Abstract
Aim
The aim of this study was to investigate the possible risk factors related with osteoporosis in women with spontaneous menopause.
Methods
Five hundred and one postmenopausal women were divided into three groups as normal, osteopenic and osteoporotic according to their bone mineral density (BMD). By face-to-face interview, parity, age at menarche, age at menopause, duration of fertility, duration of menopause, first pregnancy age, total lactation period, exercise, smoking were assessed. Women with menopause age before 40 years, surgical menopause, who had any anti-osteoporosis treatment, hormone replacement therapy at the time of BMD measurement and corticosteroid use longer than 6 months were excluded from the study.
Results
Among 501 postmenopausal women, 107 women were classified as normal, 170 as osteopenic and 224 as osteoporotic. Among demographic features of patients, there was statistically significant difference between the groups in age, BMI and parity (p < 0.001, p < 0.0001 and p = 0.002, respectively). There were statistically significant differences between the groups in case of age at menopause, duration of fertility and duration of menopause (p = 0.013, p = 0.013 and p < 0.0001, respectively). In the multivariate logistic regression analysis, BMI over 32 and fertility duration over 33 years had a statistically significant protective effect against osteoporosis (OR 0.42, CI 95 % 0.27–0.66; OR 0.36, CI 95 % 0.24–0.56, respectively), but age was positively correlated with osteoporosis (OR 1.13, CI 95 % 1.01–1.17)
Conclusions
Duration of fertility (years of menstruation) longer than 33 years and body mass index higher than 32 seem to protect against postmenopausal osteoporosis. Age is also an independent risk factor for postmenopausal osteoporosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is an important health problem characterized by compromised bone strength that leads to increased fracture risk [1]. In a recent report, the economic burden of osteoporosis in 27 European Union countries was estimated at 37 billion euro and were expected to increase 25 % in 2025 [2]. So identification of risk factors to prevent postmenopausal osteoporosis (POPS) is very important.
Since etiology of POPS is multifactorial [3], it is difficult to reveal the risk factors properly. Advancing age, female sex, low body mass index (BMI), smoking, family history, lifestyle changes and medical history have been reported as risk factors for osteoporosis [3, 4].
Despite the well-known risk factors, there is conflict about the effect of reproductive characteristics (age at menarche, age at menopause, duration of fertility, parity, age at first pregnancy and total lactation period) on POPS in the literature.
There are also conflicting reports about the effect of paracetamol and non-steroidal anti-inflammatory drugs (NSAIDs) on bone mineral density (BMD) and fracture risk [5–8]. Eating patterns and dietary habits have been found to affect the bone mass also [9–11].
In this study, we investigated the effects of reproductive characteristics, dietary habits (black tea, vegetarian status), exercise, smoking, paracetamol, NSAID consumption on BMD in postmenopausal Turkish women.
Materials and methods
Five hundred and one postmenopausal women who were admitted to Ankara Kecioren Education and Research Hospital between January 2012 and April 2013 were included in this study. The study was approved by the Ethical Committee of Ankara Kecioren Education and Research Hospital.
Women with menopause age before 40 years, surgical menopause, who had any anti-osteoporosis treatment or hormone replacement therapy at the time of BMD measurement or within the past 6 months and corticosteroid use longer than 6 months were excluded from the study.
Informed consent was provided from all the women and the data were collected by face-to-face interview. Demographic features, reproductive history (parity, age at menarche, age at menopause, duration of fertility, duration of menopause, first pregnancy age, total lactation period), dietary habits (black tea drinking, vegetarian status) and paracetamol or NSAID consumption were recorded (Table 1).
The patients were divided into three groups as normal (n = 107), osteopenic (n = 170) and osteoporotic (n = 224) according to the lowest T score of BMD values at the lumbar vertebra (L1–L4) and right femur (neck, intertrochanteric and ward triangle) by dual energy X-ray absorptiometry (DEXA) method using Hologic 4500 QDR (Discovery). Osteoporosis was defined as a T score ≤−2.5, osteopenia as T score from −1.1 to −2.4 and normal as a T score ≥−1.0 [12].
Menopause was defined as amenorrhea lasting more than one year. Exercise was defined as walking at least 30 min per day. Low socioeconomic status was defined as “monthly income lower than 1000 Turkish Liras” according to the 2012 Turkish Statistical Institute report [13]. Any gestation lasting at least 28 weeks was defined as pregnancy. Total lactation period was mentioned as months.
As dietary habits, black tea-drinking patients were described as “drinking black tea at least 1 cup in a day” and patients preferring vegetarian diet were described as vegetarian.
Paracetamol or NSAID consumption was recognized if patients were current users or received at least three or more prescriptions in last year.
Data were stored and analyzed using the Statistical Package for the Social Sciences version 13.0 (SPSS, Chicago, IL, USA). Normality was tested by Kolmogorov–Smirnov test. Non-normally distributed metric variables were analyzed by the Kruskal–Wallis test for the comparison of the variables among the groups. Chi-square test (Fischer’s exact test), student t test and Mann–Whitney U test were used for the comparison of data between the groups. For the variables that showed statistically significant differences between the groups, univariate and multivariate logistic regression models have been performed. p values less than 0.05 were considered significant.
Results
Five hundred and one postmenopausal women were classified as normal (n 107), osteopenic (n 170) and osteoporotic (n 224) according to their BMD. Demographic and reproductive characteristics of the patients are shown in Table 1. Among demographic features of patients, there was statistically significant difference between the groups in age, BMI and parity (p < 0.001, p < 0.0001 and p = 0.002, respectively). There was no statistically significant difference in vegetarian status, black tea drinking, smoking, exercise and low socioeconomic status between the groups. When we consider the reproductive characteristics of the patients, there were statistically significant differences between the groups in case of age at menopause, duration of fertility and duration of menopause (p = 0.013, p = 0.013 and p < 0.0001, respectively). There was no statistically significant difference in first pregnancy age and total lactation period between the groups.
The percentage of patients who had lactation period over 45 months was statistically significantly higher in the osteoporotic and osteopenic group than the control group (p = 0.018).
In the univariate logistic regression analysis of the variables, age, parity and lactation over 45 months were positively correlated with osteoporosis [odds ratio (OR) 1.1, 95 % confidence interval (CI) 1.07–1.14; OR 1.16, CI 95 % 1.04–1.30; OR 1.46, CI 95 % 1.02–2.09, respectively) (Table 2). On the other hand, BMI over 32 and fertility duration over 33 years were negatively correlated with osteoporosis (OR 0.48, CI 95 % 0.31–0.72; OR 0.58, CI 95 % 0.41–0.83, respectively). In the multivariate logistic regression analysis of variables for osteoporosis, BMI over 32 kg/m2 and fertility duration over 33 years had a statistically significant protective effect against osteoporosis (OR 0.42, CI 95 % 0.27–0.66; OR 0.36, CI 95 % 0.24–0.56, respectively), but age was positively correlated with osteoporosis (OR 1.13, CI 95 % 1.01–1.17) (Table 2).
Discussion
In this cross-sectional study, after the multivariate logistic regression analysis of variables for osteoporosis, BMI over 32 kg/m2 and fertility duration over 33 years had a statistically significant protective effect against osteoporosis (OR 0.42, CI 95 % 0.27–0.66; OR 0.36, CI 95 % 0.24–0.56, respectively), but age was positively correlated with osteoporosis (OR 1.13, CI 95 % 1.01–1.17) (Table 2).
In the literature, there is good evidence that lower BMI is associated with higher osteoporosis risk [14–16]. Ho et al. [15] have found that weight was the best predictor of osteoporosis at each of six skeletal sites measured. Kroger et al. [16] reported that each kilogram increase in weight resulted in an increase in BMD at the lumbar spine 0.004 g/cm2 and femur neck by 0.005 g/cm2 among 1600 perimenopausal women in Finland. In our study, women with BMI >32 kg/m2 had statistically significant protective effect against osteoporosis similar with the literature.
There is inconsistent evidence that older age is associated with lower BMD after adjustment for menopausal status [17]. Although most of the studies have reported negative association between older age and BMD [14–16], some studies found no relationship between age and BMD [18, 19]. We have found that older age is an independent risk factor for osteoporosis.
Although some studies reported negative association between older age at menarche and BMD [14, 15], in many of the studies, age at menarche was not found to be associated with BMD similar to our study [20–23]. Also in a recent review, inconsistent evidence was found between older age at menarche and BMD [17]. This might be explained by the differences in years of menopause in these studies.
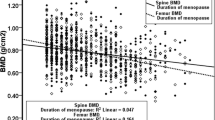
In case of menopause age, no relationship between BMD and menopausal age [22, 24], lower BMD in women with menopausal age between 40 and 44 years old [25] have been reported. In our study, age at menopause was statistically significantly lower in the osteoporotic group and duration of menopause was significantly higher in the osteoporotic group (Table 1).
In the present study, we have found that fertility duration over 33 years had a statistically significant protective effect against osteoporosis (Table 2). Similar to our study, duration of fertility was positively correlated with BMD in the literature [20, 21, 26]. Among French postmenopausal women, duration of fertility has been reported as the best predictor of spinal BMD [26] and longer duration of menstruation was associated with higher BMD in Australian postmenopausal women [27]. On the other hand, in a study from Morocco, no relationship has been reported between the duration of fertility and BMD [24]. But in this study, all the participants were not postmenopausal and nearly half of the women had low calcium intake.
In a study from Sweden consisting of 1044 postmenopausal women who were all 75 years old, it has been reported that duration of fertility did not influence BMD in old age [23]. This finding might be attributed to the very old age of the participants.
In the literature, there are conflicting results about the effect of parity on BMD. In our study in the univariate analysis of variables, parity had a negative effect on BMD, but in multivariate logistic regression model, there was no association between the parity and osteoporosis in agreement with other studies [17, 20, 28]. But in two studies from Turkey, high parity was determined as a risk factor for osteoporosis [29, 30]; however, a recent study from Turkey has found that parity has a protective effect for osteoporosis [31]. These conflicting results could be explained with the evaluation of different confounding factors (dietary calcium intake, time interval between the parities, hormone replacement therapy, anti-osteoporosis treatment, etc.) in different studies.
Effect of total lactation period on osteoporosis is another debated subject. The fetus mobilizes 30 g of maternal calcium for skeleton formation [32] which comes mostly from increased intestinal absorption and substantially mobilization from the maternal skeleton during pregnancy. But during lactation, maternal skeleton supplies most of this calcium [32]. Bone loss during lactation is transient and is reversed postpartum [33]. Recovery period depends on the length of both lactation and postpartum amenorrhea [34]. In fact, selection criteria of the patients cause this discrepancy in the literature. Schnatz et al. [35] reported that lactation decreased the incidence of POPS and Chantry et al. [36] found that lactation might be associated with greater BMD in adolescent motherhood. But both in these reports, the definition of lactation was “exclusively lactation for at least 1 month”. On the other hand, most of the patients had prolonged lactation periods in the studies that reported negative association between BMD and lactation [31, 37]. The time interval between the pregnancies was not mentioned in most of the studies [31, 35, 37]. Similar to our results, many studies did not find any correlation between lactation and BMD [20, 22]. Also in a recent review, no association between parity, lactation and lower BMD has been accepted as good evidence according to the literature [17].
Since peak bone mass (PBM) is important in the development of POPS, effect of age at first pregnancy on BMD has been studied in the recent years. Schnatz et al. [35] have found that women whose first pregnancy was after PBM (≥27 years) and who had a history of breast-feeding had the lowest prevalence of POPS. On the other hand, Okyay et al. have reported that osteoporosis risk in women who breast-fed >1 year per child under age 27 was increased 7.1-fold [31]. Ozdemir et al. [29] reported increased risk of osteoporosis in women who had a higher age at first pregnancy. In the present study, although age at first pregnancy over 24 seems to protect against bone loss in univariate logistic regression, in the multivariate logistic regression model we could not find any correlation between the age at first pregnancy and lower BMD.
Vegetarian diet has been found beneficial for bone health because of increased phytoestrogens and calcium intake [9, 38]. Contrastly, Ho-Pham et al. [39] reported that vegan diets were associated with lower BMD, newly reported normal BMD in vegetarians [40]. In this study, there was no relationship between the vegetarian status and BMD. Among 5379 postmenopausal women, there was no difference in mean broadband ultrasound attenuation of calcaneum between different dietary groups [10]. But vegetarian status of our patients was not determined by a food frequency questionnaire which might be a weakness of our study.
Recently, it has been reported that black tea might prevent early bone loss in a rat osteoporosis model [41]. We also investigated the effect of black tea drinking on BMD, but there was no association between the groups. Also in a study from Turkey, black tea drinking had no statistically significant effect on BMD in agreement with our study [11].
Effect of paracetamol and NSAID consumption on BMD and fracture risk is also not well understood. NSAIDs inhibit the synthesis of prostaglandins which increases bone resorption by osteoclasts. On the basis of this, higher BMD was found in NSAIDs users than the non-users [42, 43] but this was not associated with fracture risk reduction [42]. Paracetamol was shown to inhibit osteoblast activity in vitro [44] and paracetamol use was found as a risk factor for fracture [8]. Similar to our study Danish Osteoporosis Prevention Study (DOPS) reported no association between paracetamol, NSAID consumption and BMD [6], but NSAID was associated with an increased fracture risk in DOPS. We had no data of patients about fracture history.
The limitations of our study are the potential selection bias seen in cohort studies. Although the data were collected by face-to-face interview, recall bias might have occurred also. Some osteoporosis-related factors such as presence of connective tissue diseases and dietary sources of calcium and vitamin D were not mentioned which might be a weakness of our study.
We tried to select a homogenized group of patients who had natural menopause, and excluded patients who had anti-osteoporosis treatment and hormone replacement therapy. Although very difficult, we tried to control the possible confounding factors such as reproductive history, dietary habits, exercise, smoking and socioeconomic status in our study. These might be accepted as the strength of our study.
In conclusion, duration of fertility (years of menstruation) longer than 33 years and body mass index (BMI) higher than 32 seem to be protective against postmenopausal osteoporosis but advanced age is an independent risk factor for postmenopausal osteoporosis and bone loss. However, the multifactorial etiology of osteoporosis makes the prospective studies difficult in terms of standardization of the patients and determination of a sole risk factor.
References
(2010) Management of osteoporosis in postmenopausal women: 2010 position statement of The North American Menopause Society. Menopause 17(1):25–54
Svedbom A, Hernlund E, Ivergard M, Compston J, Cooper C, Stenmark J et al (2013) Osteoporosis in the European Union: a compendium of country-specific reports. Arch Osteoporos 8(1–2):137
Tella SH, Gallagher JC (2014) Prevention and treatment of postmenopausal osteoporosis. J Steroid Biochem Mol Biol 142:155–170
Diab DL, Watts NB (2013) Postmenopausal osteoporosis. Curr Opin Endocrinol Diabetes Obes 20(6):501–509
Vestergaard P, Rejnmark L, Mosekilde L (2006) Fracture risk associated with use of nonsteroidal anti-inflammatory drugs, acetylsalicylic acid, and acetaminophen and the effects of rheumatoid arthritis and osteoarthritis. Calcif Tissue Int 79(2):84–94
Vestergaard P, Hermann P, Jensen JE, Eiken P, Mosekilde L (2012) Effects of paracetamol, non-steroidal anti-inflammatory drugs, acetylsalicylic acid, and opioids on bone mineral density and risk of fracture: results of the Danish Osteoporosis Prevention Study (DOPS). Osteoporos Int 23(4):1255–1265
van Staa TP, Leufkens HG, Cooper C (2000) Use of nonsteroidal anti-inflammatory drugs and risk of fractures. Bone 27(4):563–568
Williams LJ, Pasco JA, Henry MJ, Sanders KM, Nicholson GC, Kotowicz MA et al (2011) Paracetamol (acetaminophen) use, fracture and bone mineral density. Bone 48(6):1277–1281
New SA, Robins SP, Campbell MK, Martin JC, Garton MJ, Bolton-Smith C et al (2000) Dietary influences on bone mass and bone metabolism: further evidence of a positive link between fruit and vegetable consumption and bone health? Am J Clin Nutr 71(1):142–151
Welch A, Bingham S, Camus J, Dalzell N, Reeve J, Day N et al (2005) Calcaneum broadband ultrasound attenuation relates to vegetarian and omnivorous diets differently in men and women: an observation from the European Prospective Investigation into Cancer in Norfolk (EPIC-Norfolk) population study. Osteoporos Int 16(6):590–596
Hamdi Kara I, Aydin S, Gemalmaz A, Akturk Z, Yaman H, Bozdemir N et al (2007) Habitual tea drinking and bone mineral density in postmenopausal Turkish women: investigation of prevalence of postmenopausal osteoporosis in Turkey (IPPOT Study). Int J Vitamin Nutr Res Internationale Zeitschrift fur Vitamin- und Ernahrungsforschung Journal international de vitaminologie et de nutrition 7(6):389–397
Prevention and management of osteoporosis. Report of a WHO Scientific Group. Geneva, World Health Organization, 2003 (WHO Technical Report Series, No. 921)
Turkish Statistical Institute Report (2013) 2012 poverty study. (http://www.tuik.gov.tr/PreHaberBultenleri.do?id=16023). Accessed 6 Dec 2013
Tuppurainen M, Kroger H, Saarikoski S, Honkanen R, Alhava E (1995) The effect of gynecological risk factors on lumbar and femoral bone mineral density in peri- and postmenopausal women. Maturitas 21(2):137–145
Ho SC, Chen YM, Woo JL (2005) Educational level and osteoporosis risk in postmenopausal Chinese women. Am J Epidemiol 161(7):680–690
Kroger H, Tuppurainen M, Honkanen R, Alhava E, Saarikoski S (1994) Bone mineral density and risk factors for osteoporosis—a population-based study of 1600 perimenopausal women. Calcif Tissue Int 55(1):1–7
Waugh EJ, Lam MA, Hawker GA, McGowan J, Papaioannou A, Cheung AM et al (2009) Risk factors for low bone mass in healthy 40–60 year old women: a systematic review of the literature. Osteoporos Int 20(1):1–21
New SA, Bolton-Smith C, Grubb DA, Reid DM (1997) Nutritional influences on bone mineral density: a cross-sectional study in premenopausal women. Am J Clin Nutr 65(6):1831–1839
Ryan PJ, Blake GM, Fogelman I (1992) Postmenopausal screening for osteopenia. Br J Rheumatol 31(12):823–828
Sioka C, Fotopoulos A, Georgiou A, Xourgia X, Papadopoulos A, Kalef-Ezra JA (2010) Age at menarche, age at menopause and duration of fertility as risk factors for osteoporosis. Climacteric 13(1):63–71
Hagemans ML, van der Schouw YT, de Kleijn MJ, van Staveren WA, Pop VJ, Leusink GL et al (2004) Indicators for the total duration of premenopausal endogenous estrogen exposure in relation to BMD. Hum Reprod 19(9):2163–2169
Hassa H, Tanir HM, Senses T, Oge T, Sahin-Mutlu F (2005) Related factors in bone mineral density of lumbal and femur in natural postmenopausal women. Arch Gynecol Obstet 273(2):86–89
Gerdhem P, Obrant KJ (2004) Bone mineral density in old age: the influence of age at menarche and menopause. J Bone Miner Metab 22(4):372–375
El Maghraoui A, Guerboub AA, Mounach A, Ghozlani I, Nouijai A, Ghazi M et al (2007) Body mass index and gynecological factors as determinants of bone mass in healthy Moroccan women. Maturitas 56(4):375–382
Francucci CM, Romagni P, Camilletti A, Fiscaletti P, Amoroso L, Cenci G et al (2008) Effect of natural early menopause on bone mineral density. Maturitas 59(4):323–328
Vico L, Prallet B, Chappard D, Pallot-Prades B, Pupier R, Alexandre C (1992) Contributions of chronological age, age at menarche and menopause and of anthropometric parameters to axial and peripheral bone densities. Osteoporos Int 2(3):153–158
Nguyen TV, Jones G, Sambrook PN, White CP, Kelly PJ, Eisman JA (1995) Effects of estrogen exposure and reproductive factors on bone mineral density and osteoporotic fractures. J Clin Endocrinol Metab 80(9):2709–2714
Kojima N, Douchi T, Kosha S, Nagata Y (2002) Cross-sectional study of the effects of parturition and lactation on bone mineral density later in life. Maturitas 41(3):203–209
Ozdemir F, Demirbag D, Rodoplu M (2005) Reproductive factors affecting the bone mineral density in postmenopausal women. Tohoku J Exp Med 205(3):277–285
Demir B, Haberal A, Geyik P, Baskan B, Ozturkoglu E, Karacay O et al (2008) Identification of the risk factors for osteoporosis among postmenopausal women. Maturitas 60(3–4):253–256
Okyay DO, Okyay E, Dogan E, Kurtulmus S, Acet F, Taner CE (2013) Prolonged breast-feeding is an independent risk factor for postmenopausal osteoporosis. Maturitas 74(3):270–275
Kovacs CS, Fuleihan Gel H (2006) Calcium and bone disorders during pregnancy and lactation. Endocrinol Metab Clin North Am 35(1):21–51
Ritchie LD, Fung EB, Halloran BP, Turnlund JR, Van Loan MD, Cann CE et al (1998) A longitudinal study of calcium homeostasis during human pregnancy and lactation and after resumption of menses. Am J Clin Nutr 67(4):693–701
Holmberg-Marttila D, Sievanen H, Laippala P, Tuimala R (2000) Factors underlying changes in bone mineral during postpartum amenorrhea and lactation. Osteoporos Int 11(7):570–576
Schnatz PF, Barker KG, Marakovits KA, O’Sullivan DM (2010) Effects of age at first pregnancy and breast-feeding on the development of postmenopausal osteoporosis. Menopause 17(6):1161–1166
Chantry CJ, Auinger P, Byrd RS (2004) Lactation among adolescent mothers and subsequent bone mineral density. Arch Pediatr Adolesc Med 158(7):650–656
Dursun N, Akin S, Dursun E, Sade I, Korkusuz F (2006) Influence of duration of total breast-feeding on bone mineral density in a Turkish population: does the priority of risk factors differ from society to society? Osteoporos Int 17(5):651–655
Tucker KL, Hannan MT, Chen H, Cupples LA, Wilson PW, Kiel DP (1999) Potassium, magnesium, and fruit and vegetable intakes are associated with greater bone mineral density in elderly men and women. Am J Clin Nutr 69(4):727–736
Ho-Pham LT, Nguyen ND, Nguyen TV (2009) Effect of vegetarian diets on bone mineral density: a Bayesian meta-analysis. Am J Clin Nutr 90(4):943–950
New SA (2004) Do vegetarians have a normal bone mass? Osteoporos Int 15(9):679–688
Das AS, Banerjee M, Das D, Mukherjee S, Mitra C (2013) Black tea may be a prospective adjunct for calcium supplementation to prevent early menopausal bone loss in a rat model of osteoporosis. J Osteoporos 2013:760586
Bauer DC, Orwoll ES, Fox KM, Vogt TM, Lane NE, Hochberg MC et al (1996) Aspirin and NSAID use in older women: effect on bone mineral density and fracture risk. Study of osteoporotic fractures research group. J Bone Mineral Res 11(1):29–35
Carbone LD, Tylavsky FA, Cauley JA, Harris TB, Lang TF, Bauer DC et al (2003) Association between bone mineral density and the use of nonsteroidal anti-inflammatory drugs and aspirin: impact of cyclooxygenase selectivity. J Bone Mineral Res 18(10):1795–1802
Diaz-Rodriguez L, Garcia-Martinez O, Arroyo-Morales M, Rubio-Ruiz B, Ruiz C (2010) Effect of acetaminophen (paracetamol) on human osteosarcoma cell line MG63. Acta Pharmacol Sin 31(11):1495–1499
Conflict of interest
The authors declare that they have no conflict of interest.
Human and Animal Rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the author.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study has been accepted as poster presentation in 23th EBCOG European Congress of Obstetrics and Gynaecology in Glasgow, Scotland (UK), on May 7th–10th 2014.
Rights and permissions
About this article
Cite this article
Cavkaytar, S., Seval, M.M., Atak, Z. et al. Effect of reproductive history, lactation, first pregnancy age and dietary habits on bone mineral density in natural postmenopausal women. Aging Clin Exp Res 27, 689–694 (2015). https://doi.org/10.1007/s40520-015-0333-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-015-0333-4