrition, sarcopenia, concurrent multiple diseases, sleep disorders, anxiety and depression [4,5,6].
Gut microbiota is defined as the community of microbes (including bacteria, archaea, and eukarya) inhabiting the GI tract which has a complicated and mutually beneficial relationship with the host [7]. The number of gut microorganisms is estimated to over 1014 [8]. Gut microbiota plays an important role in the metabolism and immune function of the host. Human gut microbiota composition is affected by multiple factors, such as age, race, disease, and medication intake [9,10,11]. Intestinal microorganisms can hydrolyze complex plant polysaccharides to promote the host’s metabolic capacity and produce short-chain fatty acids, which can improve the energy metabolism of colon cells [12]. Some short-chain fatty acids even have anti-inflammatory effects [13]. Human gut microbiota composition is relatively stable during adulthood but undergoes the age-related reduction in biodiversity [9, 14,15,16]. Changes in the composition of the gut microbiota have been reported to be related with several clinical conditions, such as obesity, diabetes, nonalcoholic fatty liver disease, allergic diseases, diabetes, gastrointestinal diseases, autoimmune diseases, and even cancer [17, 18]. Verdi et al. have shown that frailty is associated with gut microbiota in the TwinsUK British twin cohort aging over 40 years [19]. Theou et al. have reported that a prebiotic intervention can reduce frailty levels in non-demented nursing home elder over 65 years, suggesting that gut microbiota has an effect on the frailty levels in the elderly [20]. Although the change in the composition of gut microbiota has been reported in elderly [21] and in frail individuals [22]; however, studies on gut microbiota in frail elderly are still limited [23, 24], especially in late elderly (aged ≥ 75 years). The purpose of this study was to characterize the gut microbiota in late elderly patients with frailty.
Methods
Participants and grouping
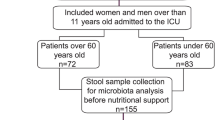
From September 2017 to February 2018, 27 elderly patients hospitalized in the Department of Geriatrics, First Affiliated Hospital of Sun Yat-sen University were enrolled. Admission reasons, dietary regimen during hospital stay, and pharmacologic treatments of the enrolled patients have been summarized in Table 1. Frail status of the patient was evaluated by Rockwood Clinical Frailty Scale [25]. The exclusion criteria were as follows: acute infectious diseases or end-stage disease; patients receiving antibiotics, prebiotics treatment or other drugs that regulate intestinal flora in the previous month; receiving gastrointestinal surgery within 6 months. This study was conducted in accordance with the Declaration of Helsinki and approved by the institutional review board of our hospital. Written informed consent was obtained from the patient.
Frailty assessment
Frail status of the patient was evaluated by modified Rockwood frailty questionnaire [26], which consisted of 50 health defects items, mainly including: disease history: hypertension, diabetes, stroke, depression, degenerative diseases, family history of cognitive impairment, etc.; self-care ability: ability to dress, bath, toilet independently, etc.; mental health: emotions problems, anxiety or depression, loneliness, etc.; auxiliary examinations: albumin, BMI, etc. Each item represented a health defect issue. The patient having the defect of the item was recorded as “1”, otherwise it was recorded as “0”. The frailty index was calculated as follows: FI = cumulative health defects/total defects (50). Patients with frailty index < 0.25 were grouped into the frailty group (n = 15), while those with frailty index ≥ 0.25 were grouped into the control group (n = 12) [26].
16S rRNA sequencing
To analyze the gut microbiota diversity, fecal samples of the participants were collected for 16S rRNA sequencing. Fresh fecal samples were collected within 3 days after enrollment. After collection, fresh stool samples were stored at − 80 °C refrigerator or liquid nitrogen tanks within 4 h. The genomic bacterial DNA was extracted from fecal samples using the freeze–thaw method. The 16S rDNA was PCR-amplified and then sequenced on the MiSeq system (Illumina, USA). The primer sequence used in the PCR were F: 5′GTGCCAGCMGCCGCGGTAA3′ and R:5′ GGACTACHVGGGTWTCTAAT3′.
Operational taxonomic unit (OTU) clustering
The sequence reads with a similarity greater than 97% were identified and clustered into an OTU using the Usearch software [27]. The Ribosomal Database Project (RDP) classifier Bayesian algorithm was applied to classify the OTUs clusters, and the community composition was analyzed in each taxonomic rank: domain, kingdom, phylum, class, order, family, genus, and species.
Rank-abundance curve
Within a single sample, the number of sequences included in every single OTU was counted, and the OTUs were sorted by the order of richness. Then the percentage of the sequences number in each OTU was plotted on the rank-abundance curve, which can be used to explain the species abundance and uniformity.
Rarefaction curve
Rarefaction curves were created by repeatedly randomly re-sampling the whole population multiple times and then plotting the average number of species found in each sample. It can be used to compare the species richness between samples, and it can also be used to indicate whether the sampling size of the sample is reasonable.
Intra-sample diversity analysis (alpha-diversity)
The single sample diversity analysis (alpha diversity) can reflect the richness and diversity of microbial communities, including a series of statistical analysis indices to estimate the species abundance and diversity of communities. The community richness indexes included Chao1, Ace, and SOBs, while the community diversity indexes were Shannon and Simpson. Sequencing depth was evaluated by coverage.
Statistical analysis
Continuous variables were presented as mean ± standard deviation (SD) while categorical data were expressed as number and percentage (%). Student’s independent t test was used to compare the differences between groups. If normality of continuous variables was not assumed, non-parametric analysis Mann–Whitney U test would be used instead. Chi square test and Fisher’s exact test (if any expected value lower than 5 was observed) were used for categorical data. Principal co-ordinates analysis (PCoA) and non-metric multidimensional scaling (NMDS) were used to analyze the beta diversity. Hierarchical and K means clustering methods were used to examine the grouping results from diversity index. A p value lower than 0.05 would be recognized as significant. All analyses were performed using IBM SPSS Version 20 (SPSS Statistics V20, IBM Corporation, Somers, New York). Bioinformatics results were analyzed using R software (version 3.5.2).
Results
Patient’s clinical characteristics and laboratory index
A total of 27 patients (17 males and 10 females, mean age 81.63 ± 7.90) were enrolled and divided into frailty group (frailty index < 0.25, n = 15) and control group (frailty index ≥ 0.25, n = 12). Patient’s demographic and clinical characteristics were summarized in Table 2. Frailty index was significantly higher in the frailty group than in the control group (0.35 ± 0.09 vs. 0.14 ± 0.07; p < 0.001). However, there was no significant difference in other outcomes, including all demographic and clinical characteristics, and all blood biochemical indexes (all p > 0.05, Table 2), These results indicated the comparability between the two groups.
Operational taxonomic unit (OTU) clustering and community richness
To characterize gut microbiota, fecal samples were collected for 16S rRNA-amplicon sequencing. The 16S rRNA sequencing reads were clustered using the Usearch software with a 97% sequence similarity threshold. A total of 2501 OTUs were detected (range 187–794). Figure 1 showed the rank-abundance curve of each sample. As the number of OTUs increased, the relative abundance decreased. Most samples had an abundance between 200 and 600.
The Venn diagram showed the number of common microbial taxonomies between the frailty and control groups (Fig. 2). There were 1282 OTUs overlapping between the two groups, while 621 and 598 OTUs were exclusively belonging to the control and frailty groups, respectively.
Alpha diversity analyses
Rarefaction curve indicates the results of observed species corresponding to random sampling sequences per sample (Fig. 3). In all samples, a flat trend was observed as the number of sequences increased, indicating that maximum sequencing level had been reached and the sequencing results were reliable.
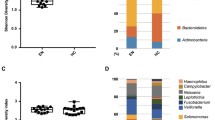
For alpha diversity analysis, we calculated the Shannon index and Simpson index. The Shannon index of frailty and control groups were 4.39 ± 1.34 and 4.63 ± 0.65, respectively (p = 0.722, Fig. 4a); while the Simpson index of frailty and control groups were 0.84 ± 0.13 and 0.89 ± 0.04, respectively (p = 0.887, Fig. 4b). These results suggested that there was no statistical difference in alpha diversity of microbiota community between the two groups.
Beta diversity analyses
For beta diversity analysis, principal co-ordinates analysis (PCoA) and non-metric multidimensional scaling (NMDS) were performed. Although a major overlapping was observed in the PCoA plot, the PC2 axis shows a relatively larger distribution in the frailty group than the control group (Fig. 5a). Likewise, in the NMDS plot, the frailty group had a relatively larger distributed area than the control group. These results suggested that the frailty group had a larger beta diversity than the control group.
Gut microbiota diversity analysis in the phylum and genus level
The diversity of intestinal microbe between the frailty and control groups were analyzed on phylum (Fig. 6a) and genus (Fig. 6b). The top-10 taxonomy categories and abundances of gut microbiota were similar between the two groups.
The top-10 abundant phyla were Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, Verrucomicrobia, Fusobacteria, Acidobacteria, Chloroflexi, Patescibacteria, and Epsilonbacteraeota. The top-10 abundant genera included Bac Bacteroides, Lac Lachnospiraceae UCG-008, Lac Blautia, Rum Faecalibacterium, Lac Lachnoclostridium, Str Streptococcus, Lac Roseburia, Tan Parabacteroides, Rik Alistipes, and Bif Bifidobacterium.
The community richness at family and genus levels were compared between the frailty and control groups. As shown in Table 3, there were 4 and 17 significant the gut microbiota composition at family and genus levels, respectively (p < 0.05), suggesting that microbial diversity and distribution are different between the frailty and control groups.
Clustering analyses
Hierarchical clustering and K-means clustering methods were used to investigate the grouping by microbial diversity indexes, including Shannon index, Simpson index, chao1, ACE, and goods coverage. As indicated in Fig. 7, the hierarchical clustering correctness rates of the frailty and control groups were 60% and 72.7%, respectively.
The K means clustering correctness rates of the frailty and control groups were 55.6% and 75%, respectively. These results suggested that the frailty and control groups indeed possessed different gut microbiota diversity, and the diversity indexes could be used to discriminate the frailty and non-frailty elderly.
Discussion
In this study, we investigated the gut microbiota of the frail elderly. The results showed that there were 1903 and 1880 OTUs identified in the control and frailty groups, respectively, with 1282 OTUs overlap between the two groups. The alpha diversity of microbiota community was similar between the two groups, whereas the frailty group had larger beta diversity than the control group. The top-10 taxonomy categories and the abundances of gut microbiota between two groups were mainly identical. As for the gut microbiota composition, however, 4 families and 17 genera were significantly different between the two groups. Taken together, these results suggested that frailty can affect gut microbiota diversity and compositions in late elderly hospitalized patients.
The gut microbiota composition and function are stable in adulthood up to 65–70 years, and then the inter-individual variability would be increased with the decline of biodiversity and a tendency to dysbiosis, leading to significant disturbance in host physiology [28]. Animal studies have demonstrated that dysbiosis of gut microbiota can elevate intestinal permeability [29], which promote endotoxin and other microbial products [e.g., lipopolysaccharide (LPS)] entering the circulation. Circulating LPS induces skeletal muscle inflammation and insulin resistance [30], which impairs both the metabolic and contractile characteristics of skeletal muscle. The relevant systemic inflammation has been demonstrated in the pathophysiology of sarcopenia patients [31]. These findings suggest the possibility of a “gut–muscle axis”, namely gut microbiota act as the mediator of the effects of nutrition on muscle cells. This phenomenon leads to a decrease in muscle strength and quality, further enhances insulin resistance, and ultimately leads to sarcopenia and physical frailty [32, 33].
Accumulating evidence has revealed the correlation between gut microbiota and frailty [34,35,36], as well as the composition of gut microbiota in the elderly [37,38,39], but little attention has been paid to the gut microbiota in frail elderly. In a small pilot study, van Tongeren et al. report that the abundance of Bacteroides/Prevotella and the Faecalibacterium prausnitzii is significantly reduced in frailer elderly, while the abundance of Enterobacteriaceae which can cause opportunistic infections is significantly increased in frailer individuals [23]. Jackson et al. have reported that frailty is negatively associated with the alpha diversity of gut microbiota. Species more abundant in the frail elderly included Eubacterium dolichum and Eggerthella lenta, while Faecalibacterium prausnitzii is less abundant in frail elderly [24]. F. prausnitzii is a key butyrate producer [35, 36] and has an anti-inflammatory effect on the gut [40]. The abundance of F. prausnitzii is decreased in patients with inflammatory bowel disease [41]. Likewise, Haran et al. have demonstrated that the nursing home elder with increasing frailty has lower abundances of butyrate-producing bacteria [42]. Nevertheless, the composition of the gut microbiota is greatly affected by diet, environment, and ethnicity [9,10,11], and may exhibit varying patterns in different countries and geographic regions.
The dominant gut microbial phyla in human are Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, in which Firmicutes and Bacteroidetes representing 90% of gut microbiota [43]. Supporting this notion, our results demonstrated that the common dominant phyla between the two groups were Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria, while the common dominant genera included Bac Bacteroides, Lac Lachnospiraceae UCG-008, Lac Blautia, and Rum Faecalibacterium. Of note, there was no significant difference in the top-10 taxonomy categories and the abundances of gut microbiota at both phylum and genus levels between the two groups. According to the Shannon index and the Simpson index, we found that the alpha diversity of gut microbiota was similar between the frailty group and the control group, which is inconsistent with Jackson et al.’s report [24]. This discrepancy may be attributed to the small sample size of this study. While in the beta diversity analysis, the frailty group had a relatively larger distribution area in both PCoA and NMDS plots as compared with the control group. One possible explanation might be that the various etiologies of frailty contributed to a larger difference in the beta diversity among the 15 frail elderly patients.
Comparing the gut microbiota at the genus level between the two groups, we found that Gemella, Lachnoanaerobaculum, Eubacterium[U1]_ruminantium_group, Azospira, Tyzzerella, Cloacibacterium, and EU455341_g genera were significantly less abundant in the frailty group, while the Acetanaerobacterium, Catenibacterium, [Ruminococcus]_torques_group, DQ801572_g, Ruminococcaceae_UCG-011, Prevotella_9, Olsenella, EF434341_g, KF843164_g, and Pseudoxanthomonas were more abundant in the frailty group as compared with the frailty group. Family Moraxellaceae was more abundant in the frailty group. These results indicated that the composition of gut microbiota shows different patterns in different countries and geographic regions. Of them, genera Prevotella, Olsenella, Catenibacterium, and Tyzzerella are reported to be associated with a lifetime risk of cardiovascular disease [44]. On the other hand, Olsenella bacteria are involved in endodontic infections in humans [45]. The pathogenic role of these microbiomes may be involved in the development of frailty in the elderly. However, it is unknown whether these relationships are the causes or consequences of frailty, and the underlying mechanism remains to be further investigated.
Reduction in microbiota-related metabolic capacity, such as lower short-chain fatty acids (SCFAs) levels may be associated with age-related frailty [46]. SCFAs mainly include acetic acid, propionic acid, and butyric acid, which stimulate the secretion of mucin by inducing hypoxia-inducible factor (HIF) in the intestinal epithelial cells, thereby strengthening the intestinal mucosal barrier. Meanwhile, SCFAs promote IL-8 release, accelerating repair of the intestinal mucosal barrier and modulate inflammation, oxidative stress [47]. High frail individuals are reported to have a decrease in the abundance of Eubacteria, Faecalibacterium, and Lactobacilli [48]. We also found that Eubacteria was less abundant in the frailty elderly (p = 0.023); however, there was no significant difference in Lactobacilli and Faecalibacterium between the two groups. Eubacterium is considered as protective colonic bacteria by producing SCFAs (especially butyrate) [49] and modulating the immune system [50]. Thereby, a decrease in Eubacterium abundance may negatively affect gut health or induce systemic effects. The Eubacterium decreased in the frail elderly may support the protective effect of SCFAs on human guts. However, the detailed mechanism is needed to be further investigated. Our result showed that genus Prevotella was more abundant in the frail group, which is in line with van Tongeren et al.’s report [23]. For other bacterial genera with an abundant change in our study, their potential correlations with the development of frailty remain to be elucidated.
Several limitations of this study should be pointed out. First, the sample size of this study is small, and the findings of this study should be validated in a large study. In addition, hospitalization may have an impact on gut microbiota composition due to exposure to nosocomial pathogens, disruption of usual dietary and lifestyle habits, presence of acute diseases and need for acute treatments. Nevertheless, this study was conducted in the elderly health care ward of our hospital, and all patients were admitted to a single ward. In addition, the patients with acute infectious diseases, antibiotics or probiotics were excluded. Thus, the impact of hospitalization could be minimized. Moreover, we cannot determine whether frailty is the cause, consequence or bystander of the changes in gut microbiota diversity and composition. All these studies should be addressed in the future study.
In summary, our study demonstrated that frailty can change the abundance and composition of gut microbiota in Chinse frail late elderly hospitalized patients, further highlighting an association between the gut microbiota alterations and development of frailty. Our finding may provide a theoretical basis for further study on the pathological mechanism for the development of frailty.
References
Department of Economic and Social Affairs, Population Division UN (2013) World population ageing. United Nations, New York
Huang Y, Tong X (2012) Current situation of population aging in China. Chin J Gerontol 32:4853–4855
Chen X, Mao G, Leng SX (2014) Frailty syndrome: an overview. Clin Interv Aging 9:433–441. https://doi.org/10.2147/CIA.S45300
Shi J, Song X, Yu P et al (2011) Analysis of frailty and survival from late middle age in the Beijing Longitudinal Study of Aging. BMC Geriatr 11:17
Jürschik P, Nunin C, Botigué T et al (2012) Prevalence of frailty and factors associated with frailty in the elderly population of Lleida, Spain: the FRALLE survey. Arch Gerontol Geriatr 55:625–631
de Albuquerque Sousa ACP, Dias RC, Maciel Álvaro CC et al (2012) Frailty syndrome and associated factors in community-dwelling elderly in Northeast Brazil. Arch Gerontol Geriatr 54:e95–e101
Hold GL, Hansen R (2019) Impact of the gastrointestinal microbiome in health and disease: co-evolution with the host immune system. Curr Top Microbiol Immunol 421:303–318
Bäckhed F, Ley RE, Sonnenburg JL et al (2005) Host-bacterial mutualism in the human intestine. Science 307:1915–1920
Odamaki T, Kato K, Sugahara H et al (2016) Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol 16:90
Chen L, Zhang YH, Huang T, Cai YD (2016) Gene expression profiling gut microbiota in different races of humans. Sci Rep 6:23075
Biagi E, Rampelli S, Turroni S et al (2017) The gut microbiota of centenarians: Signatures of longevity in the gut microbiota profile. Mech Ageing Dev 165:180–184
Flint HJ, Duncan SH, Scott KP et al (2007) Interactions and competition within the microbial community of the human colon: links between diet and health: minireview. Environ Microbiol 9:1101–1111
Macfarlane GT, Steed H, Macfarlane S (2008) Bacterial metabolism and health-related effects of galacto-oligosaccharides and other prebiotics. J Appl Microbiol 104:305–344
Biagi E, Franceschi C, Rampelli S et al (2016) Gut microbiota and extreme longevity. Curr Biol 26:1480–1485
Claesson MJ, Jeffery IB, Conde S et al (2012) Gut microbiota composition correlates with diet and health in the elderly. Nature 488:178–184
OToole PW, Jeffery IB (2015) Gut microbiota and aging. Science 350:1214–1215
Clemente JC, Ursell LK, Parfrey LW et al (2012) The impact of the gut microbiota on human health: an integrative view. Cell 148:1258–1270
Sekirov I, Russell SL, Antunes LC et al (2010) Gut microbiota in health and disease. Physiol Rev 90:859–904
Verdi S, Jackson MA, Beaumont M et al (2018) An investigation into physical frailty as a link between the gut microbiome and cognitive health. Front Aging Neurosci 10:398
Theou O, Jayanama K, Fernández-Garrido J et al (2018) Can a prebiotic formulation reduce frailty levels in older people? J Frailty Aging 8:48–52
Ticinesi A, Milani C, Lauretani F et al (2017) Gut microbiota composition is associated with polypharmacy in elderly hospitalized patients. Sci Rep 7:11102
Di Sabatino A, Lenti MV, Cammalleri L et al (2018) Frailty and the gut. Dig Liver Dis 50:533–541
Van Tongeren SP, Slaets JP, Harmsen HJ et al (2005) Fecal microbiota composition and frailty. Appl Environ Microbiol 71:6438–6442
Jackson MA, Jeffery IB, Beaumont M et al (2016) Signatures of early frailty in the gut microbiota. Genome Med 8:8
Juma S, Taabazuing MM, Montero-Odasso M (2016) Clinical frailty scale in an acute medicine unit: a simple tool that predicts length of stay. Can Geriatr J 19:34–39
Joseph B, Zangbar B, Pandit V et al (2016) Emergency general surgery in the elderly: too old or too frail? J Am Coll Surg 222:805–813
Edgar RC (2010) Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26:2460–2461. doi:10.1093/bioinformatics/btq461
Ticinesi A, Nouvenne A, Tana C et al (2018) The impact of intestinal microbiota on bio-medical research: definitions, techniques and physiology of a “new frontier”. Acta Biomed 89:52–59
Thevaranjan N, Puchta A, Schulz C et al (2017) Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe 21:455.e4–466.e4
Choi Y, Kwon Y, Kim DK et al (2015) Gut microbe-derived extracellular vesicles induce insulin resistance, thereby impairing glucose metabolism in skeletal muscle. Sci Rep 5:15878
Bano G, Trevisan C, Carraro S et al (2017) Inflammation and sarcopenia: a systematic review and meta-analysis. Maturitas 96:10–15
Poggiogalle E, Lubrano C, Gnessi L et al (2019) The decline in muscle strength and muscle quality in relation to metabolic derangements in adult women with obesity. Clin Nutr 38:2430–2435
Sachs S, Zarini S, Kahn DE et al (2019) Intermuscular adipose tissue directly modulates skeletal muscle insulin sensitivity in humans. Am J Physiol Endocrinol Metab 316:E866–E879
Maffei VJ, Kim S, Blanchard E et al (2017) Biological aging and the human gut microbiota. J Gerontol A Biol Sci Med Sci 72:1474–1482
Tran TTT, Cousin FJ, Lynch DB et al (2019) Prebiotic supplementation in frail older people affects specific gut microbiota taxa but not global diversity. Microbiome 7:39
Ticinesi A, Tana C, Nouvenne A (2019) The intestinal microbiome and its relevance for functionality in older persons. Curr Opin Clin Nutr Metab Care 22:4–12
Park SH, Kim KA, Ahn YT et al (2015) Comparative analysis of gut microbiota in elderly people of urbanized towns and longevity villages. BMC Microbiol 15:49
Shin JH, Sim M, Lee JY et al (2016) Lifestyle and geographic insights into the distinct gut microbiota in elderly women from two different geographic locations. J Physiol Anthropol 35:31
Milani C, Ticinesi A, Gerritsen J et al (2016) Gut microbiota composition and Clostridium difficile infection in hospitalized elderly individuals: a metagenomic study. Sci Rep 6:25945
Sokol H, Pigneur B, Watterlot L et al (2008) Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci 105:16731–16736
Cao Y, Shen J, Ran ZH (2014) Association between Faecalibacterium prausnitzii reduction and inflammatory bowel disease: a meta-analysis and systematic review of the literature. Gastroenterol Res Pract. https://doi.org/10.1155/2014/872725
Haran JP, Bucci V, Dutta P et al (2018) The nursing home elder microbiome stability and associations with age, frailty, nutrition and physical location. J Med Microbiol 67:40–51
Rinninella E, Raoul P, Cintoni M et al (2019) What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 7:E14
Kelly TN, Bazzano LA, Ajami NJ et al (2016) Gut microbiome associates with lifetime cardiovascular disease risk profile among bogalusa heart study participants. Circ Res 119:956
Singh H (2016) Microbiology of endodontic infections. J Dent Oral Health Sci 2:2–5
Nagpal R, Mainali R, Ahmadi S et al (2018) Gut microbiome and aging: physiological and mechanistic insights. Nutr Heal aging 4:267–285
Feng W, Ao H, Peng C (2018) Gut microbiota, short-chain fatty acids, and herbal medicines. Front Pharmacol 9:1354
Theou O, Rockwood K (2015) Frailty in aging: biological, clinical, and social implications. Karger Medical and Scientific Publishers, Basel
Rivière A, Selak M, Lantin D et al (2016) Bifidobacteria and butyrate-producing colon bacteria: importance and strategies for their stimulation in the human gut. Front Microbiol 7:979
Tedelind S, Westberg F, Kjerrulf M et al (2007) Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: a study with relevance to inflammatory bowel disease. World J Gastroenterol 13:2826–2832
Funding
This study was supported by the National Natural Science foundation of China (81670226).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Statement of human and animal rights
This study was conducted in accordance with the Declaration of Helsinki and approved by the institutional review board of the First Affiliated Hospital, Sun Yat-Sen University. Written informed consent was obtained from the patient.
Informed consent
The written informed consent was waived by the IRB due to the retrospective nature of this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, L., Liao, J., Chen, Q. et al. Characterization of the gut microbiota in frail elderly patients. Aging Clin Exp Res 32, 2001–2011 (2020). https://doi.org/10.1007/s40520-019-01385-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-019-01385-2