Abstract
Background
Metabolic dysfunction and obesity rates are on the rise. Although the central modes of circadian disruption has been studied in relation to the risk of obesity, the role of eating time has remained unclear. Here, we aimed to assess circadian behavioral phenotypes and their association with the risk of elevated body mass index (BMI).
Methods
This was a prospective cross-sectional study of individuals presenting for colorectal cancer screening colonoscopy. Participants completed demographic questionnaires, The Munich ChronoType Questionnaire (MCTQ), and Food Timing Screener (FTS). The primary outcome of the study was the association between circadian phenotypes and elevated BMI.
Results
A total of 488 individuals completed the survey, with a mean (SD) age of 57.5 (10.8) years. The mean body mass index (BMI) was 28.8 (6.1) kg/m2, with 72.3% of individuals met criteria for elevated BMI. Four circadian behavioral phenotypes were generated: early chronotype with regular food timing (ER) (34.7%), early chronotype with irregular food timing (EI) (11.7%), intermediate/late chronotype with regular food timing (LR) (33.9%), and intermediate/late chronotype with irregular food timing (LI) (19.7%). In a multivariable regression analysis, LI phenotype had 2.9 times higher odds of elevated BMI as compared to ER phenotype (OR 2.9, 95% CI 1.3–6.7, P = 0.01).
Conclusion
The combination of late chronotype and irregular food timing, representative of a behavioral circadian rhythm disruption, is associated with higher rates of elevated BMI. The majority of individuals with this abnormal circadian phenotype were younger than 60 years old. This observation is especially relevant because of the ongoing rise in the obesity rates among young adults.
Level III
Evidence obtained from well-designed cohort or case–control analytic studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The circadian rhythm is roughly a 24-h physiologic sleep/wake cycle, which is generated by a self-sustained endogenous circadian clock [1]. The clock is regulated by external cues, such as light/dark cycles allowing the organism to anticipate changes in the external environment [1]. These changes enable the body to adapt to its environment though physiologic and behavioral responses, which is vital for survival. Besides the central circadian clock, the peripheral clocks located in most organs regulate the physiologic function of these organs based on environmental stimuli [2, 3]. The circadian clock in the central nervous system typically controls the clock in the periphery. This orchestrated central–peripheral circadian clock forms a state of healthy circadian homeostasis which is crucial in synchronizing physiological and biological processes, such as body temperature, metabolism, local, and systemic immunity, optimizing response to the surrounding environment [1, 4]. The peripheral clock could be influenced by other external cues such as food. For example, the time of meal consumption (i.e., timing of nutrient availability) actively regulates circadian clocks in the intestine and liver [4,5,6,7]. The modern lifestyle significantly affects the individual circadian rhythm by forcing it to adapt to the working or social environment. Although working at night shifts and frequent traveling across time zones are typical forms of circadian disruption, recent works by our group and others indicate that abnormal and irregular time of eating could also desynchronize the central and peripheral clock, leading to circadian rhythm disruption (CRD) [8,9,10]. The close interaction of circadian rhythms and metabolism [11] explains the deleterious effects of CRD in experimental models of metabolic dysfunction and obesity [4, 6, 7]. Controlled human studies have further supported the contribution of CRD to metabolic dysfunction [2, 7, 12]. While central modes of CRD, such as shift work has been well studied, data on the other forms of behavioral determinants of CRD (i.e., food timing) on obesity is still scarce. A number of epidemiological studies suggest that eating patterns such as breakfast skipping and eating large portions closer to the rest time in the evening could be associated with metabolic syndromes and obesity independent of the food type [7, 13,14,15]. However, such findings were reported as secondary outcomes of those studies that were otherwise designed for noncircadian primary outcomes. Here we aimed to comprehensively assess the behavioral circadian phenotypes (i.e., chronotype and food timing) in a cohort of consecutive individuals who presented for colon cancer screening in association with elevated body mass index (BMI).
Methods
Study design and data collection
A prospective cross-sectional study was conducted at a tertiary medical center. Individuals who presented to the endoscopy unit for colorectal cancer screening colonoscopy were approached. Patients were excluded for any of the following: lynch syndrome, familial adenomatous polyposis, inflammatory bowel disease, and any active malignancy under therapy. Participants completed questionnaires of demographics, the Munich ChronoType Questionnaire (MCTQ), and Food Timing Screener (FTS) [16, 17]. Demographics and clinical data, including body mass index (BMI) was extracted from the electronic medical records. Informed consent was obtained from all participants who agreed to participate in the study. There was no missing data as all the surveys were examined by the research assistant who assured the surveys were completed fully by participants. The study was approved by Institutional Review Board at Rush University (15011602).
The Munich chronotype questionnaire (MCTQ)
We assessed the chronotype of patients using the MCTQ [16]. It consists of questions about the individual’s typical sleep behavior during work days and free days. The MCTQ provides information about sleep patterns in work and free days which allow to calculate different outcomes like chronotype, social jetlag, weekly sleep loss, and weekly light exposure. Chronotype is divided into three groups; early, intermediate, and late and was calculated based on the Mid-Sleep in free days (MSF) in patients who did not use the alarm to wake up on free days. Participants were considered early chronotype if their MSF fell before 3:00 am, intermediate chronotype if MSF fell between 3:00 am and 5:00 am, and late chronotype if MSF fell beyond 5:00 am [16].
Food timing screener
The Food Timing Screener (FTS) is a concise questionnaire to capture food timing that was validated using seven, 24-h recalls [17]. The FTS was also found to accurately assess timing of food intake compared to a more extensive Food Timing Questionniare, both of which were developed by experts in gastroenterology and nutrition at Rush University Medical Center [17]. The FTS was designed to be a concise form of the FTQ to be used independently when time to complete the full FTQ is limited. As with the FTQ, sleep–wake time is included to be able to identify the relation between food intake and sleep timing. Using the FTS, we collected information about exact meal times, and largest meal of the day in typical work days and free days. We calculated the average time for all the meals during work days and free days. Irregular food timing was defined as having more than a 2-h difference in the average meal time between work days and free days.
The FTS is a self-report questionnaire that assesses an individual’s usual eating and sleep habits categorized into two types of days: school/work days and free days/days off. Respondents report, in one sitting, time of eating for three meals (breakfast, lunch, and dinner) and up to three snacks (snack 1, snack 2, and snack 3), the times of awakening and falling asleep, whether s/he awakens from sleep to eat, and the largest (self-defined most food) eating event of the day on each type of day.
In order to correct for the potential effects of the food types in our participants, we included major food categories (wheat, vegetables, fruit, fried food, pickled food, and red meat) that have been linked to the risk of metabolic syndrome from population based studies [18,19,20]. Each participant reported the consumption of food types as regular, occasional, or never.
Study outcomes and statistical analysis
The aim of this hypothesis generating study was to test the association between circadian phenotypes and elevated BMI. Age was dichotomized into two groups (60 years and over and less than 60 years of age) because it was not normally distributed, so we created comparable age groups in numbers. Elevated BMI was defined as having a BMI ≥ 25 kg/m2 [21]. We combined “occasional” and “never” categories of food types due to the low numbers in these categories. We also combined “intermediate” and “late” categories of chronotype because the majority of participants had early chronotype. We defined the behavioral circadian phenotypes based on the combination of two chronotypes and two eating habits, which resulted in four behavioral circadian phenotypes: early chronotype and regular food timing (ER), early chronotype and irregular food timing (EI), intermediate/late chronotype and regular food timing (LR), and intermediate/late chronotype and irregular food timing (LI).
Categorical variables were reported as counts and frequencies and continuous variables were reported as means and standard deviations. For categorical variables, we used χ2 test or Fisher’s exact test, as appropriate. For continuous variables, we used t test and one-way ANOVA, as appropriate. A univariate logistic regression analysis of the association between elevated BMI and other factors was performed. Furthermore, a multivariable logistic regression analysis of elevated BMI was conducted including all variables. A P value < 0.05 was considered statistically significant. All statistical analyses were performed using STATA/IC (version 14.2; StataCorp LLC, College Station, TX, USA).
Results
A total of 488 patients were recruited and included in the analysis (Table 1). The age ranged between 21 and 79 years with a mean (SD) of 57.5 (10.8) years. The majority (55.5%) of participants were under 60 years old. The mean BMI was 28.8 kg/m2 (6.1), with 72.3% of individuals meeting criteria for elevated BMI (BMI ≥ 25 kg/m2) (Table 1). A majority of the participants consumed vegetables, fruits, and foods containing wheat, while less consumed foods linked with cancers of the gastrointestinal tract (Table 1). There were no colorectal cancer cases in our cohort.
Circadian characteristics of participants
Table 2 shows the circadian characteristics of participants stratified by age, sex, and BMI status. There were 196 (53.5%) participants who had intermediate/late chronotype. Younger participants (< 60 years old) had a higher rate of intermediate/late chronotype than older (≥ 60 years old) participants (122 (58.1%) vs. 74 (47.4%), P = 0.04). There was no significant association between chronotype and sex.
Dinner was the largest meal of the day in 335 (73.8%) participants, and only 64 (13.3%) participants skipped breakfast. Irregular food timing was present in 30.7% of participants and was associated with younger age (40.2% vs 18.9% in < 60 years old versus ≥ 60 years old, respectively; P < 0.01). There was no significant association between food timing and sex.
Circadian characteristics and elevated BMI
Participants with elevated BMI had a higher rate of intermediate/late chronotype than participants with normal BMI (56.6% vs. 44.7%, respectively; P = 0.04) (Table 2). Similarly, participants with elevated BMI tended to have a higher rate of irregular food timing compared to participants with normal BMI, but the results were not statistically significant (32.8% vs. 25.2%, respectively; P = 0.10).
Combining two behavioral determinants of circadian phenotypes based on the chronotype and food timing habits resulted in four behavioral circadian phenotypes: Early chronotype and regular food timing (ER, 34.7%), early chronotype and irregular food timing (EI, 11.7%), intermediate/late chronotype and regular food timing (LR, 33.9%), and intermediate/late chronotype and irregular food timing (LI, 19.7%) (Table 3). These phenotypes were distributed unequally according to the participants’ age. While the majority of participants in LI group (76.4%) were < 60 years old, only 44% of the ER group were < 60 years old (P < 0.001).
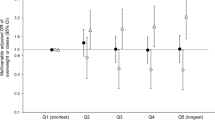
There was an association of elevated BMI with circadian phenotypes (Fig. 1), where individuals in the LI group had the highest rates of having elevated BMI compared to those in the ER group (84.7% vs. 68.5%, P = 0.01).
Behavioral circadian phenotypes and elevated BMI
In a univariate analysis of the factors associated with elevated BMI, in addition to the LI group, regular consumption of fried food was positively associated with elevated BMI, while fruit consumption was negatively associated with the odds of having elevated BMI (Table 4). In a multivariable logistic regression analysis adjusted for all factors, LI group had 2.9 times higher odds of elevated BMI compared to ER group (OR 2.9, 95% CI 1.3–6.7, P = 0.01) (Table 5).
Discussion
Circadian rhythm is closely linked to metabolic homeostasis, and once disrupted could lead to metabolic dysfunction [22]. This is supported by experimental models that showed the causal link between circadian rhythm disruption (CRD) and metabolic dysfunction and obesity in animals [11, 14, 23, 24]. Subsequently, evidence from human studies linked the CRD with chronic diseases including metabolic dysfunction and obesity [25]. Although prior studies mostly focused on the central modes of CRD, the data are still limited on the possible association of food timing habits on metabolism and obesity in humans [2, 12, 26,27,28]. In our study, using a complementary circadian phenotyping using both chronotype and food timing habits in 488 individuals, we found that irregular food timing particularly in those with intermediate/late chronotype was an independent factor for elevated BMI after adjusting for other factors. This circadian phenotype was more common among younger individuals. The rate of elevated BMI was increasing from one extrem phenotype (ER, 68.5%) to the other extreme phenotype (LI, 84.7%). Therefore, we compared these two phenotypes in a multivariable regression analysis and found that individuals with LI phenotype have 2.9 higher odds of elevated BMI compared to ER phenotype individuals.
The circadian clock has an essential role in metabolic health; circadian rhythm disruption can cause metabolic dysfunction, which in turn can result in weight gain and other chronic metabolic diseases [29]. Prior studies evaluated the role of circadian rhythm in metabolic homeostasis, where late chronotype was associated with metabolic dysfunction and obesity [8, 28, 30,31,32]. In a systematic review of the literature, Antunes et al. reported that the disruption of the circadian rhythm represented by rotating shift work schedule can increase the risk of obesity, diabetes, and cardiovascular diseases. They hypothesized that both abnormal timing of sleep and eating may have contributed to this observed association [29]. Our study showed that elevated BMI was more prevalent among individuals with intermediate/late chronotype compared with early chronotype group, which is consistent with previous studies [8, 29]. Shift work in younger individuals results in the interruption of sleep during work days, which may lead to sleep loss. Sleep loss especially in individuals with late chronotype predispose them to circadian rhythm disruption, metabolic dysfunction and obesity [8].
Beside the effects of light/dark cycle on the circadian clock, food entrains the circadian rhythm in the digestive tract and plays a crucial role in metabolic homeostasis [22, 25, 33]. Animal studies suggest that abnormal time of eating (i.e. during inactive phase) predisposes mice to metabolic dysfunction and obesity [14, 23].
In humans, meal timing, independent from calorie intake and sleep patterns has been linked with metabolic dysfunction and body weight [2, 12, 31, 32]. Eating close to the rest time has been associated with decreased resting-energy expenditure, decreased glucose tolerance and body weight [33, 34]. Consistent with recently published studies [35], most participants in our study reported dinner as their largest meal of the day (70.1%). Some but not all previous studies suggested an association between obesity and late evening eating [13, 35]; however, we did not find the habit of dinner as the largest meal of the day to be associated with elevated BMI.
While inconsistent time of sleep between work and free days is considered an abnormal circadian phenotype [8, 29], there is not enough data regarding the effect of consistency of meal timing. In animals, regular feeding times are associated with healthier circadian rhythms and lower risk for metabolic dysfunction [14]. Our animal data also supports that inconsistent meal timing (i.e., rest phase eating during weekdays and ad-lib during weekends mimicking human condition) could lead to circadian rhythm disruption [7] and predispose to metabolic dysfunction and weight gain [36]. Recently, irregular food timing in humans was found to be associated with worsening inflammation among patients with inflammatory bowel disease [4]. Here, we found that irregular food timing as defined by inconsistent eating during work vs. free days tended to be more frequent among individuals with elevated BMI, a finding that did not reach statistical significance likely due to the sample size. When combined with central circadian phenotype, irregular food timing increased the risk of elevated BMI in subjects with intermediate/late chronotypes, an association which remained significant after adjusting for all other factors. The link between abnormal circadian phenotypes and elevated BMI in our study could be explainable by the role of circadian rhythm disruption in metabolic dysfunction [2, 7, 12, 35, 36], which could be in part mediated via changes in intestinal microbiota [6, 37,38,39].
Chronotype changes throughout the life from childhood to elderliness. Children usually have early chronotype that progresses to later chronotype by puberty and adolescence. In elderly individuals, chronotype continues to progress until it cycles back to early chronotype similar to children [40]. We also observed higher rates of intermediate/late chronotype among younger adults compared to older adults who had higher rates of early chronotype. Interestingly, the same group of younger adults also had higher rates of irregular food timing. This is particularly relevant to the current obesity epidemic in the modern societies including the US that is affecting the younger population, as younger adults could be more exposed to lifestyle habits such as CRD, which could predispose them to metabolic dysfunction [41, 42].
Our study has several limitations. It is a cross-sectional study and there are inherited limitations including recall bias and selection bias, especially that our study sample included indivudals who presented for colonoscopy. We expect that having a larger sample size with participants outside the clinical settings may result in similar finidngs to our study. This is a survey-based study which limits the ability to evaluate for other potential confounding factors as calorie intake, exercise, and other factors that may affect the BMI. It is noteworthy that our findings should be verified in younger population; for the current study we recruited healthy individuals at the screening age for colorectal cancer which explains why our study population was overall older.
In conclusion, we found that the behavioral circadian phenotypes illustrated by intermediate/late chronotype and irregular food timing is associated with higher rates of elevated BMI. The majority of individuals with this risky circadian phenotype were younger. Identifying risk factors for elevated BMI can help us develop new strategies in mitigating the obesity epidemic. Irregular food timing is a novel factor that could contribute to the risk of obesity, especially among younger adults; further studies in a younger population with larger sample size are needed to confirm our results.
What is already known on this subject?
The rise in obesity is closely linked to our lifestyle. Disruption of circadian clock which is a common lifestyle habit in our 24/7 modern society has been linked to predisposition to metabolic dysregulation and obesity. Shift in the light/dark cycles as occurs in shift workers is a widely studied model of central circadian disruption which can increase risk of obesity. Besides, studies from our lab and others show that eating during or close to the rest time could also lead to circadian rhythm disruption by desynchronizing the central from peripheral circadian clock. Animal studies show that the time of eating (independent from the type of diet) affects the risk of obesity.
What does this study add?
The combination of late chronotype and irregular food timing is associated with obesity. The association of behavioral circadian rhythm phenotypes with obesity is more evident in nonelderly population, an intriguing finding in light of the ongoing rise in the obesity rates among younger adults. Our observations introduce additional “targetable” lifestyle habits to decrease the risk of obesity.
References
Sládek M, Rybová M, Jindráková Z et al (2007) Insight into the circadian clock within rat colonic epithelial cells. Gastroenterology 133(4):1240–1249. https://doi.org/10.1053/j.gastro.2007.05.053
Johnston JD, Ordovás JM, Scheer FA, Turek FW (2016) Circadian rhythms, metabolism, and chrononutrition in rodents and humans. Adv Nutr 7(2):399–406. https://doi.org/10.3945/an.115.010777
Bishehsari F, Levi F, Turek FW, Keshavarzian A (2016) Circadian rhythms in gastrointestinal health and diseases. Gastroenterology 151(3):e1–e5. https://doi.org/10.1053/j.gastro.2016.07.036
Chakradeo PS, Keshavarzian A, Singh S et al (2018) Chronotype, social jet lag, sleep debt and food timing in inflammatory bowel disease. Sleep Med 52:188–195. https://doi.org/10.1016/j.sleep.2018.08.002
Forsyth CB, Voigt RM, Burgess HJ, Swanson GR, Keshavarzian A (2015) Circadian rhythms, alcohol and gut interactions. Alcohol 49(4):389–398. https://doi.org/10.1016/j.alcohol.2014.07.021
Voigt RM, Forsyth CB, Green SJ, Engen PA, Keshavarzian A (2016) Circadian rhythm and the gut microbiome. Int Rev Neurobiol 131:193–205. https://doi.org/10.1016/bs.irn.2016.07.002
Bishehsari F, Engen PA, Voigt RM et al (2020) Abnormal eating patterns cause circadian disruption and promote alcohol-associated colon carcinogenesis. Cell Mol Gastroenterol Hepatol 9(2):219–237. https://doi.org/10.1016/j.jcmgh.2019.10.011
Roenneberg T, Allebrandt KV, Merrow M, Vetter C (2012) Social jetlag and obesity. Curr Biol 22(10):939–943. https://doi.org/10.1016/j.cub.2012.03.038
Vetter C, Fischer D, Matera JL, Roenneberg T (2015) Aligning work and circadian time in shift workers improves sleep and reduces circadian disruption. Curr Biol 25(7):907–911. https://doi.org/10.1016/j.cub.2015.01.064
Pagel R, Bär F, Schröder T et al (2017) Circadian rhythm disruption impairs tissue homeostasis and exacerbates chronic inflammation in the intestine. FASEB J 31(11):4707–4719. https://doi.org/10.1096/fj.201700141RR
Turek FW, Joshu C, Kohsaka A et al (2005) Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308(5724):1043–1045. https://doi.org/10.1126/science.1108750
Scheer FA, Hilton MF, Mantzoros CS, Shea SA (2009) Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A 106(11):4453–4458. https://doi.org/10.1073/pnas.0808180106
Kant AK, Schatzkin A, Ballard-Barbash R (1997) Evening eating and subsequent long-term weight change in a national cohort. Int J Obes Relat Metab Disord 21(5):407–412. https://doi.org/10.1038/sj.ijo.0800422
Kessler K, Pivovarova-Ramich O (2019) Meal timing, aging, and metabolic health. Int J Mol Sci 20(8):1911. https://doi.org/10.3390/ijms20081911
Horikawa C, Kodama S, Yachi Y et al (2011) Skipping breakfast and prevalence of overweight and obesity in Asian and Pacific regions: a meta-analysis. Prev Med 53(4–5):260–267. https://doi.org/10.1016/j.ypmed.2011.08.030
Roenneberg T, Wirz-Justice A, Merrow M (2003) Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms 18(1):80–90
Chakradeo P (2018) Validity and reliability of the food timing questionnaire (FTQ) and food timing screener (FTS). [Order no. 10749081]. Rush University, Chicago
Aekplakorn W, Satheannoppakao W, Putwatana P et al (2015) Dietary pattern and metabolic syndrome in thai adults. J Nutr Metab 2015:468759. https://doi.org/10.1155/2015/468759
Panagiotakos DB, Pitsavos C, Skoumas Y, Stefanadis C (2007) The association between food patterns and the metabolic syndrome using principal components analysis: the ATTICA Study. J Am Diet Assoc 107(6):979–997. https://doi.org/10.1016/j.jada.2007.03.006
Yeo R, Yoon SR, Kim OY (2017) The Association between food group consumption patterns and early metabolic syndrome risk in non-diabetic healthy people. Clin Nutr Res 6(3):172–182. https://doi.org/10.7762/cnr.2017.6.3.172
Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC Jr, Spertus JA, Costa F, American Heart Association; National Heart, Lung, and Blood Institute (2005) Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 112(17):2735–2752. https://doi.org/10.1161/CIRCULATIONAHA.105.169404. Erratum in: Circulation. 2005;112(17):e297. Erratum in: Circulation. 2005;112(17):e298
Bishehsari F, Voigt RM, Keshavarzian A (2020) Circadian rhythms and the gut microbiota: from the metabolic syndrome to cancer. Nat Rev Endocrinol 16(12):731–739. https://doi.org/10.1038/s41574-020-00427-4
Fonken LK, Lieberman RA, Weil ZM, Nelson RJ (2013) Dim light at night exaggerates weight gain and inflammation associated with a high-fat diet in male mice. Endocrinology 154(10):3817–3825. https://doi.org/10.1210/en.2013-1121
Yoon JA, Han DH, Noh JY et al (2012) Meal time shift disturbs circadian rhythmicity along with metabolic and behavioral alterations in mice. PLoS ONE 7(8):e44053. https://doi.org/10.1371/journal.pone.0044053
Bishehsari F, Saadalla A, Khazaie K et al (2016) Light/dark shifting promotes alcohol-induced colon carcinogenesis: possible role of intestinal inflammatory milieu and microbiota. Int J Mol Sci 17(12):2017. https://doi.org/10.3390/ijms17122017
Ma Y, Bertone ER, Stanek EJ et al (2003) Association between eating patterns and obesity in a free-living US adult population. Am J Epidemiol 158(1):85–92. https://doi.org/10.1093/aje/kwg117
Colles SL, Dixon JB, O’Brien PE (2007) Night eating syndrome and nocturnal snacking: association with obesity, binge eating and psychological distress. Int J Obes (Lond) 31(11):1722–1730. https://doi.org/10.1038/sj.ijo.0803664
Fonken LK, Workman JL, Walton JC et al (2010) Light at night increases body mass by shifting the time of food intake. Proc Natl Acad Sci U S A 107(43):18664–18669. https://doi.org/10.1073/pnas.1008734107
Antunes LC, Levandovski R, Dantas G, Caumo W, Hidalgo MP (2010) Obesity and shift work: chronobiological aspects. Nutr Res Rev 23(1):155–168. https://doi.org/10.1017/S0954422410000016
Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW (2009) Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring) 17(11):2100–2102. https://doi.org/10.1038/oby.2009.264
Ekmekcioglu C, Touitou Y (2011) Chronobiological aspects of food intake and metabolism and their relevance on energy balance and weight regulation. Obes Rev 12(1):14–25. https://doi.org/10.1111/j.1467-789X.2010.00716.x
Allison KC, Goel N, Ahima RS (2014) Delayed timing of eating: impact on weight and metabolism. Curr Obes Rep 3(1):91–100. https://doi.org/10.1007/s13679-013-0084-5
Garaulet M, Gómez-Abellán P, Alburquerque-Béjar JJ, Lee YC, Ordovás JM, Scheer FA (2013) Timing of food intake predicts weight loss effectiveness. Int J Obes (Lond) 37(4):604–611. https://doi.org/10.1038/ijo.2012.229
Bandín C, Scheer FA, Luque AJ et al (2015) Meal timing affects glucose tolerance, substrate oxidation and circadian-related variables: a randomized, crossover trial. Int J Obes (Lond) 39(5):828–833. https://doi.org/10.1038/ijo.2014.182
Wang JB, Patterson RE, Ang A, Emond JA, Shetty N, Arab L (2014) Timing of energy intake during the day is associated with the risk of obesity in adults. J Hum Nutr Diet 27(Suppl 2):255–262. https://doi.org/10.1111/jhn.12141
Bishehsari F, Engen PA, Adnan D, Sarrafi S, Wilber S, Shaikh M, Green SJ, Naqib A, Giron LB, Abdel-Mohsen M, Keshavarzian A (2020) Abnormal food timing and predisposition to weight gain: role of barrier dysfunction and microbiota. Transl Res. https://doi.org/10.1016/j.trsl.2020.11.007
Moossavi S, Bishehsari F (2019) Microbes: possible link between modern lifestyle transition and the rise of metabolic syndrome. Obes Rev 20(3):407–419. https://doi.org/10.1111/obr.12784
Bishehsari F, Keshavarzian A (2019) Microbes help to track time. Science 365(6460):1379–1380. https://doi.org/10.1126/science.aaz0224
Rácz B, Dušková M, Stárka L, Hainer V, Kunešová M (2018) Links between the circadian rhythm, obesity and the microbiome. Physiol Res 67(Suppl 3):S409–S420. https://doi.org/10.33549/physiolres.934020
Roenneberg T, Kuehnle T, Pramstaller PP et al (2004) A marker for the end of adolescence. Curr Biol 14(24):R1038–R1039. https://doi.org/10.1016/j.cub.2004.11.039
Wang Y, Beydoun MA (2007) The obesity epidemic in the United States—gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev 29:6–28. https://doi.org/10.1093/epirev/mxm007
Ng M, Fleming T, Robinson M et al (2014) Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384(9945):766–781. https://doi.org/10.1016/S0140-6736(14)60460-8
Acknowledgements
We thank Shahab Khan for his help in the recruitment of participants.
Funding
FB was supported by NIH/AA025387 and Rush Translational Sciences Consortium/Swim Across America Organization, and is grateful for the support of the Brinson Foundation. MA was supported by the National Center For Advancing Translational Sciences of the National Institutes of Health under Award Number TL1TR002388. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by Institutional Review Board at Rush University (15011602).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alsayid, M., Khan, M.O., Adnan, D. et al. Behavioral circadian phenotypes are associated with the risk of elevated body mass index. Eat Weight Disord 27, 1395–1403 (2022). https://doi.org/10.1007/s40519-021-01276-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40519-021-01276-4