Abstract
Purpose
We aimed to study the occurrence of long-term changes in appetite, taste, smell perceptions, and food aversion in patients following bariatric surgery. Additionally, we compared two surgery types, excess weight loss, rate of weight regain, and time since surgery.
Methods
This cross-sectional study included 146 post-bariatric patients who were without regular medical follow-up (126 post-Roux-en-Y gastric bypass [RYGB] and 20 post-sleeve gastrectomy [SG]), aged 42 ± 8 years, BMI of 32.6 ± 6.3 kg/m2, with excess weight loss of 87.5 ± 20.2%, rate of weight regain (RWR) of 15.4 [3.9–30.9]% and time since surgery of 5.0 ± 4.0 years. They answered a questionnaire about sensory and food perceptions at their first medical appointment at our unit.
Results
Changes in appetite (76%), taste (48.6%), and an increased sensation for sweet taste (60.2%) frequently occurred in our sample. Sensory and food aversion perceptions, taste changes to specific foods, and loss level of taste and smell were similar between RYGB and SG. No differences between patients with or without changes in appetite, taste, smell, and food aversion perceptions concerning excess weight loss were observed. The RWR in post-RYGB was lower in those with changes in taste and smell (P = 0.05). Sensory changes were noted in those with shorter time since surgery for both surgeries (P ≤ 0.05).
Conclusion
Changes in appetite and taste occurred frequently in our patients even in the long term. Post-RYGB patients with lower RWR had more changes in taste and smell while a shorter time since surgery showed more frequent changes in appetite, taste, and smell.
Level of evidence
Level V, cross-sectional study.
Trial registration number
ClinicalTrials.gov (NCT04193384).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bariatric surgery is effective in treating obesity showing sustained weight loss. Additionally, it improves or prevents obesity-related comorbidities and also reduces mortality rates [1, 2]. Several mechanisms contribute to weight loss after surgery, including reducing caloric intake by restricting food, malabsorption of nutrients, and altered secretion of gastric and intestinal peptides [3, 4]. Changes in appetite, taste, and smell perceptions resulting from adipose–gut–brain axis modifications [5] appear to be another possible contributing mechanism that affects food intake, caloric consumption, and subsequent weight loss after surgery [6, 7].
Rapid and substantial weight loss may occur within the first two years after the procedure [8, 9]. Unfortunately, some of these patients regain weight [10, 11]. Magro et al. reported rates of weight regain (RWR) varying from 46 to 63% between 2 and 4 years post surgery, respectively [11]. RWR as high as 75% within six years post surgery were also described [10]. Weight recidivism is multifactorial, and a relationship between regaining weight and recurrence of obesity-related comorbidities or even difficulty in managing them is already known [12].
Surgical failure, metabolic and hormonal imbalances, mental disorders, nutritional non-adherence, and physical inactivity are involved alone or together in the multifactorial etiology of weight regain. However, many possible factors are still unclear [13]. It is already known that post-bariatric patients may have changes in appetite, taste, and smell perceptions [7, 14], and maybe these factors could be predictors for weight recidivism. Lynch et al. showed that for gastric bypass patients, the maintenance of healthy eating habits for the long term is hard to accomplish [15]. An altered taste perception has an essential role in food preferences and eating behavior [16, 17]. Therefore, it is possible that changes in taste and smell perceptions can influence these food preference patterns [18], which supports the importance of assessing sensory changes and their possible influence on weight management. Maybe higher rates of sensory and appetite changes may positively influence weight maintenance after surgery. Some points on this area are still unclear, like possible sensory and appetite changes that may occur regardless of the type of surgery [19, 20] or even if it is sustained for several years [19] and if these changes could also be influenced by the weight variations [13].
Optimizing long-term weight loss and minimizing weight recidivism in patients following bariatric surgery challenge health professionals [20]. We hypothesize that sensory and appetite changes would be frequently presented after surgery, especially in those with lower RWR. Additionally, we suppose that these altered perceptions vary according to the bariatric procedure employed and time since surgery. Therefore, we aimed to study appetite, taste and smell perceptions, and food aversion in a cohort of post-bariatric patients. We also aimed to compare patients subjected to Roux-en-Y gastric bypass (RYGB) vs. Sleeve Gastrectomy (SG) and to investigate the excess weight loss (EWL), the RWR, and time since surgery in patients with sensory and food changes vs. counterparts without these changes.
Subjects and methods
During two years, we enrolled 269 volunteers previously subjected to bariatric surgery in private or public health care units who were without any clinical follow-up, as previously described [21]. Of those, 146 patients (126 post-RYGB and 20 post-SG) entered the study. Recruitment was performed at the Obesity Unit for outpatient care at their first appointment on it. The study was approved by the local Ethics Committee (CAAE: 07,662,918.1.0000.5259) and registered in ClinicalTrials.gov (NCT04193384). All procedures involving the participants were performed according to the principles outlined in the Declaration of Helsinki.
The inclusion criterion was to be a post-bariatric patient after one year since surgery. Exclusion criteria were: (a) use any medication or gastrointestinal disease that might affect appetite, taste, and smell; 9b) incomplete information on anthropometric and surgery data (procedure data and pre- and postoperative weight); (c) unanswered questionnaires or (d) questionnaires not adequately fulfilled.
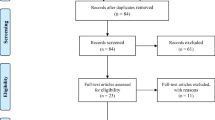
This group was subsequently divided into two groups according to the type of bariatric procedures employed (RYGB or SG). In each of the latter groups, patients were then reallocated into new subgroups, according to the presence or absence of appetite, taste, smell, and food aversion changes. Figure 1 presents the flowchart of the enrollment, allocation, and follow-up of participants.
Participants were subjected to a pre-participation screening before being included in the study. In this visit, the research protocol was explained, and the written informed consent was obtained from all volunteers. Afterward, a clinical history, physical examination, demographic, anthropometric, heart rate, and blood pressure measurements, and questionnaire application were performed. All patients confirmed the surgery procedure by digestive endoscopy.
Body mass and height were measured by a calibrated electronic scale and stadiometer (Welmy™ W300A, São Paulo, SP, Brazil). BMI was calculated. Waist circumference was measured at the umbilicus after expiration and hip at the widest circumference around the gluteal region. BMI and waist-to-hip ratio were calculated. Neck circumference was measured along the inferior margin of the laryngeal prominence and perpendicular to the long axis of the neck. All measures were performed twice by the same trained nurse. Preoperative weight and minimum postoperative weight were obtained by self-reporting in the first visit and after three months [21].
EWL and RWR were calculated, respectively: (preoperative weight–minimum postoperative weight)/(preoperative weight–ideal weight for BMI of 25 kg/m2) × 100% and (current weight–minimum weight postoperative)/(preoperative weight–minimum weight postoperative) × 100% and expressed as percentages. Blood Pressure (BP) was measured twice in a sitting position by a semi-automated oscillometric device (G-Tech™ BSP11, Hangzhou, Zhejiang, China). At the end of these measurements, they got the questionnaire and were followed to a quiet room to answer it while waiting for the medical appointment. This questionnaire was adapted from a previously used and validated one by Tichansky et al. [14], modified by Graham et al.[7]. It has 33 questions about postoperative sensory and appetite changes with a subjective assessment of postoperative changes in appetite, the taste of food or drink, smell, and food aversion. This tool starts with four simple questions about these perceptions with direct answers and subscale sequences to grade them (for detailed information, go to supplementary material).
Data were expressed as mean ± standard deviation (SD) or median [percentiles 25–75]. The Shapiro–Wilk test tested data normality. The statistical power was calculated using G-Power (Universität Kiel, Kiel, Germany) through the differences between two independent means [two groups, according to differences in RWR[22] with a priori power analysis, an effect size of 0.5, α error probability of 0.05, sample power of 0.80, allocation ratio of 1 into two groups; resulting in a total sample size of 106 subjects. Chi-square tests were used for categorical data and presented as percentages of the total. Between-groups differences were determined by unpaired Student’s t test or Mann–Whitney U test, as appropriate. All calculations were performed using the NCSS™ statistical software (LLC, Kaysville, Utah, USA), and statistical significance was set at P < 0.05.
Results
Out of 269 individuals enrolled, 151 patients answered the questionnaire. A total of 146 (96.7%) patients adequately fulfilled them and were included in the final analysis (126 post-RYGB and 20 post-SG). Of these, 130 females/16 males, aging 42 ± 8 years, body mass index (BMI) of 32.6 ± 6.3 kg/m2, EWL of 87.5 ± 20.2%, RWR of 15.4 [3.9–30.9]%, with time since surgery of 5.0 ± 4.0 years. The pooled sample (PS) represents all the patients included in the final analysis (n = 146).
Table 1 presents the demographic characteristics, clinical and surgical data of the patients. The groups were similar for all variables (P ≥ 0.09 for all comparisons), except for age, preoperative weight, preoperative BMI, and time since surgery, all higher in RYGB than SG.
Sensory and food perceptions
Data for sensory and food perceptions in the pooled sample (PS) and following RYGB and SG are depicted in Table 2. In the PS, the highest percentages of changes were reported in appetite (76%), in taste (48.6%) perception, and also in an increased sensation for sweet taste (60.2%), followed by salty taste (30.2%). No difference between types of surgery was observed for sensory and food changes, taste changes to a specific food, or loss level of taste and smell (P ≥ 0.10, for all comparisons). An expressive percentage of patients reported changes in appetite (RYGB = 75.4% and SG = 80%), in taste (RYGB = 46% and SG = 65%), and also an increased sensation for sweet taste (RYGB = 57.4% and SG = 76.5%) in both types of surgery.
Excess weight loss, rate of weight regain, and surgery time
Table 3 exhibits results of EWL, RWR, and time since surgery of patients with and without changes in sensory and food perceptions. No difference was observed between those with or without changes in appetite, taste, smell, and food aversion perceptions and EWL in the PS or according to the type of surgery (P ≥ 0.06, for all comparisons). In the PS, there was a significant difference between the change in smell and lower RWR (P = 0.02) and between changes in appetite (P = 0.03), taste (P = 0.004), and smell (P < 0.001) and less time since surgery. The RWR was lower in patients with changes in taste (P = 0.05) and smell (P = 0.05) in the RYGB as well as for PS. In the former, time since surgery was shorter in patients with taste and smell changes (P < 0.001, for both), and also in those with changes in appetite (P = 0.05). On the counterpart in the SG, no difference was detected between sensory and food variables and RWR (P ≥ 0.11, for all comparisons). As for time analyses, time since surgery was shorter in patients with changes in smell (P = 0.03).
Discussion
Our findings showed high frequencies of changes in appetite, taste, smell, and food aversion, taste changes to the specific food (sweet, salty, and sour), and a loss of level of taste and smell in post-bariatric patients in the long term. These data concur with prior research showing that changes in sensory and food perceptions are common in patients after RYGB and SG [7, 14, 23]. Since both surgical groups had a similar and high frequency of changes in the investigated variables, no difference in these variables between groups was noted. Maybe if we had tested a non-surgical group, this would significantly affect the surgical group vs. the controls. The main point is that some changes were highly prevalent in the patients investigated, especially those related to appetite, taste, and sweet taste (reported by 76%, 48.6%, and 60.2% of all patients, respectively).
Bariatric procedures alter adipose tissue, gastrointestinal tract, and central nervous system and explain the sensory and food changes with possible influence on weight loss [4, 16, 24, 25]. Additionally, they lead to critical changes in gastrointestinal peptides, like glucose-dependent insulinotropic polypeptide (GIP), glucagon-like peptide-1 (GLP-1), peptide YY (PYY), ghrelin, and cholecystokinin (CCK), and leptin. These peptides interact with the nervous system and integrate signals of hunger and satiety, regulating energy balance. Moreover, some gut hormones, like GLP-1, CCK, PYY, and ghrelin, may also act in gustative receptors of the tongue and or the olfactory neurons, modulating taste, smell, and food preferences [4, 16, 24, 25]. Another aspect of this pathophysiology negatively relates the adipose tissue-derived peptide, named leptin, to taste and smell function [26]. Therefore, it is possible to infer that bariatric surgery with critical influences on gut peptides and weight loss would cause sensory, appetite, and food preference changes. The resultant altered feeding behavior and modified caloric intake would favor a negative energy balance, loss of weight, or even lower rates of weight regain, especially in those who keep these altered perceptions in the long term.
Our results in the pooled sample that compared EWL, RWR, and time since surgery in patients with and without sensory and food changes, reflect data extracted predominantly from RYGB group. Possibly, the SG group did not seem to influence them. From auto-referred data, we could assure that all patients had an effective surgery [21], and concerning EWL, no difference between those with and without sensory and appetite changes was observed. Previous studies investigating changes in appetite, taste, smell, and weight loss in the post-bariatric patients were performed during the first year post-surgery [23, 25, 27, 28], and the authors speculated that these changes have a higher impact precisely at this period than afterward. On the counterpart, we opted to test patients after the first year post surgery.
Regarding the influence of food changes in EWL, our study noted a percentage relatively low of post-bariatric patients with food aversion compared with those observed by Graham et al. (73% of 103 patients with a median of 19 months post RYGB) [7]. Besides, as this variable was associated with more significant weight loss in patients ten months post surgery [23], it is feasible to suppose that temporal changes were possibly responsible for our findings. Other potential mechanisms by which bariatric surgery leads to weight loss should be considered [4, 8, 28] and may explain in part the observed effective EWL, independently whether the patients have sensory and appetite changes. Indeed, we observed that post-RYGB patients with changes in appetite, taste, and smell and post-SG with smell changes presented a shorter time since surgery than those without them. Although the surgery is known to promote sensory alterations in the short term [6, 19], long-term effects still need to be better elucidated. Our findings suggest that sensory and appetite changes could gradually decrease over time due to the known gut–adipose–brain axis's physiological adaptation [29].
In respect to weight regain, we noticed that those post-RYGB with changes in taste and smell presented lower RWR than those without them. This finding may suggest that the smell and taste of foods have influenced their preferences and choices and possibly the ingested calories. Dietary habit changes with a healthier and more balanced diet were observed in patients after an RYGB procedure [30]. Since obesity etiopathogenesis is multifactorial, we should highlight the multiple aspects that command taste and smell [28] and weight loss and regain.
Our major limitation is the cross‐sectional design, damping causal relationships and not allowing us to follow how the sensory and appetite changes occurred over time. The lack of a non-surgical control group, and the inclusion of patients with type 2 diabetes, although at low prevalence (7.5%), should both be acknowledged as limitations too. The sensory and appetite changes were self-reported and collected by questionnaires. Therefore, complex understanding and inadequately completed responses should be considered. Nevertheless, a high return rate of questionnaires (96.7%) occurred in our study, especially considering other studies in post-bariatric patients [7, 14]. Since our recruited patients were those without regular medical follow-up attended in their first appointment, several bariatric procedures and their minimal specificities in technical operations regarding surgeon's techniques were involved. Independently, an adequate mean EWL was successfully achieved. We had a total sample of 146 patients, which was adequately powered, particularly to the PS and post-RYGB patients. However, data on the SG group should be viewed with caution since they did not reach the ideal sample size for definitive conclusions. We had 126 post-RYGB patients and 20 post-SG ones, a proportion of 6.3 to 1. However, our sample reflects in part the reality in our country since the number of SG performed here is lower than RYGB [20], and trials including large sample sizes of SG are minimal. We recognized it as a limitation of our study. Despite our limitations, we should emphasize that our study sample reflects a real-world model of post-bariatric patients in our country, who even undergoing bariatric surgery were all unfortunately without regular medical follow-up.
In conclusion, in the post-bariatric patients, the highest percentages of changes were reported in appetite and taste in the long term. Similar changes in appetite, taste, smell, and food aversion occurred between surgeries. Additionally, they also showed taste changes to specific foods (sweet, salty, and sour) and smell, independently of the type of surgery. Of note, the RWR was lower in those with changes in taste and smell, while those with shorter time since surgery had higher frequencies of changes in appetite, taste, and smell. Longitudinal studies will clarify if the sensory and appetite changes are predictive factors for long-term success on weight in post-bariatric patients.
What is already known on this subject?
Most previous studies analyzed the changes in appetite, taste, smell, and food aversion of post-bariatric patients during the first year after surgery.
What does this study add?
Using data from post-bariatric patients without regular clinical follow-up, we showed some sensorial changes during the long-term period, which is relevant for analyzing the relationship with weight regain.
Availability of data and material
The data used to support the findings of this study are available upon reasonable request.
References
Reges O, Greenland P, Dicker D, Leibowitz M, Hoshen M, Gofer I, Rasmussen-Torvik LJ, Balicer RD (2018) Association of bariatric surgery using laparoscopic banding, Roux-en-Y gastric bypass, or laparoscopic sleeve gastrectomy vs usual care obesity management with all-cause mortality. JAMA 319(3):279–290. https://doi.org/10.1001/jama.2017.20513
Sjöström CD, Lissner L, Wedel H, Sjöström L (1999) Reduction in incidence of diabetes, hypertension and lipid disturbances after intentional weight loss induced by bariatric surgery: the SOS Intervention Study. Obes Res 7(5):477–484. https://doi.org/10.1002/j.1550-8528.1999.tb00436.x
Sweeney TE, Morton JM (2014) Metabolic surgery: Action via hormonal milieu changes, changes in bile acids or gut microbiota? A summary of the literature. Best Pract Res Clin Gastroenterol 28(4):727–740. https://doi.org/10.1016/j.bpg.2014.07.016
Dimitriadis GK, Randeva MS, Miras AD (2017) Potential hormone mechanisms of bariatric surgery. Curr Obes Rep 6(3):253–265. https://doi.org/10.1007/s13679-017-0276-5
Behary P, Miras AD (2015) Food preferences and underlying mechanisms after bariatric surgery. Proce Nutr Soc 74(4):419–425. https://doi.org/10.1017/S0029665115002074
Ahmed K, Penney N, Darzi A, Purkayastha S (2018) Taste changes after bariatric surgery: a systematic review. Obes Surg 28(10):3321–3332. https://doi.org/10.1007/s11695-018-3420-8
Graham L, Murty G, Bowrey DJ (2014) Taste, smell and appetite change after Roux-en-Y gastric bypass surgery. Obes Surg 24(9):1463–1468. https://doi.org/10.1007/s11695-014-1221-2
Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K (2004) Bariatric surgery: a systematic review and meta-analysis. JAMA 292(14):1724–1737. https://doi.org/10.1001/jama.292.14.1724
Sjöström L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjöström CD, Sullivan M, Wedel H (2004) Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 351(26):2683–2693. https://doi.org/10.1056/NEJMoa035622
Lauti M, Kularatna M, Hill AG, MacCormick AD (2016) Weight regain following sleeve gastrectomy-a systematic review. Obes Surg 26(6):1326–1334. https://doi.org/10.1007/s11695-016-2152-x
Magro DO, Geloneze B, Delfini R, Pareja BC, Callejas F, Pareja JC (2008) Long-term weight regain after gastric bypass: a 5-year prospective study. Obes Surg 18(6):648–651. https://doi.org/10.1007/s11695-007-9265-1
Cambi MPC, Baretta GAP, Magro DO, Boguszewski CL, Ribeiro IB, Jirapinyo P, de Moura DTH (2021) Multidisciplinary approach for weight regain-how to manage this challenging condition: an expert review. Obes Surg 31(3):1290–1303. https://doi.org/10.1007/s11695-020-05164-1
Karmali S, Brar B, Shi X, Sharma AM, de Gara C, Birch DW (2013) Weight recidivism post-bariatric surgery: a systematic review. Obes Surg 23(11):1922–1933. https://doi.org/10.1007/s11695-013-1070-4
Tichansky DS, Boughter JD Jr, Madan AK (2006) Taste change after laparoscopic Roux-en-Y gastric bypass and laparoscopic adjustable gastric banding. Surg Obes Relat Dis 2(4):440–444. https://doi.org/10.1016/j.soard.2006.02.014
Lynch A (2016) “When the honeymoon is over, the real work begins:” Gastric bypass patients’ weight loss trajectories and dietary change experiences. Soc Sci Med 151:241–249. https://doi.org/10.1016/j.socscimed.2015.12.024
Behary P, Miras AD (2015) Food preferences and underlying mechanisms after bariatric surgery. Proc Nutr Soc 74(4):419–425. https://doi.org/10.1017/S0029665115002074
Bryant EJ, Malik MS, Whitford-Bartle T, Waters GM (2020) The effects of bariatric surgery on psychological aspects of eating behaviour and food intake in humans. Appetite 150:104575. https://doi.org/10.1016/j.appet.2019.104575
Guyot E, Dougkas A, Robert M, Nazare JA, Iceta S, Disse E (2021) Food Preferences and their perceived changes before and after bariatric surgery: a cross-sectional study. Obes Surg 31(7):3075–3082. https://doi.org/10.1007/s11695-021-05342-9
Altun H, Hanci D, Altun H, Batman B, Serin RK, Karip AB, Akyuz U (2016) Improved gustatory sensitivity in morbidly obese patients after laparoscopic sleeve gastrectomy. Ann Otol Rhinol Laryngol 125(7):536–540. https://doi.org/10.1177/0003489416629162
Welbourn R, Hollyman M, Kinsman R, Dixon J, Liem R, Ottosson J, Ramos A, Våge V, Al-Sabah S, Brown W, Cohen R, Walton P, Himpens J (2019) Bariatric surgery worldwide: baseline demographic description and one-year outcomes from the fourth IFSO global registry report 2018. Obes Surg 29(3):782–795. https://doi.org/10.1007/s11695-018-3593-1
Romagna EC, Lopes KG, Mattos DMF, Farinatti P, Kraemer-Aguiar LG (2021) Physical activity level, sedentary time, and weight regain after bariatric surgery in patients without regular medical follow-up: a cross-sectional study. Obes Surg. https://doi.org/10.1007/s11695-020-05184-x
Zhang Y, Nagarajan N, Portwood C, Smith KR, Kamath V, Carnell S, Moran TH, Steele KE (2020) Does taste preference predict weight regain after bariatric surgery? Surg Endosc 34(6):2623–2629. https://doi.org/10.1007/s00464-019-07033-0
Zerrweck C, Zurita L, Alvarez G, Maydon HG, Sepulveda EM, Campos F, Caviedes A, Guilbert L (2016) Taste and olfactory changes following laparoscopic gastric bypass and sleeve gastrectomy. Obes Surg 26(6):1296–1302. https://doi.org/10.1007/s11695-015-1944-8
Mulla CM, Middelbeek RJW, Patti ME (2018) Mechanisms of weight loss and improved metabolism following bariatric surgery. Ann N Y Acad Sci 1411(1):53–64. https://doi.org/10.1111/nyas.13409
Karamanakos SN, Vagenas K, Kalfarentzos F, Alexandrides TK (2008) Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg 247(3):401–407. https://doi.org/10.1097/SLA.0b013e318156f012
Uygun B, Kiyici S, Ozmen S, Gul Z, Sigirli D, Cavun S (2019) The association between olfaction and taste functions with serum ghrelin and leptin levels in obese women. Metab Syndr Relat Disord 17(9):452–457. https://doi.org/10.1089/met.2019.0037
Holinski F, Menenakos C, Haber G, Olze H, Ordemann J (2015) Olfactory and gustatory function after bariatric surgery. Obes Surg 25(12):2314–2320. https://doi.org/10.1007/s11695-015-1683-x
Pepino MY, Bradley D, Eagon JC, Sullivan S, Abumrad NA, Klein S (2014) Changes in taste perception and eating behavior after bariatric surgery-induced weight loss in women. Obesity (Silver Spring) 22(5):E13-20. https://doi.org/10.1002/oby.20649
Sinclair P, Brennan DJ, le Roux CW (2018) Gut adaptation after metabolic surgery and its influences on the brain, liver and cancer. Nat Rev Gastroenterol Hepatol 15(10):606–624. https://doi.org/10.1038/s41575-018-0057-y
Ernst B, Thurnheer M, Wilms B, Schultes B (2009) Differential changes in dietary habits after gastric bypass versus gastric banding operations. Obes Surg 19(3):274–280. https://doi.org/10.1007/s11695-008-9769-3
Funding
Coordination for the Improvement of Higher Education Personnel (CAPES, process 88882.463218/2019–01), Brazilian Council for Technological and Scientific Development (CNPq, process 304335/2019–3, recipient LGK-A), and Carlos Chagas Filho Foundation for the Research Support in the State of Rio de Janeiro (FAPERJ, process 250304) supported this work with scholarships for KGL, GPS, and LGK-A.
Author information
Authors and Affiliations
Contributions
Conception and design of the study: GPS and LGK-A; administration and supervision of the study: KGL and LGK-A; patient recruitment and data collection: KGL, GPS, ECR, DMFM, TGB, CBC; analysis or interpretation of data: KGL, DMFM, LGK-A, and draft the manuscript: KGL and GPS; revising the work: KGL and LGK-A. KGL and GPS had the same participation in drafting the present work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no competing interest.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the local Ethics Committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to participate
Written informed consent was provided by all participants included in the study.
Consent for publication
All authors have read and approved the publication of this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lopes, K.G., dos Santos, G.P., Romagna, E.C. et al. Changes in appetite, taste, smell, and food aversion in post-bariatric patients and their relations with surgery time, weight loss and regain. Eat Weight Disord 27, 1679–1686 (2022). https://doi.org/10.1007/s40519-021-01304-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40519-021-01304-3