Abstract
Purpose
The potential dietary inflammatory index (DII) and the phenomenon of obesity have been linked in recent studies, but it is unclear whether this connection is dependent on metabolic status. Therefore, it was thought that this research would be useful in establishing the relationship between obesity phenotypes and DII.
Methods
The 5956 people who took part in the Ravansar non-communicable diseases (RaNCD) cohort research (MHNO) were put into four groups: metabolically unhealthy obesity (MUO), metabolically healthy obesity (MHO), metabolically unhealthy non-obesity (MUNO), and metabolically healthy non-obesity. According to the International Diabetes Federation's criteria, MUO exhibits at least two metabolic disorders and have a body mass index of 30 kg/m2 or higher. DII was extracted from the participant's dietary consumption data.
Results
When possible confounders like age, gender, smoking, drinking alcohol, and exercise were taken into account, more adherence to DII was linked to a higher odds of MHO compared to MHNO (OR: 1.44; CI 95% 1.18, 1.75). Additionally, we discovered that greater adherence to DII was significantly related to higher odds for MUO compared to MHNO (OR: 1.67; CI 95% 1.3, 2.15). However, we found no association between adherence to DII and MUNO.
Conclusions
Our findings indicated that greater adherence to DII was significantly associated with higher odds of MUO. However, it substantially increased the chances of both phenotypes of obesity.
Level of evidence Level V–Cross-sectional observational study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is an inflammatory metabolic phenomenon that appears as a result of excess fat accumulation and is considered a worldwide public health burden [1]. Nowadays, one-third of the world's population suffers from overweight and obesity [2]. As stated by the World Health Organization (WHO) reports, the prevalence of overweight and obesity among global adults was 46% and 13%, whereas in Iran it was 62.3% and 26.1%, respectively, by 2016 [3]. If this trend persists, projections indicate that by 2025, obesity will affect over 18% of men, 21% of women, and 124 million children and adolescents globally [4].
Studies have demonstrated that oxidative stress and pro-inflammatory effects of obesity can cause metabolic disorders such as dyslipidemia and insulin resistance, which lead to several diseases such as cardiovascular diseases, various cancers, type 2 diabetes mellitus, and non-alcoholic fatty liver [5,6,7]. Despite various studies showing that a group of overweight and obese individuals do not suffer from chronic disease, they are referred to as "Metabolically Healthy Obese (MHO) (MHO)" [8]. These individuals have favorable metabolic profiles, which include insulin sensitivity, a lipid profile, low blood pressure, and lower levels of circulating C-reactive protein (CRP). Therefore, they not only have a lower risk of mortality but also have a better prognosis compared to their metabolically unhealthy obese (MUO) phenotype [9, 10].
Because diet is a significant modifiable risk factor for obesity and its consequences, several dietary scores have recently been developed to examine the relationship between overall diet quality and different diseases. The Dietary Inflammatory Index (DII) is an emerging analysis approach to a literature-based algorithm scoring tool that was first introduced in 2009 to measure the inflammatory potential of a diet. DII consists of 45 pro-inflammatory and anti-inflammatory dietary components, categorized into healthy and unhealthy (Western) patterns. The negative score indicates anti-inflammatory effects of the diet, while the positive score shows the inflammatory effects [11]. Many studies showed the relationship between DII and chronic diseases such as cardiovascular diseases [5], certain types of cancers [7], and diabetes mellitus (5).
According to previous studies, the high prevalence of overweight and obesity was seen in the Kurdish population [12, 13]. Thus we conducted this study to find out whether DII has any effect on healthy versus unhealthy obese phenotypes or not in the Ravansar non-communicable diseases (RaNCD) cohort study.
Methods
Study design and participants
In a nutshell, the PERSIAN (Prospective Epidemiological Research Studies in Iran) mega cohort study is a large-scale, multi-center investigation of non-communicable diseases (NCDs) in Iran, and its protocol has been approved by the ethics committees of the Ministry of Health and Medical Education, the Digestive Diseases Research Institute, and Tehran University of Medical Sciences [14, 15]. The RaNCD study is the first cohort study to look into NCDs in a Kurdish community (4764 men and 5283 women) aged 35–65 in Ravansar, Kermanshah province, Western Iran. It is one of the sites supported by the PERSIAN mega cohort study. The Kermanshah University of Medical Sciences Ethics Committee gave its permission to all of the study's procedures (ethics approval number: IR.KUMS.REC.1402.251). Additionally, a written informed permission form was voluntarily signed by each participant.
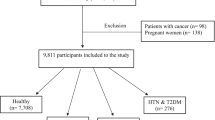
We used data from the RaNCD cohort study requirement phase with 5956 participants for the present cross-sectional analysis. Participants with CVDs (n = 1684), diabetes mellitus (n = 490), hypertension (n = 372), thyroid disease (n = 507), cancer (n = 57), and expectant and nursing women (n = 138) were excluded. Furthermore, we excluded participants whose calorie intake was less than 800 kcal/day or 4200 kcal/day for males and 600 kcal/day or 3500 kcal/day for women (n = 788). After we excluded 55 individuals due to insufficient data, we were left with 5956 individuals with evaluable data.
Measurements
We obtained demographic data (age, gender, and socio-economic status (SES)), physical activity data, smoking and drinking history, blood pressure assessment, anthropometric measurements, biochemical indices, as well as dietary intake. Anthropometric measurements were assessed using standard protocols in the RaNCD cohort center. Moreover, blood samples were collected to assess biochemical indices using standard protocols designed by Persian mega cohort. The levels of total cholesterol (TC), high-density lipoprotein (HDL), triglycerides (TG), and low-density lipoprotein (LDL) were checked using enzymatic kits, and the levels of fasting blood sugar (FBS) were checked using the glucose oxidase technique (Pars Azmun, Iran). Earlier studies published the specifics of these measurements [15, 16].
Dietary inflammatory index
DII was calculated using dietary intake from a standardized semi-quantitative food frequency questionnaire (FFQ) [17, 18]. Shivapa et al. [19] have detailed the DII's design and development. They introduced 45 dietary parameters to impact inflammatory and pro-inflammatory factors such as interleukins (IL-1B, IL-4, IL-6, IL-10), tumor necrosis factor-a (TNF-a), and CRP.
In the current study, we only had access to 31 of the 45 food parameters introduced by Shivapa, which included: vitamin A, vitamin B6, vitamin B12, vitamin C, vitamin D, vitamin E, folic acid, niacin, iron, zinc, selenium, magnesium, beta-carotene, caffeine, thiamin, riboflavin, onion, garlic, tea, omega 3, omega 6, trans fat, saturated fats (SFAs), cholesterol, and monounsaturated. To create the DII, the "standard global mean" was subtracted from the actual amount of dietary intake and divided by its standard deviation. The Z-scores were then converted to a percentile by doubling the number and subtracting 1. Lastly, the total of the DII scores was calculated. Furthermore, DII were divided into three groups to examine the connection between various variables. The first and third tertiles reflect the less inflammatory diet and the more pro-inflammatory diet, respectively.
Outcome
We defined MUO as having a BMI of more than 30 kg/m2 and at least two metabolic disorders, such as HDL levels of 40 mg/dl in men and 50 mg/dl in women, TG levels of more than 150 mg/dl, SBP levels of more than 130 mmHg or DBP levels of more than 80 mmHg or taking antihypertensive medication, and FBS levels of more than 100 mg/dl or taking diabetes medication [20]. MHO was defined as having a BMI greater than 30 kg/m2 and having at least one of the metabolic disorders listed above. The MUNO phenotype is characterized by a BMI less than 30 kg/m2 and at least two of the aforementioned metabolic disorders. Finally, MHNO participants were classified as either healthy or having at least one metabolic disorder.
Statistical analysis
We used mean ± standard deviation (SD) and frequency (%) to describe all variables. We described each measure using its mean, standard deviation (SD), and frequency (%). Using the Chi-square and ANOVA tests, the basic features of the study subjects were revealed based on the tertiles of DII and the obesity phenotypes. We used multinomial logistic regression, along with crude and modified odds ratios (OR) and 95% confidence intervals (CI), to look into the link between the tertiles of DII and the different types of obesity. In modified model 1, the age (continuous) and sex variables were controlled (categorical). Additionally, we took into account sex (categorical), history of drinking and smoking, SES (categorical), menopause status (categorical), and physical activity (continuous) as possible confounders in adjusted Model 2. The reference group for DII was thought to be the first tertile. On top of that, binary logistic regression with crude and adjusted odds ratios and 95% confidence intervals was used to find the link between different aspects of obesity across tertiles of DII. Along with logistic regression correction for the above variables, a figure of linear regression OR across elevated DII and parts of the obesity phenotypes was also looked at to help show this link even more clearly. The significance threshold for all statistical analyses was set at 0.05 using Stata, version 14, and SPSS 20, both from IBM Corp. in Chicago, Illinois, and College Station, Texas, respectively.
Results
51.9% of the 5956 participants who fulfilled our study's inclusion criteria were men. In comparison to other phenotypes, the mean of weight, BMI, and WC was significantly greater in the MUO phenotype (p < 0.001). In comparison to other phenotypes, the mean PA in the MHNO phenotype was considerably higher (p < 0.001). Both MHO and MUO were found to have higher levels of inflammatory diets, but MUO had the substantially highest DII score (p < 0.001) of any phenotype. Based on obesity phenotypes, the basic characteristics of the study subjects are described in Table 1. On the other hand, we observed that greater adherence to DII was significantly associated with higher weight, BMI, an inappropriate glycemic profile, blood pressure, and a lipid profile, as well as these factors (Table 2). Our research had 5956 participants, 51.9% of whom were men. The MUO phenotype had substantially higher mean weight, BMI, and WC than the other phenotypes (p < 0.001). The MHNO phenotype had a substantially higher mean PA than the other phenotypes (p < 0.001). Both MHO and MUO had more inflammatory dietary habits, but the MUO phenotype had the highest DII score (p < 0.001). In total, 14.5% of the studied women were in menopause, and its distribution was not significantly different between obesity phenotypes and DII categories. Table 2 describes the basic characteristics of the research participants based on their DII categories.
Higher adherence to DII, on the other hand, was strongly associated with increased weight, BMI, an inappropriate glycemic profile, blood pressure, and lipid profile.
Higher adherence to DII was significantly associated with higher odds for MHO compared to MHNO (OR: 1.28; CI 95% 1.06, 1.54). This was demonstrated by multinomial regression logistic odds ratios and 95% confidence intervals for obesity phenotypes across DII tertiles. This association was strengthened after adjusting for potential confounders like age, gender, smoking, drinking alcohol, SES, menopause, and physical activity (OR: 1.44; CI 95% 1.18, 1.75). Additionally, we discovered that in both crude and adjusted models, increased adherence to DII was significantly associated with higher odds for MUO when compared to MHNO (OR: 1.53; CI 95% 1.2, 1.95) and (OR: 1.67; CI 95% 1.3, 2.15), respectively. The link between adherence to DII and MUNO, however, was not observed. Despite this, no relationship could be detected after adjusting for potential confounders (P-trend = 0.302). (Table 3).
As in the present study, we assessed the relationship between adherence to DII and the components of obesity phenotypes (Table 4). Figure 1 shows that higher adherence to DII was significantly associated with a higher risk of obesity (OR: 1.49; CI 95% 1.28, 1.76) (Fig. 1a), lower HDL (OR: 1.26; CI 95% 1.11, 1.44) (Fig. 1b), and higher blood pressure (OR: 1.42; CI 95% 1.06, 1.9) (Fig. 1c).
Discussion
The present study used DII as a representative of dietary inflammation. As our results indicated, higher adherence to DII was significantly associated with higher odds for both MHO and MUO. Moreover, the odds for BMI, blood pressure, and low HDL were increased with greater following DII. As expected, we also observed higher adherence to DII was accompanied by higher weight, BMI, unappropriated glycemic profile, blood pressure, as well as, lipid profile. However, odds for MUNO were increased; this association was not significant.
Since obesity is accompanied by an increased risk of NCDs, it boosted the burden of health system costs [21, 22]. Furthermore, numerous studies have established a connection between the inflammatory potential of a diet and factors such as overweight, obesity, cardiovascular disease, and cancer [23, 24]. Considering the high prevalence of obesity in the Kurdish ethnicity [12] and its importance in the occurrence of NCDs [8], it is essential to study both obesity phenotypes and diet.
As Saghafi-Asl and colleagues mentioned, higher adherence to DII was related to the lower healthy food intake and a higher BMI [25]. A cross-sectional study of obese individuals mirrored our findings, showing a positive correlation between a higher DII score and MUO phenotypes [26]. In addition, a cohort study indicated that higher adherence to DII was associated with a higher body fat percentage [27]. It is due to the mutual association between inflammation and body fat that higher body fats may increase inflammatory cytokines and pre-inflammatory diets may increase adipose tissue [28]. However, the underlying mechanisms of DII on BMI are not clearly understood. In the animal models, it has been observed that a high-fat diet increases the production of inflammatory factors that potentially induce inflammation or ER stress markers in NPY/AgRP neurons, which stimulate appetite in the hypothalamus, and play a role in increasing appetite [29, 30]. Since the increase in fat consumption is an important factor in DII calculation, its consumption modification should be recommended. One study found that diets with a higher DII score do increase the level of CRP and the risk of metabolic syndrome [31]. Another theory asserted that diet plays a role in causing inflammation. Diet could be an amplifier for the level of oxidative stress in the body, which alters the inflammatory status. For example, a high-fat diet activated a pro-inflammatory pathway that increased inflammatory cytokines [32]. Whereas obesity is propounded as an inflammatory status, a feasible mechanism for the relationship between obesity and chronic disease is based on the theory of inflammation and inflammatory markers like TNF-α, CRP, and IL-6 [33]. For instance, in obesity situations, the excessive adipose tissues not only increase leptin and cytokines production but also decrease anti-inflammatory immune cells, which cause inflammation [34]. That is why adherence to DII leads in obesity. Moreover, as is presented in this study, higher adherence to DII was related to lower HDL levels, higher blood pressure, and serum triglyceride, which is correlated with metabolic syndrome and CVDs in obese individuals [35]. Despite the unknown mechanism, a theory declared that a pro-inflammatory diet may increase the level of cytokines, which lead to attraction and migration of inflammatory cells into vascular tissues [36]. A systematic review and meta-analyses showed that a higher DII is strongly linked to MetS and its components, such as abdominal obesity, high blood pressure, hyperglycemia, and hyperglycerolaemia [37]. Our study aligns with previous research, showing a significant correlation between a higher DII score and an increased risk of metabolic syndrome and its associated complications [38].
Strength and limits
Our study had some limitations, the most important of which was the cross-sectional design, which does not specify cause and effect. Also, we did not have information about all of the items that Shivapa mentioned to calculate DII, so the range of DII in our study may be different from other studies. On the other hand, FFQ data were used for dietary intake, which may have recall bias, although a trained nutritionist was used to collect nutritional information to reduce this error.
Conclusion
In conclusion, our findings demonstrated that higher adherence to DII was significantly associated with higher odds for MUO compared to MHNO. Nevertheless, it significantly increased the odds of both phenotypes of obesity. Furthermore, a higher DII score was linked to higher weight, BMI, an inappropriate glycemic profile, blood pressure, and a lipid profile, while it did decrease the level of HDL. However, further studies are necessary to clarify the potential effects of DII on the four types of obesity.
What is already known on this subject?
In general, the prevalence of obesity is increasing. There are many obese people who are metabolically healthy and are called metabolically healthy obesity (MHO). But some people with obesity have an unhealthy metabolic profile, which is called metabolically unhealthy obesity (MUO), and these people are at a greater risk of developing NCDs. Knowing the dietary factors affecting them can be helpful in reducing the risk of developing NCDs.
What this study adds?
This present study indicated that higher adherence to DII was significantly associated with higher odds for MUO compared to MHNO. Nevertheless, it significantly increased the odds of both phenotypes of obesity. In addition to that, a greater score in DII was associated with higher weight, BMI, inappropriate glycemic profile, blood pressure, as well as lipid profile, but it does lower the level of HDL.
Availability of data and materials
Data will be available upon request from the corresponding author. No datasets were generated or analysed during the current study.
References
Paczkowska-Abdulsalam M, Kretowski A (2021) Obesity, metabolic health and omics: current status and future directions. World J Diabetes 12(4):420
Hojjat TA (2021) Introduction: the magnitude of the obesity problem. The economics of obesity. Springer, Cham, pp 1–9
Karimbeiki R, Alipoor E, Yaseri M, Shivappa N, Hebert JR, Hosseinzadeh-Attar MJ (2021) Association between the dietary inflammatory index and obesity in otherwise healthy adults: role of age and sex. Int J Clin Pract 75(10):e14567
Kelly T, Yang W, Chen C-S, Reynolds K, He J (2008) Global burden of obesity in 2005 and projections to 2030. Int J Obes 32(9):1431–1437
Hariharan R, Odjidja EN, Scott D, Shivappa N, Hébert JR, Hodge A, de Courten B (2022) The dietary inflammatory index, obesity, type 2 diabetes, and cardiovascular risk factors and diseases. Obes Rev 23(1):e13349
Moradi F, Heidari Z, Teimori A, Ghazvini M, Imani ZF, Naeini AA (2022) The association between the dietary inflammatory index (DII) and some serum oxidative stress markers in Non-Alcoholic fatty liver disease: case-control. Int J Prevent Med 13:93
Syed Soffian SS, Mohammed Nawi A, Hod R, Jaa’far MH, Isa ZM, Chan H-K, Hassan MRA (2022) Meta-analysis of the association between dietary inflammatory index (DII) and colorectal cancer. Nutrients 14(8):1555
Hamzeh B, Pasdar Y, Moradi S, Darbandi M, Rahmani N, Shakiba E, Najafi F (2022) Metabolically healthy versus unhealthy obese phenotypes in relation to hypertension incidence; a prospective cohort study. BMC Cardiovasc Disord 22(1):1–7
Matta J, Nasreddine L, Jomaa L, Hwalla N, Mehio Sibai A, Czernichow S, Itani L, Naja F (2016) Metabolically healthy overweight and obesity is associated with higher adherence to a traditional dietary pattern: a cross-sectional study among adults in Lebanon. Nutrients 8(7):432
Cheng D, Zhao X, Yang S, Cui H, Wang G (2021) Metabolomic signature between metabolically healthy overweight/obese and metabolically unhealthy overweight/obese: a systematic review. Diabetes Metab Syndr Obes Targets Ther 14:991
Pawlow X, Ott R, Winkler C, Ziegler A-G, Hummel S (2021) A new mathematical approach to improve the original dietary inflammatory index (DII) calculation. PLoS ONE 16(11):e0259629
Pasdar Y, Moradi S, Abdollahzad H, Hamzeh B, Najafi F, Nachvak SM, Mostafai R (2019) Accuracy of waist to hip ratio calculated by bioelectric impedance device in the Ravansar non-communicable disease cohort study. Top Clin Nutr 34(4):269–276
Pasdar Y, Moradi S, Moludi J, Saiedi S, Moradinazar M, Hamzeh B, Jafarabadi MA, Najafi F (2020) Waist-to-height ratio is a better discriminator of cardiovascular disease than other anthropometric indicators in Kurdish adults. Sci Rep 10(1):1–10
Pasdar Y, Najafi F, Moradinazar M, Shakiba E, Karim H, Hamzeh B, Nelson M, Dobson A (2019) Cohort profile: ravansar non-communicable disease cohort study: the first cohort study in a Kurdish population. Inter J Epidemiol 48(3):682–683f
Poustchi H, Eghtesad S, Kamangar F, Etemadi A, Keshtkar A-A, Hekmatdoost A, Mohammadi Z, Mahmoudi Z, Shayanrad A, Roozafzai F (2018) Prospective epidemiological research studies in Iran (the PERSIAN Cohort Study): rationale, objectives, and design. Am J Epidemiol 187(4):647–655
Pasdar Y, Najafi F, Moradinazar M, Shakiba E, Karim H, Hamzeh B, Nelson M, Dobson A (2019) Cohort profile: Ravansar non-communicable disease cohort study: the first cohort study in a Kurdish population. Int J Epidemiol. https://doi.org/10.1093/ije/dyy296
Moradi S, Pasdar Y, Hamzeh B, Najafi F, Nachvak SM, Mostafai R, Niazi P, Rezaei M (2018) Comparison of 3 nutritional questionnaires to determine energy intake accuracy in Iranian adults. Clin Nutrit Res 7(3):213–222
Mirmiran P, Esfahani FH, Mehrabi Y, Hedayati M, Azizi F (2010) Reliability and relative validity of an FFQ for nutrients in the Tehran lipid and glucose study. Public Health Nutr 13(5):654–662
Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR (2014) Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr 17(8):1689–1696
Alberti KGM, Zimmet P, Shaw J (2005) The metabolic syndrome—a new worldwide definition. Lancet 366(9491):1059–1062
Desai K, Arora P (2023) Burden of infectious diseases and strategies of prevention. In: viral, parasitic, bacterial, and fungal infections. Elsevier, Amsterdam, pp 49–61
Leggio M, Lombardi M, Caldarone E, Severi P, D’emidio S, Armeni M, Bravi V, Bendini MG, Mazza A (2017) The relationship between obesity and hypertension: an updated comprehensive overview on vicious twins. Hypertens Res 40(12):947–963
Zahedi H, Djalalinia S, Asayesh H, Mansourian M, Abdar ZE, Gorabi AM, Ansari H, Noroozi M, Qorbani M (2020) A higher dietary inflammatory index score is associated with a higher risk of incidence and mortality of cancer: a comprehensive systematic review and meta-analysis. Int J Prevent Med 11:15
Farhangi MA, Vajdi M (2020) The association between dietary inflammatory index and risk of central obesity in adults: an updated systematic review and meta-analysis. Int J Vitamin Nutrit Res. https://doi.org/10.1024/0300-9831/a000648
Saghafi-Asl M, Mirmajidi S, Asghari Jafarabadi M, Vahid F, Shivappa N, Hébert JR, Ebrahimzadeh Attari V (2021) The association of dietary patterns with dietary inflammatory index, systemic inflammation, and insulin resistance, in apparently healthy individuals with obesity. Sci Rep 11(1):7515. https://doi.org/10.1038/s41598-021-86993-7
Abdurahman AA, Azadbakhat L, Rasouli M, Chamari M, Qorbani M, Dorosty AR (2019) Association of dietary inflammatory index with metabolic profile in metabolically healthy and unhealthy obese people. Nutr Diet 76(2):192–198
Gholamalizadeh M, Ahmadzadeh M, BourBour F, Vahid F, Ajami M, Majidi N, Hajipour A, Doaei S, Kalantari N, Alizadeh A, Jarrahi AM (2022) Associations between the dietary inflammatory index with obesity and body fat in male adolescents. BMC Endocr Disord 22(1):115. https://doi.org/10.1186/s12902-022-01001-x
Ruiz-Canela M, Zazpe I, Shivappa N, Hébert JR, Sánchez-Tainta A, Corella D, Salas-Salvadó J, Fitó M, Lamuela-Raventós RM, Rekondo J, Fernández-Crehuet J, Fiol M, Santos-Lozano JM, Serra-Majem L, Pinto X, Martínez JA, Ros E, Estruch R, Martínez-González MA (2015) Dietary inflammatory index and anthropometric measures of obesity in a population sample at high cardiovascular risk from the PREDIMED (PREvención con DIeta MEDiterránea) trial. Br J Nutr 113(6):984–995. https://doi.org/10.1017/s0007114514004401
Dalvi P, Chalmers J, Luo V, Han D-Y, Wellhauser L, Liu Y, Tran D, Castel J, Luquet S, Wheeler M (2017) High fat induces acute and chronic inflammation in the hypothalamus: effect of high-fat diet, palmitate and TNF-α on appetite-regulating NPY neurons. Int J Obes 41(1):149–158
Briggs DI, Lockie SH, Benzler J, Wu Q, Stark R, Reichenbach A, Hoy AJ, Lemus MB, Coleman HA, Parkington HC, Tups A, Andrews ZB (2014) Evidence that diet-induced hyperleptinemia, but not hypothalamic gliosis, causes ghrelin resistance in NPY/AgRP neurons of male mice. Endocrinology 155(7):2411–2422. https://doi.org/10.1210/en.2013-1861
Wirth MD, Burch J, Shivappa N, Violanti JM, Burchfiel CM, Fekedulegn D, Andrew ME, Hartley TA, Miller DB, Mnatsakanova A, Charles LE, Steck SE, Hurley TG, Vena JE, Hébert JR (2014) Association of a dietary inflammatory index with inflammatory indices and metabolic syndrome among police officers. J Occup Environ Med 56(9):986–989. https://doi.org/10.1097/jom.0000000000000213
Duan Y, Zeng L, Zheng C, Song B, Li F, Kong X, Xu K (2018) Inflammatory links between high fat diets and diseases. Front Immunol 9:2649. https://doi.org/10.3389/fimmu.2018.02649
Taylor EB (2021) The complex role of adipokines in obesity, inflammation, and autoimmunity. Clin Sci 135(6):731–752
Kawai T, Autieri MV, Scalia R (2021) Adipose tissue inflammation and metabolic dysfunction in obesity. Am J Physiol Cell Physiol 320(3):C375–C391
Welty FK (2013) How do elevated triglycerides and low HDL-cholesterol affect inflammation and atherothrombosis? Curr Cardiol Rep 15(9):400. https://doi.org/10.1007/s11886-013-0400-4
Li X, Chen B, Zhang J, Li M, Zhang Z, Zhu Y, Zeng XF, Li H, Wang Y, Wang SF, Hu A, Zhao Q, Yang W (2022) Association of dietary inflammatory potential with risk of overall and cause-specific mortality. Br J Nutr 127(12):1878–1887. https://doi.org/10.1017/s0007114521002907
Yi Q, Li X, He Y, Xia W, Shao J, Ye Z, Song P (2021) Associations of dietary inflammatory index with metabolic syndrome and its components: a systematic review and meta-analysis. Public Health Nutr 24(16):5463–5470. https://doi.org/10.1017/s1368980021000288
Abdollahzad H, Pasdar Y, Nachvak SM, Rezaeian S, Saber A, Nazari R (2020) The relationship between the dietary inflammatory index and metabolic syndrome in ravansar cohort study. Diabetes Metab Syndr Obes Targets Ther 13:477
Acknowledgements
RaNCD is part of PERSIAN national cohort and we would like to thank Professor Reza Malekzadeh, Deputy of Research and Technology at the Ministry of Health and Medical Education of Iran and Director of the PERSIAN cohort, and also Dr. Hossein Poustchi Executive Director of PERSIAN cohort for all their supports during design and running of RaNCD.
Funding
This study was supported by Ministry of Health and Medical Education of Iran and Kermanshah University of Medical Science (Grant No: 4020512).
Author information
Authors and Affiliations
Contributions
SM and YP equally contributed to the conception and design of the research; FN, BA, and YP contributed to data collection; SM, MS and SR contributed to the acquisition and analysis of the data; SM and MS contributed to the interpretation of the data; and SM, AS, DS, MS and YP contributed to draft the manuscript. SM, SC, and MS contributed to critically revise the manuscript. All authors are in agreement with the manuscript and declare that the content has not been published elsewhere.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the Ethics Committee of Kermanshah University of Medical Sciences (ethics approval number: IR.KUMS.REC.1402.251).
Informed consent
Written informed consent was obtained from each studied subject after explaining the purpose of the study. The right of subjects to withdraw from the study at any time and subject’s information is reserved and will not be published.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Pasdar, Y., Sharifi, M., Saber, A. et al. Dietary inflammatory index and its relationship with obesity phenotypes: a cross- sectional analysis from RaNCD cohort study. Eat Weight Disord 29, 56 (2024). https://doi.org/10.1007/s40519-024-01686-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40519-024-01686-0