Abstract
Anxiety and obesity are two current phenomena. They are among the important public health problems with increasing prevalence worldwide. Although it is claimed that there are strong relations between them, the mechanism of this relationship has not been fully clarified yet. On the other hand, the effect of this relationship on the offspring has been another research subject. In this study, obese zebrafish were obtained by feeding two different diets, one containing high amount of lipid (HF) and the other containing high amount of carbohydrate (HK), and their anxiety levels were evaluated. To establish a relationship between these two phenomena, in addition to histopathological and immunohistochemical analysis in the brain tissues of fish, the transcription levels of some genes related to lipid and carbohydrate metabolisms were determined. In addition, offspring were taken from obese zebrafish and studied to examine the effect of parental obesity on offspring. As a result, it was observed that the HC diet, causing more weight increase than the HF diet, showed an anxiolytic while the HF diet an anxiogenic effect. It was suggested that the probable cause of this situation may be the regulatory effect on the appetite-related genes depending on the upregulation severity of the PPAR gene family based on the diet content. In addition, it was also suggested that it may have contributed to this process in neuron degenerations caused by oxidative stress. Regarding effects on offspring, it can be concluded that HF diet-induced obesity has more negative effects on the next generation than the HC diet.
Level of evidence
No Level of evidence: animal study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of obesity and overweight both adults and children has been increasing dramatically all over the world [1]. According to the World Health Organization (WHO) reports, it is estimated that about 7% of world children population under five years of age (around 43 million) were overweight. It is also suggested that the adverse effect of childhood-onset obesity persists into adulthood [2]. Moreover, obesity and overweight are associated with numerous common health problems, such as diabetes (type 2), anxiety and certain cancers, besides cardiovascular disease [3,4,5]. It is also known that obesity, one of the most important public health problems facing the world, causes mitochondrial dysfunction [6, 7], oxidative stress [8, 9], inflammation [10, 11], and resulting in apoptosis [12,13,14].
Anxiety disorders are the most common type of psychiatric disorders worldwide with a lifetime prevalence of 28.8% [15]. Although the complex pathophysiology of anxiety is not fully elucidated, it is known that the hypothalamus–pituitary–adrenal (HPA) axis regulates responses to danger and stress by arranging sympathetic and parasympathetic autonomic functions [16]. Increased corticotropin-releasing hormone (CRH), the major hypothalamic activator of the HPA axis released from the paraventricular nucleus of the hypothalamus, responds with increased adrenocorticotropin (ACTH) and cortisol in the periphery, leading to cardiovascular and endocrine responses of anxiety [17]. Under stress conditions, neuronal nitric oxide synthase (nNOS) expressed in also neurons and/or astrocytes regulates sympathetic activation by central CRH and may affect HPA axis activity [18]. In zebrafish (Danio rerio), a model organism for behavioral researches, the HPA axis is fundamental to stress responses and involves a cascade of hormones from nNOS to Glial fibrillary acidic protein (GFAP), which is one of the important markers for astrocyte changes [19, 20]. It is also reported that there are irregularities and impairments in these axis functions in the pathophysiology of anxiety [17]. The hypothalamus also plays a key role in the regulation of appetite and energy. Hypothalamus is the place hosting the organization of orexigenic and anorexigenic peptides, such as ghrelin, agouti-related protein (AgRP), neuropeptide Y (NPY) and proopiomelanocortin (POMC), and leptin, which also regulates lipid metabolism and shows its effect on the central nervous system through the hypothalamus [17]. Moreover, it is reported that some of these molecules have associated with the etiology and pathophysiology of anxiety disorders [21].
Scientists have suggested that based on human epidemiological and animal model studies, the risk of obesity in the offspring in later life is strongly associated with maternal obesity [22,23,24,25]. But more animal studies especially based on molecular are needed to support this suggestion and to clarify the potential mechanism of childhood obesity [26]. To address this scientific gap, choosing the best model animal is important in terms of investigating the relationship between parental obesity and early-life-stage lipid metabolism. Many signalling pathways associated with both lipid metabolism and energy homoeostasis are overlapped among vertebrates and are similar in a number of model species, including humans, rodents and zebrafish [27, 28]. Supplying nutrient from the maternally deposited yolk up to first 5–6 days in the early-life stage and being transparency of the larval stage makes zebrafish been a good model for this type of studies [28, 29]. Regarding this, Meguro and his colleagues established an obesity model for zebrafish using a lipid-modified special diet [29].
One of the main reasons for obesity is an unbalanced diet, which causes a positive energy imbalance between caloric intake and energy expenditure [30]. The extra calories intake via the excess nutrient depot as triglycerides or neutral lipids in the fat cells. Most of the animal model studies aimed at clarifying the potential effect mechanism of obesity especially on anxiety, high-fat (HF) diets were used. On the other hand, the number of studies investigating the effects of high-carbohydrate (HC) diets induced obesity on this mechanism is limited. Noronha and colleagues suggested that the influence of obesity as a relevant cause for the dysregulation of brain circuits, which could lead to anxiety-like disorders, is not completely understood [4]. The aim of the current study is to comparatively evaluate potential effect mechanism of HF and HC diet-induced obesity on anxiety in terms of behavioral and molecular perspective and to investigate the molecular and behavioral effects of parental obesity on offspring using obese zebrafish models.
Materials and methods
Experimental setup, zebrafish husbandry and fish feeding
AB strain zebrafish (Danio rerio) were obtained from Oregon State University and kept in Aquatic Habitats (imported by Akuamaks Co., Turkey) zebrafish system which maintained standard water condition and light–dark photo-period as described in The Zebrafish Book [31]. Zebrafish embryos were obtained from spawning adults in groups of about 20 males and 10 females in the breeding tanks overnight. Embryos were examined under a dissecting microscope, and unfertilized and death embryos were removed. Health embryos were kept in E3 medium (5 mM NaCI, 0.17 mM KCI, 0.33 mM CaCI2, 0.33 mM MgSO4, % 0.01 methylene blue) until 5 days post fertilization (dpf), then transferred to system water in the separated baby tanks and fed with chicken egg yolk until 12 dpf. Then, larvae were transferred to larvae tanks in the system and fed with Artemia salina four times a day until 20 dpf. From 20th day to 4 months, fish were fed with Artemia salina twice and flake diet once a day. By the end of the 4th month, fish were measured and weighed, and they divided into two groups by their gender. Each gender group allocated to three independent diet groups (control, high carbohydrate (HC) and high fat (HF)) which include seven fish of each with similar body mass index (BMI). Control groups were fed with standard flake diet (SFD) (Sera Co.), whereas other groups with formulated HC and HF diets (Table 1). Preparation of diets and proximate analyses were performed as previously described [29, 32]. Each tank of seven fish received 70 mg of the experimental and control diet twice daily during 6 weeks [29]. During feeding, water inflow was paused for 30 min and the fish were allowed to consume all their diet. During the 6-week experiment time, fishes were measured and weighed at second, fourth and the last week. To analyse effects of obesity on offspring, by the end of the 6th week, randomly selected male and female fish pairs belonging to the same diet group were put into the breeding tank. All embryos obtained from seven pairs of each group were pooled and kept in E3 medium until 5 dpf in the 28.5 °C incubator for further analysis. Each diet group included approximately 450 (± 75) embryo. After behavior tests following breeding, adult fish were anaesthetized and dissected under the microscope, and sampled brain tissues for further analysis. All procedures were performed in accordance with the approved Institutional Ethical Rules of Atatürk University, and fallowed OECD guidelines (TG 305 and 236).
Histopathology and immunofluorescence assays of adult brain
Five of seven fish in each group, whose reproduction and behavioral analyses were completed, were anesthetized and their brains were sampled under a microscope. The brain samples of adult zebrafish were detected in 4% paraformaldehyde solution for 48 h. Then, samples embedded in paraffin blocks following routine tissue tracking. 4 μm thickness serial sections of hypothalamic regions were taken from each block (Fig. 5). 17–20 serial sections were taken from each brain tissue. One of the sections was taken on the slides in turn, one for histopathology and three for immunohistochemical analysis. Hematoxylin–eosin was used for histopathological analysis while three different antibodies for immunohistochemical analysis. Therefore, for each analysis, at least 4–5 sections from each brain tissue were examined. For histopathological evaluation, preparations were stained with hematoxylin–eosin (HE) and examined with the light microscope. For immunofluorescence evaluation, sections taken to the slides were de-paraffinized and dehydrated and then washed with PBS. After de-paraffinization, the sections were dipped in 3% H2O2 for 10 min to block endogenous peroxidase activity. To unmask the antigen, slides were boiled in antigen retrieval solution 4 times for 5 min and then allowed to cool at room temperature. After cooling, they were washed with PBS and the protein block was dropped and then waited for 10 min. Primary antibodies (8-hydroxy-2′ -deoxyguanosine (8-OHdG) (sc-66036, Santa Cruz); glial fibrillary acidic protein (GFAP) (ab68428, Abcam); 8-hydroxy-2′ -deoxyguanosine (nNOS) (ab16650, Abcam) prepared in appropriate reconstitution (1/50 for 8-OHdG and 1/100 for the others) were dropped and incubated for 1 h at the 37 °C and then they were washed with PBS. Immunofluorescence antibody (FITC) (ab6785, Abcam; at a dilution of 1/500) was used as a secondary marker and after application, samples were incubated for 45 min at the 37 °C in dark and then washed with distilled water. Afterwards, DAPI (ab104140; Abcam) was dropped, and after incubation for 5 min in the dark, then tissues were washed with distilled water and covered with a coverslip. Samples were examined and imaged under a laser scanning microscope (Zeiss LSM 710) [31, 33]. Findings in the examined images were scored according to the severity of the findings as none (−), mild (+), moderate (+ +), severe (+ + +).
Whole mount staining of offspring larvae
Apoptosis detection assay of offspring larvae
To observe apoptotic cell in the live larvae, acridine orange (AO) staining, a nucleic acid selective metachromatic dye that interacts with DNA and RNA by intercalation or electrostatic attractions, was used for 10 fish (with three biological replicates, 30 fish in total) selected randomly from each group larvae pool [34]. To do this, 5 dpf larvae were rinsed three times with PBS and incubated in 5 mg/mL concentration and 2 mL amount of AO for 30 min in the dark at 28 °C, followed by three times of rinse in PBS [35, 36]. Stained larvae were examined under fluorescence microscope (Zeiss, Discovery V12, Germany). The fluorescence intensity of larvae was measured and quantified using ZEN (blue edition) software (Zeiss, Germany). To eliminate differences of intensity that come from analyzing different images, even they were taken at the same configurations, the fluorescence intensity of larvae was measured and quantified from images taken together of three larvae which belong to one of the three groups of each [35].
Reactive oxygen species (ROS) detection assay of offspring larvae
To detect reactive oxygen species (ROS) in the live larvae, 10 fish (with three biological replicates, 30 fish in total) were randomly selected from each group larvae pool and washed with ultra-pure H2O three times, and excess water was removed. Then, larvae were incubated in 1 μg/mL chloromethyl-20, 70-dichlorodihydro fluorescein diacetate (CM-H2DCFDA, Invitrogen) for 2 h in the dark at 28 °C and washed again with ultra-pure H2O three times, then immobilized in 3% methylcellulose [37]. CM-H2DCFDA reacts with many different ROS and gives fluorescent DCF signal, so it has use as a general indicator of oxidative stress (Invitrogen). Larvae imaging and quantification of the fluorescence intensity were performed according to description in apoptosis detection assay.
Lipid accumulation detection assay of offspring larvae
To make lipid droplets visible in the live larvae, Nile Red, which is an organic heterotetracyclic compound and can interact with intracellular lipid droplets [38], was used for 10 fish (with three biological replicates, 30 fish in total) selected randomly from each group larvae pool. In the experiment, 5 dpf larvae were incubated overnight with 10 ng/mL Nile Red in the dark at 28 °C. Larvae imaging and quantification of the fluorescence intensity were performed according to description in apoptosis detection assay.
Behavioral tests
Adult behavioral test
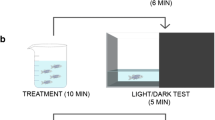
Behavior and anxiety-like behavior measurement were performed in all seven adult zebrafish of each group as reported by Cachat et al. [39]. For this purpose, adult zebrafish in a narrow and transparent diving tank divided into two equal virtual horizontal zones (top and bottom) were placed in the test room for 1 h due to habitation before the test. The behavior of the fish was recorded for 5 min using a video camera. The recorded videos were analyzed for total distance, latency, zone transmissions, time in upper half, average velocity and number of erratic movement and freezing bouts of each fish via Ethovision software (Noldus Co.).
Larvae behavioral test
The behavior of zebrafish larvae was evaluated by standard protocol. For this purpose, to monitor the activities of 24 zebrafish larvae (with three biological and two technical replicates, 144 larvae in total) selected randomly from each group pool with an infrared analog camera (25 frames per second), the 24-well plate containing one larva (6 dpf) in each well was placed in the DanioVision Observation Chamber (Noldus Co.). The temperature condition of observation chamber was carried out at 28 °C using a heating/cooling system (Noldus Co.). Behavior analysis was made especially in the afternoon due to the more stable movements. Dark/light periods were applied to create behavioral effects (swimming activity) in response to changing dark and light conditions. Following 10 min habitation in dark, 10 min light and 10 min dark conditions were applied for images to be recorded for a total of 50 min [40,41,42,43]. The anxiety index of the larvae, thigmotaxis, was measured as reported by Schnörr et al. [44]. For this purpose, each well of the 24-well plate containing the one larvae is divided into two zones (3 mm in outer), equivalent to the body length of the larvae. Thigmotaxis was calculated as the ratio between the total distance moved (TDM) in the outer zone and in the entire test arena (including inner and outer zone). Monitoring and evaluation of the behavior of zebrafish larvae was carried out using the EthoVision software (Noldus Co.). The analyses were repeated by three independent replications.
Larval measurements
To detail evaluation of the effects of parental obesity on offspring, some morphological and physiological properties were measured, such as survival and hatching rate, body malformations, body length, eye size, pericardial area, heart rate, blood flow rate using DanioScope software (Noldus Co.) and stereomicroscope (Zeiss Co.). To screen for morphological abnormalities (included pericardial edema, yolk sac edema, body malformations and curved body axis), obtained embryos from obese and control pairs were examined under the stereomicroscope in 24 h intervals during the 96 h and images were recorded in same configurations. Survival and hatching rates were determined during the relevant period. Mortality was identified by coagulation of the embryos, missing heartbeat, failure to develop somites and a non-detached tail [45]. Dead zebrafishes were recorded and promptly removed from the solution during observations. 3% methylcellulose was used for immobilizing the larvae during imaging. Body length, eye size and pericardial area of larvae were measured using DanioScope software from images taken in the same magnification under the stereomicroscope. Morphological observation, survival and hatching rates were determined as a result of examining at least 200 embryos larvae (with three biological replicates, 600 fish in total) from each group. 20 larvae (with three biological replicates, 60 larvae in total) were randomly selected from each group pool for pericardial area, eye size and body length measurements. For heart rate and blood flow, 10 larvae (with three biological replicates, 30 fish in total) randomly selected from each group pool were anaesthetized with anaesthetic agent MS-222 (tricaine, 0.10 mg/mL) for 5 min. Videos of 3 min were recorded in a suitable format for software from the pericardiac region of anesthetized larvae under the stereomicroscope. Videos were transferred to DanioScope and determined heart and blood vessel regions on video captures. Then software automatically calculates the number of beats per second/min (BPS/BPM) in heart and measures the flow activity in determined blood vessel.
Gene expression assay
Total RNA was isolated from the muscle tissue of all adult fish and randomly selected 17 larvae from each diet group pools with three biological replicates using RNA isolation kit (ECO-TECH Co. Turkey) according to manufacturer instructions. RNA concentrations and quality were verified by means of spectrophotometer (Epoch) and RNA gel electrophoresis, respectively. cDNA was synthesised from three adult RNAs and three larva pool RNAs which are 260/280 ratio closest to 2 (> 1.9) in each group using the cDNA Synthesis Kit (Bio-Rad) according to the manufacturer instructions. All cDNAs were stored at − 20 °C until use. Quantification of gene expression by real-time PCR analysis was performed using Bio-Rad CFX96 thermal cycler system and SYBR Green Real-time PCR Buffer (Bio-Rad) according to manufacturer instructions. Primer sets are given in Supplementary File. Amplification and detection of the samples and the standards were performed using the following thermal cycling conditions: 98 °C for 2 min for polymerase activation and cDNA denaturation followed by 40 cycles of denaturation at 95 °C for 15 s and annealing and extension at 60 °C for 30 s. Then, the reaction was finalized by standard melt curve analysis which from 65 to 95 °C and 0.5 °C increments at 2 s/step. Real-Time PCR data were analyzed using the efficiency (e)(−ΔΔCt) method [46], which is used to determine mean fold changes in gene expression against control group and reference gene GAPDH. The expression stability of the reference gene was confirmed. Analytical sensitivity was confirmed by running standard curves. Amplification efficiency (e) was calculated based on the slopes of the curves using the formula e = 10(−1/slope) [46, 47]. All Real-Time PCR reactions were performed using three biological replicates, and each biological replicate included three technical replicates. Finally, the average value for each group was considered for comparative analysis. The heat map of gene expressions was generated via the Heatmapper software online (http://heatmapper.ca/expression/).
Statistical analysis
Each experimental group (HF, HC and Control) composed of three biological replicates, and each biological replicate included at least two technical replicates to assess experimental accuracy and precision. Statistical analysis of the data obtained in the study was done using SPSS (SPSS Inc., Chicago, IL, USA) program. A one-way analysis of variance (ANOVA, among more than two groups) with post hoc Tukey studentized range honestly significant difference (HSD) test (Dunnett T3 or Kruskal–Wallis test for data not following normal distribution) and t-test (between two groups) was used to identify differences in all parameters. The specifics of each statistical analysis and the results are presented in relevant figure legend.
Results and discussion
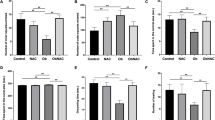
Although a standard diet for zebrafish has not yet been formulated in diet-induced obesity models, in this study, obese zebrafish were obtained using HF and HC diets, considering previous studies [29]. According to the results, it was observed that the HC diet caused more BMI increase (Fig. 1). Epidemiological studies have shown that dietary fat intake is positively associated with obesity in humans and laboratory animals. Meguro et al. [29] found that significantly higher total body fat volumes in high-fat diet-fed groups than those fed with the low-fat diet in a zebrafish. Although saturated or unsaturated fat in dietary ingredients has been associated with weight gain, previous studies have shown that body fat accumulation in zebrafish [29], in spayed dogs [48] and in Wistar rats [49] revealed that it is affected by the amount of dietary fat, but not the type of dietary fat. However, dietary lipid sources significantly affected the lipid accumulation in South American catfish, Pseudoplatystoma fasciatum, with lower whole body lipid content in fish fed diets with higher amount of polyunsaturated fatty acids [50]. It should be taken into account at this point that freshwater fish, such as zebrafish, have a mechanism which has the ability to synthesize long-chain saturated fatty acids from short-chain polyunsaturated fatty acids, through two separate pathways involving desaturation and elongation of their respective precursors [51]. This ability enables fish to preferentially utilize, convert or deposit the lipids depending on the dietary fatty acid profile and physiological priorities/requirements which are determined by several abiotic and biotic factors including the different life stages of the fish [32].
At the end of the feeding experiment, body mass index (BMI) were calculated to evaluate the degree of obesity. As shown in the Figure, BMI in female fish fed both HF and HC diets were significantly higher than the control group. As known that BMI is a useful marker for obesity in human but not sufficient alone for zebrafish. Hence, we have supported our obesity-related findings by evaluating the expression of some genes related to both obesity and lipid metabolism. In addition to transcriptional evaluation, we have observed excessive intraperitoneal lipid accumulation in all experiment fish against the control group. Data are expressed as means ± S.D. N = 3 biological replicates, N = 7. Statistical analyses were performed using Tukey post hoc tests (ANOVA) to compare the HF, HC and control groups. “*” indicate statistical significance between groups (p < .05)
On the other hand, dietary starches are classified as rapidly digestible, slowly digestible and resistance according to their digestibility characteristics. These different fractions of starch have important physiological results that affect metabolic hormones related to energy intake and toughness, especially body weight [52]. In this context, the pre-gelatinized starch we use in the present research is highly digestible. It has been reported that in case of excessive daily consumption of such starches may result in obesity and iron-deficiency anemia in human subjects [53]. Under normal circumstances, hepatic lipogenesis is upregulated following the ingestion of diet with high-carbohydrate and low-fat content to produce fat from dietary carbohydrate [54]. In our case, lipid was replaced with carbohydrate in HC diet, which seems to contribute to the elevated lipogenesis, thence the higher BMI in HC fed fish (Fig. 1). Similar results were reported by Dias et al. [55] in juvenile Senegalese sole (Solea senegalensis) depositing higher whole body lipid when fed diets with high carbohydrate level in comparison to those fed diets with high lipid content. Indeed, Castillo et al. [56] reported a higher final weight gain in rats fed with high-digestible starch diet than those fed with high-unsaturated fat diet. Moreover, dietary carbohydrate level and source can lead to unbalanced lipid + carbohydrate/protein ratio, which can negatively affect body composition, food use, lipid accumulation, metabolism and overall health, especially growth and behavior [57].
One of the interesting findings from this study was that according to the Nile Red staining results, the amount of fat accumulated in the body of the offspring from these obese parents was significantly higher than control (Fig. 2). According to the BMI results obtained from the parents, we would expect to detect more fat in the whole body of the offspring of fish fed with HC diet. Because we know that the excess carbohydrate taken into the body is stored as fat and we would expect the mother to transfer this extra fat to her eggs more than the group with less BMI. But, although the HC diet caused more weight gain in the parents than the HF diet, the fat accumulation in the whole body was found to be nearly equal in the offspring of the fish fed with both diets. Moreover, significantly more fat accumulation was detected in the abdomen of offspring of parents fed with the HF diet. Even more interesting was the significant difference in the number of lipid droplets detected in the bodies of offspring of their parents fed with the HF diet. According to these results, it was shown that obese zebrafish parents transferred fat to their offspring through yolk sacs and that the transferred fat could be localized differently in the bodies of the offspring depending on the diet of the parents. Studies investigating the effects of parental diet on the lipid metabolism of offspring support these results [58]. In this study, we completed our analysis of the offspring during the time the offspring lived dependent on the yolk sacs. If this study can be continued with the diets to be prepared for the offspring larvae and the offspring can be followed also in their juvenile and adult periods, it can provide us with more information about the effects of parental obesity on childhood obesity.
Whole mount staining of offspring. In panel A, apoptotic cells were determined using acridine orange (AO) staining in 5 dpf larvae. The graph represents the relative fluorescence intensity of dead cell detected by acridine orange staining. In panel B, CMH2DCFDA staining in vivo of produced ROS in 5 dpf larvae and relative fluorescence graph of the signals. In panel C, the lipid accumulation was determined Nile Red staining in 5 dpf larvae. The graph in the left represents the relative fluorescence intensity of whole larvae by Nil Red staining. The graph in the right represents the mean pixel numbers of Red fluorescence in the Nile Red staining were calculated from the whole body of larvae except abdomen. Data are expressed as means ± S.D. Statistical analyses were performed using Tukey post hoc tests (ANOVA) to compare the HF, HC and control groups. “*” indicate statistical significance between groups (p < .05). Each image in the figure was chosen from the average value range representing the group
Similar to the Nile red staining results, it was found that offspring of parents fed with HF diet had more free oxygen radicals in the abdomen (Fig. 2). However, according to the results of acridine orange staining, it was observed that HF and HC-diet-induced obesity in zebrafish had no effect on apoptosis in the next generation (Fig. 2). On the other hand, according to the morphological and physiological observations on the larvae, it can be said that HF-diet-induced obesity has more negative effects in the next generation than the HC diet (Fig. 3A). These results clearly showed us that the effect of obesity induced by different diets on the offspring is different in zebrafish model.
Some morphological and physiological properties (A) and locomotor activity (B) of the offspring larvae of obese and control parents. Panel C represents a heat map image showing the total distance covered by the offspring at the end of one of the locomotor activity tests. The graph in panel D shows the result of the Thigmotaxis analysis of offspring larvae, known as an anxiety test in the larval fish. Data are expressed as means ± S.D. Statistical analyses were performed using Tukey post hoc tests (ANOVA) to compare the HF, HC and control groups. Different letters (a, b and c) indicate statistically significant differences between treatment groups (p < .05)
Besides a positive association between obesity and anxiety, there is evidence that anxiety can provoke an increase in appetite [59], but it is still unclear how to affect these two syndromes each other. On the other hand, the relationship between dietary fat and calorie intake, age and cognitive decline is still controversial, but obesity is reported to be strongly linked to neural degeneration [30]. This shows that the long- and/or short-term behavioral and neuropathological effects of high-fat and high-carbohydrate diets should be revealed in more detail. In literature, most of the studies have focused on the high-fat diet model which leads to obesity, but there is a lack of study examining the linkage between high-carbohydrate-diet-induced obesity and anxiety.
Zebrafish is rapidly gaining popularity in neuroscience and behavioral research because it provides important information regarding neural pathways, physiological biomarkers, and genetic bases of normal and pathological brain function [29, 60]. In the present study, to evaluate the anxiety level, we have used the novel tank test for parents and the thigmotaxis for offspring. Evaluation of anxiety degree of adult fish was performed using seven parameters obtained observation/calculation of video records via Ethovision software as described previously [19, 39]. According to this test model, the anxiety levels of the zebrafish were determined by examining the behaviors, such as the part where the fish spend more time in the tank (upper half or lower half), transition between zones, erratic movement and freezing movement. For example, the fish spending more time in the lower part of the tank and/or the latency in the transition to the upper half is considered as an indicator of anxiety (See legend of Fig. 4). It has been clearly figured out from these adult behavioral results given in Fig. 4 that HF diet showed an anxiogenic effect on zebrafish, while HC diet anxiolytic. In the new generation, while there was no difference in thigmotaxis results, it was determined that the offspring of the parents fed with the HC diet were less active (Fig. 3B, C). Consistent with our results, anxiety caused by a high-fat diet has also been reported in male rats [61], mice [62] and zebrafish [63]. Additionally, Meguro et al. [63] suggested that the high-fat diet impairs cognitive function in zebrafish. It has been also reported that there is a positive relationship between impair cognitive performance and anxiety [64]. To the best of our knowledge, although there are some confusing records in the literature that both low-carb [65] and high-carb [66] diets can cause anxiety, there is no detailed molecular study that examines the effects of high-carb and low-fat diets on anxiety. In this context, we think that the present study is important for clarifying this scientific lack. It was also tested how anxiety behaviors caused by diet-induced obesity change in offspring. According to the results, it was observed that the diet-induced obesity of the parents had no effect on the anxiety of the offspring (Fig. 3D).
Analysis of novel tank diving swimming behavior of fish fed experimental diets. Evaluation of anxiety degree of each group fish individually was performed using seven parameters obtained observation/calculation of video records via software as described previously [19, 39, 60]. Total erratic movements and total freezing bouts indicating increased fear/anxiety were significantly higher in the HF group. Another important behavioral anxiety parameter for zebrafish is the latency to upper half. When zebrafish put into a novel tank, as a general behavioral response, they firstly dive to the bottom of the tank and gradually explore to the whole tank as it habituates to the new environment. The longer latency to upper half indicates higher anxiety levels. Total time spent in the upper half of the novel tank is another important behavioral anxiety parameter for zebrafish. A longer duration in the upper half of the tank indicates lower anxiety levels. Similarly, more entries to the upper half indicate lower anxiety levels. According to all these results, we can say that HF diet which contains a high amount of sunflower oil has an anxiogenic effect on fish fed with this diet, while the HC diet which contains a high amount of pre-gelatinized starch has an anxiolytic effect. During the behavioral experiments, we did not observe any significant difference between genders. Values are means ± SD. N = 3 biological replicates, N = 2 technical replicates. Statistical analyses were performed using Tukey post hoc tests to compare the HF, HC and control groups at each time-point. Different letters (a, b and c) indicate statistically significant differences between treatment groups (p < .05)
Studies have suggested that obesity disrupts cognition and contributes to neuropsychiatric diseases, such as anxiety, due to reasons, such as oxidative stress and inflammation [5, 30, 67]. Our results, which necrosis, degeneration, hyperemia and immunopositivity were detected in the brain sections, are severe in the HF group while mild in the HC group, confirm this suggestion (The scoring table in Fig. 5). On the other hand, it is known that there is a direct relationship between the amount of adiposity and the level of oxidative stress in the brain and degeneration of the neuron [67], but its mechanism has not been fully illuminated yet.
Laser scanning microscopy images of immunofluorescence reactions for 8 − OHdG (B), GFAP (C) and nNOS (D) (FITC/GFP) in the brain tissues of zebrafish fed the experimental diets. Cell nuclei labelled with DAPI. And microscopic images of the brain tissues of zebrafish stained with hematoxylin–eosin (H&E) (E). The drawings in the panel A show the dorsal (left) and lateral (right) view of the adult zebrafish brain, and the lines on the right illustrate the position of the serial sections. (Tel telencephalon, CCe corpus cerebelli, TeO tectum opticum, OB olfactory bulb, Hyp Hypothalamus regions). The green-coloured zones in the scoring table at the bottom and on the right indicate the scoring the severity of the immunopositivity, while the purple-coloured zones show the scoring the severity of the degeneration and necrosis (D&N) and hyperemia (HYP) findings in the histopathological results (none (−), mild (+), moderate (+ +), severe (+ + +)). Arrowheads show D&N in neutrophils. The larger images were given in the Supplementary File for better evaluation by readers. We did not observe any significant difference between genders. The images in the figure were chosen from the average value range representing the group in which they are found
Oxidative stress, characterized by increased free oxygen radicals, is an important factor in the pathology of neurodegenerative disorders. 8-OHdG is a common biomarker used to find DNA lesions due to oxidative stress [68] and there are studies that suggest that it become an obesity marker due to its strong linkage with obesity [69, 70]. In the present study, especially 8-OHdG immunopositivity in HF diet supports this recommendation (Fig. 5). Meanwhile, it is worth noting that 8-OHdG immunopositivity detected in brain tissue was lower, although the weight gain was higher in fish fed with HC diet than HF diet (Fig. 5). One of the causes of neuronal DNA lesions is reported to be nitric oxides (NO) produced by nNOS in the brain [71]. In the present study, the observation of 8-OHdG and nNOS immunopositivities in fish brain tissues with similar intensities in the same groups makes us think that the nNOS/NO product and/or derivative is partially mediated by the oxidative DNA damage there (Fig. 5). Studies have also shown that there may be a relationship between anxiety caused by HF diet and activity in the hippocampal nNOS/NO pathway [72]. On the other hand, the oxidative stress from NO leads to the disruption of astrocytes, which represent the most abundant cell type in the central nervous system (CNS) and critically affect the homeostasis of neuronal cells [73]. Increased GFAP levels are considered one of the main markers of the commonly observed astrocyte reaction after CNS damage [74]. Metabolic disorders like diet-induced obesity have been shown to affect the structure and function of astrocytes in the hypothalamus, which is the metabolic center. For example, astrocyte and microglial GFAP gene expressions have been reported to increase in mice fed with HF diet [75, 76]. Astrocytes play a role in coordinating immune responses, maintaining neuronal functions, and regulating metabolic changes through the blood–brain barrier [77]. For these reasons, our results supported the hypothesis claiming that astrocytic functions are related to cases that may occur in cognitive and affective behavior [76].
Here, to give a chance to the readers’ deeper evaluation on the linkage between diet-induced obesity and anxiety, we have also analyzed the mRNA expression levels of some genes that could be associated with lipid/carbohydrate metabolism and obesity. According to the quantitative PCR results performed at approximately 100% (± 5) efficiency, we have obtained important differences between both these two obesity models and their offspring in terms of the transcription level of these genes (Fig. 6). Interestingly, the gene expression profiles of the offspring and their parents were quite similar, although the RNA was isolated from the whole body in offspring while muscle in adults. We normally expect melanocortin signals to come from the brain rather than the muscle. For the transcription analysis in this study, we first tried the adult muscle to make a better comparison between parents and offspring, since RNA from larvae pool is isolated from the whole body, where the amount of tissue is the most muscle. When we got satisfactory signals from the muscle, we continued the study in this way.
Heat map analysis of lipid and carbohydrate metabolism and appetite-related gene expressions between the diets in parents and offspring. The heat map of gene expression results was generated via the Heatmapper software online. The z-score was also determined by the software. The expression levels are shown on a log scale from low (red) to high (green). The calculated real-time PCR efficiency was approximately 100% (± 5). Values are means ± SD. Statistical analyses were performed using Tukey post hoc tests (ANOVA) and t-test to compare genes expression levels between the HF, HC and control groups in parents and offspring. The letters in the upper right corners of the boxes indicate statistical differences. Different letters (a, b and c) indicate statistically significant differences between treatment groups (p < .05)
It is known that lipids are metabolized for ATP production by the process of fatty acid oxidation, which is modulated by a number of signaling pathways including activation of the PPAR, FASN and LPL [54, 78, 79]. PPARs are nuclear receptor transcription factors regulating the lipid and carbohydrate metabolisms with three main subtypes: PPAR-α (alpha), PPAR-δ (delta) and PPAR-γ (gamma). PPAR-α which is the main regulator of lipid metabolism and functions as a fatty acid sensor having an important role in switching from the satiety to the hunger condition [80] besides regulating energy expenditure, while PPAR-γ plays a key role in the initiation and maintenance of the adipogenesis process. PPAR-δ is involved in the promotion of mitochondrial fatty acid oxidation and energy expenditure [81]. FASN is the key enzyme for the lipid synthesis which is promoted by ingestion of dietary carbohydrates to convert carbohydrates to lipids, mostly in triglyceride form packaged in very-low-density lipoproteins which would be hydrolyzed by LPL when needed, then transported to the adipose tissue as long-term energy storage [54, 79]. In our study, we have observed that all PPARs genes upregulated in both diets against the control group; however, the expression levels of all these upregulated genes in the HC group were significantly higher than the HF group (Fig. 4). Although there are a limited number of publications suggesting that PPAR family has an effect on anxiety, its mechanism of action has not been fully revealed [82, 83]. On the other hand, studies show that the effect of Leptin, Ghrelin, POMC and NPY/AgRP which regulates food intake, energy homeostasis and appetite on anxiety may be more direct via on hypothalamus [84,85,86,87,88]. In addition, it was found that activation of AgRP neurons decreases anxiety levels [89]. This information supports our findings of increased AgRP expression and low anxiety observed in the group fed on HC diet. Looking at gene expression profiles of diets, one of which is anxiolytic and the other is anxiogenic, most of these genes have been downregulated in the fish fed on HF diet that causes the anxiety. This shows us that PPARs may act on anxiety through these genes not directly.
Although there are animal model studies of diet-induced obesity in the literature, the number of studies comparing fat and carbohydrate as energy sources in the diets used is limited. Especially, the effect of using the only carbohydrate as an energy source on the parameters examined has not been fully clarified. In this study, the effects of obesity obtained using diets prepared with these two energy sources in the zebrafish model were investigated comparatively and more importantly, its effects on the next generation at the molecular level were examined for the first time.
Conclusion
Although it is known that there is a strong relationship between food intake and anxiety, anxiety is affected by many factors. Moreover, since the individual effects of most of these factors are still uncertain, it would not be right to focus on the cause of anxiety over a single factor. The obtained results in this study have shown us that the excess amounts of dietary carbohydrates like highly digestible starches are first converted, then stored as triglyceride and if the dietary fat cannot meet the required energy, it may be met by oxidation of this storage lipids. Moreover, this mechanism has been shown to have serious effects on anxiety. It is hypothesized that the PPAR family, which is active as a result of the excessive occurrence of triglycerides, known to directly affect the rewarding process in fish fed on the HC diet, causes anxiolytic effect through the regulation of genes related to appetite. Furthermore, in fish fed on the HF diet that causes anxiogenic effects, the occurrence of anxiety as a result of relatively less activation of PPAR family resulting in opposite regulation of these appetite-related genes supports this hypothesis. In addition, it is undoubted that the severity of the damage in the hypothalamus region as a result of oxidative stress caused by diet-induced obesity contributes to these processes. When an organism is considered as a whole, we can easily say that all systems are directly or indirectly interconnected with each other. It is worth mentioning that the clarification of these connections depends on new in vivo studies to be conducted in light of the findings obtained from such studies and new hypotheses to be developed.
Finally, it can be said that parental obesity is strongly associated with offspring lipid metabolism and lipid accumulation in the zebrafish model. Similarly, it was found that parental diet-induced obesity in animals [25, 90, 91] and humans [24] may trigger obesity in the next generations. Consequently, in light of all the results, we can conclude that although it causes more weight gain, high-carbohydrate-induced obesity is less harmful than those caused by high-fat-induced obesity in terms of anxiety, the brain damage and maybe childhood obesity.
References
WHO (2017) Guideline: assessing and managing children at primary health-care facilities to prevent overweight and obesity in the context of the double burden of malnutrition. World Health Organization, Geneva
Freedman DS, Dietz WH, Tang R, Mensah GA, Bond MG, Urbina EM, Srinivasan S, Berenson GS (2004) The relation of obesity throughout life to carotid intima-media thickness in adulthood: the Bogalusa Heart Study. Int J Obes 28:159–166. https://doi.org/10.1038/sj.ijo.0802515
Reilly J.J., Kelly J.: Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. International journal of obesity (2005), 35, 891–898, 2011 DOI: https://doi.org/10.1038/ijo.2010.222.
de Noronha SR, Campos GV, Abreu AR, de Souza AA, Chianca DA, de Menezes RC (2017) High fat diet induced-obesity facilitates anxiety-like behaviors due to GABAergic impairment within the dorsomedial hypothalamus in rats. Behav Brain Res 316:38–46. https://doi.org/10.1016/j.bbr.2016.08.042
Gariepy G, Nitka D, Schmitz N (2010) The association between obesity and anxiety disorders in the population: a systematic review and meta-analysis. Int J Obesity 34:407–419. https://doi.org/10.1038/ijo.2009.252
Putti R, Sica R, Migliaccio V, Lionetti LJFIP (2015) Diet impact on mitochondrial bioenergetics and dynamics. Front Physiol 6:109. https://doi.org/10.3389/fphys.2015.00109
Heinonen S, Buzkova J, Muniandy M, Kaksonen R, Ollikainen M, Ismail K, Hakkarainen A, Lundbom J, Lundbom N, Vuolteenaho K, Moilanen E, Kaprio J, Rissanen A, Suomalainen A, Pietiläinen KH (2015) Impaired mitochondrial biogenesis in adipose tissue in acquired obesity. J Diabetes 64:3135–3145. https://doi.org/10.2337/db14-1937%
Vincent HK, Taylor AGJIJOO (2006) Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. Int J Obes 30:400. https://doi.org/10.1038/sj.ijo.0803177
Noeman SA, Hamooda HE, Baalash AAJD (2011) Syndrome m: Biochemical study of oxidative stress markers in the liver, kidney and heart of high fat diet induced obesity in rats. Diabetol Metab Syndr 3:17. https://doi.org/10.1186/1758-5996-3-17
Maury E, Brichard SM (2010) Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol 314:1–16. https://doi.org/10.1016/j.mce.2009.07.031
Mraz M, Haluzik M (2014) The role of adipose tissue immune cells in obesity and low-grade inflammation. J Endocrinol 222:R113. https://doi.org/10.1530/joe-14-0283
de Mello AH, Costa AB, Engel JDG, Rezin GT (2018) Mitochondrial dysfunction in obesity. Life Sci 192:26–32. https://doi.org/10.1016/j.lfs.2017.11.019
Pintus F, Floris G, Rufini A (2012) Nutrient availability links mitochondria, apoptosis, and obesity. Aging 4:734–741. https://doi.org/10.18632/aging.100505
Tinahones FJ, Coín AL, Murri M, Oliva OW, Mayas Torres MD, Barbarroja N, Gomez HR, Malagón MM, El Bekay R (2013) Caspase induction and BCL2 inhibition in human adipose tissue. A potential relationship with insulin signaling alteration. J Diabetes Care 36:513–521. https://doi.org/10.2337/dc12-0194%
Dalvie S, Koen N, Stein DJ (2020) Chapter 24 - Genomic contributions to anxiety disorders. In: Baune BT (ed) Personalized psychiatry. Academic Press, San Diego, pp 297–306
Hryhorczuk C, Décarie-Spain L, Sharma S, Daneault C, Rosiers CD, Alquier T, Fulton S (2017) Saturated high-fat feeding independent of obesity alters hypothalamus-pituitary-adrenal axis function but not anxiety-like behaviour. Psychoneuroendocrinology 83:142–149. https://doi.org/10.1016/j.psyneuen.2017.06.002
Charney D (2017) Sadock’s Comprehensive Textbook of Psychiatry. 10. 1 & 2; Chapter 14. Anxiety disorders. Wolters Kluwer, New York
Wang J, Li J, Yu M, Wang Y, Ma Y (2019) An enhanced expression of hypothalamic neuronal nitric oxide synthase in a rat model of simulated transport stress. BMC Vet Res 15:323. https://doi.org/10.1186/s12917-019-2071-x
Egan RJ, Bergner CL, Hart PC, Cachat JM, Canavello PR, Elegante MF, Elkhayat SI, Bartels BK, Tien AK, Tien DH, Mohnot S, Beeson E, Glasgow E, Amri H, Zukowska Z, Kalueff AV (2009) Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav Brain Res 205:38–44. https://doi.org/10.1016/j.bbr.2009.06.022
Meng FT, Zhao J, Fang H, Zhang LF, Wu HM, Liu YJ (2017) Upregulation of mineralocorticoid receptor in the hypothalamus associated with a high anxiety-like level in apolipoprotein E4 transgenic mice. Behav Genet 47:416–424. https://doi.org/10.1007/s10519-017-9843-5
Li Q, Bartley AF, Dobrunz LE (2017) Endogenously released neuropeptide Y suppresses hippocampal short-term facilitation and is impaired by stress-induced anxiety. J Neurosci 37:23–37. https://doi.org/10.1523/jneurosci.2599-16.2016
Dahlhoff M, Pfister S, Blutke A, Rozman J, Klingenspor M, Deutsch M, Rathkolb B, Fink B, Gimpfl M, de Angelis MH (2014) Peri-conceptional obesogenic exposure induces sex-specific programming of disease susceptibilities in adult mouse offspring. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 1842:304–317. https://doi.org/10.1016/j.bbadis.2013.11.021
O’Reilly JR, Reynolds RM (2013) The risk of maternal obesity to the long-term health of the offspring. Clin Endocrinolo 78:9–16. https://doi.org/10.1111/cen.12055
Mingrone G, Manco M, Mora MEV, Guidone C, Iaconelli A, Gniuli D, Leccesi L, Chiellini C, Ghirlanda GJDC (2008) Influence of maternal obesity on insulin sensitivity and secretion in offspring. Diabetes Care 31:1872–1876. https://doi.org/10.2337/dc08-0432
Masuyama H, Hiramatsu YJPO (2014) Additive effects of maternal high fat diet during lactation on mouse offspring. PLoS ONE 9:e92805. https://doi.org/10.1371/journal.pone.0092805
Han L, Ren C, Li L, Li X, Ge J, Wang H, Miao Y-L, Guo X, Moley KH, Shu WJNG (2018) Embryonic defects induced by maternal obesity in mice derive from Stella insufficiency in oocytes. Nat Genet 50:432. https://doi.org/10.1038/s41588-018-0055-6
Seebacher F, Tallis J, McShea K, James RS (2017) Obesity-induced decreases in muscle performance are not reversed by weight loss. Int J Obes 41:1271–1278. https://doi.org/10.1038/ijo.2017.81
Bergen WG, Mersmann HJ (2005) Comparative aspects of lipid metabolism: impact on contemporary research and use of animal models. J Nutr 135:2499–2502. https://doi.org/10.1093/jn/135.11.2499%
Meguro S, Hasumura T, Hase T (2015) Body fat accumulation in zebrafish is induced by a diet rich in fat and reduced by supplementation with green tea extract. PLoS ONE 10:e0120142. https://doi.org/10.1371/journal.pone.0120142
Elsayed E-SS, Husseini Atteia H (2015) Chapter 102 - obesity and neurodegeneration: a focus on dietary influence. In: Martin CR, Preedy VR (eds) Diet and nutrition in dementia and cognitive decline. Academic Press, San Diego, pp 1097–1105. https://doi.org/10.1016/B1978-1090-1012-407824-407826.400102-407826
Westerfield M (1993) The zebrafish book : a guide for the laboratory use of zebrafish (Brachydanio rerio). M. Westerfield, Eugene
Arslan M, Alaybasi S, Altun E, Gulen S, Sirkecioglu N, Atasever A, Haliloglu HI, Aras M (2016) Changes in lipids, fatty acids, lipid peroxidation and antioxidant defence system during the early development of wild brown trout (salmo trutta). Turk J Fish Aquat Sci 16:703–714. https://doi.org/10.4194/1303-2712-v16_3_24
Kinoshita S, Ceyhun SB, Md A, Siddique BS, Akolkar DB, Asakawa S, Watabe S (2018) Promoter analysis of the fish gene of slow/cardiac-type myosin heavy chain implicated in specification of muscle fiber types. Fish Physiol Biochem 44:679–691. https://doi.org/10.1007/s10695-018-0463-7
Hu YL, Qi W, Han F, Shao JZ, Gao JQ (2011) Toxicity evaluation of biodegradable chitosan nanoparticles using a zebrafish embryo model. Int J Nanomed 6:3351–3359. https://doi.org/10.2147/ijn.S25853
Sökmen TÖ, Sulukan E, Türkoğlu M, Baran A, Özkaraca M, Ceyhun SB (2020) Polystyrene nanoplastics (20 nm) are able to bioaccumulate and cause oxidative DNA damages in the brain tissue of zebrafish embryo (Danio rerio). Neurotoxicology 77:51–59. https://doi.org/10.1016/j.neuro.2019.12.010
Duan J, Yu Y, Shi H, Tian L, Guo C, Huang P, Zhou X, Peng S, Sun Z (2013) Toxic effects of silica nanoparticles on zebrafish embryos and larvae. PLoS ONE 8:e74606. https://doi.org/10.1371/journal.pone.0074606
Kim MS, Louis KM, Pedersen JA, Hamers RJ, Peterson RE, Heideman W (2014) Using citrate-functionalized TiO2 nanoparticles to study the effect of particle size on zebrafish embryo toxicity. Analyst 139:964–972. https://doi.org/10.1039/c3an01966g
Jones KS, Alimov AP, Rilo HL, Jandacek RJ, Woollett LA, Penberthy WT (2008) A high throughput live transparent animal bioassay to identify non-toxic small molecules or genes that regulate vertebrate fat metabolism for obesity drug development. Nutr Metab (Lond) 5:23. https://doi.org/10.1186/1743-7075-5-23
Cachat J, Stewart A, Grossman L, Gaikwad S, Kadri F, Chung KM, Wu N, Wong K, Roy S, Suciu C, Goodspeed J, Elegante M, Bartels B, Elkhayat S, Tien D, Tan J, Denmark A, Gilder T, Kyzar E, Dileo J, Frank K, Chang K, Utterback E, Hart P, Kalueff AV (2010) Measuring behavioral and endocrine responses to novelty stress in adult zebrafish. Nat Protoc 5:1786–1799. https://doi.org/10.1038/nprot.2010.140
Liang X, Adamovsky O, Souders CL, Martyniuk CJ (2019) Biological effects of the benzotriazole ultraviolet stabilizers UV-234 and UV-320 in early-staged zebrafish (Danio rerio). Environ Pollut 245:272–281. https://doi.org/10.1016/j.envpol.2018.10.130
Massarsky A, Abdel A, Glazer L, Levin ED, Di Giulio RT (2018) Neurobehavioral effects of 1,2-propanediol in zebrafish (Danio rerio). Neuro Toxicol 65:111–124. https://doi.org/10.1016/j.neuro.2018.02.007
Wang XH, Souders CL, Zhao YH, Martyniuk CJ (2018) Paraquat affects mitochondrial bioenergetics, dopamine system expression, and locomotor activity in zebrafish (Danio rerio). Chemosphere 191:106–117. https://doi.org/10.1016/j.chemosphere.2017.10.032
Zhou S, Chen Q, Di Paolo C, Shao Y, Hollert H, Seiler T-B (2019) Behavioral profile alterations in zebrafish larvae exposed to environmentally relevant concentrations of eight priority pharmaceuticals. Sci Total Environ 664:89–98. https://doi.org/10.1016/j.scitotenv.2019.01.300
Schnörr SJ, Steenbergen PJ, Richardson MK, Champagne DL (2012) Assessment of thigmotaxis in larval zebrafish. In: Kalueff AV, Stewart AM (eds) Zebrafish protocols for neurobehavioral research. Humana Press, Totowa, pp 37–51. https://doi.org/10.1007/1978-1001-61779-61597-61778_61773
Shi X, Du Y, Lam PK, Wu RS, Zhou B (2008) Developmental toxicity and alteration of gene expression in zebrafish embryos exposed to PFOS. Toxicol Appl Pharmacol 230:23–32. https://doi.org/10.1016/j.taap.2008.01.043
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. https://doi.org/10.1093/nar/29.9.e45
Ceyhun SB, Aksakal E, Kırım B, Atabeyoğlu K, Erdoğan O (2012) Chronic toxicity of pesticides to the mRNA expression levels of metallothioneins and cytochrome P450 1A genes in rainbow trout. Toxicol Ind Health 28:162–169. https://doi.org/10.1177/0748233711409482
Truett AA, Borne AT, Monteiro MP, West DB (1998) Composition of dietary fat affects blood pressure and insulin responses to dietary obesity in the dog. Obes Res 6:137–146. https://doi.org/10.1002/j.1550-8528.1998.tb00328.x
Hill JO, Lin D, Yakubu F, Peters JC (1992) Development of dietary obesity in rats: influence of amount and composition of dietary fat. Int J Obes Relat Metab Disord 16:321–333
Arslan M, Rinchard J, Dabrowski K, Portella MC (2008) Effects of different dietary lipid sources on the survival, growth, and fatty acid composition of South American catfish, Pseudoplatystoma fasciatum, surubim, juveniles. J World Aquacult Soc 39:51–61. https://doi.org/10.1111/j.1749-7345.2007.00133.x
Ishak SD, Tan S-H, Khong H-K, Jaya-Ram A, Enyu Y-L, Kuah M-K, Shu-Chien AC (2008) Upregulated mRNA expression of desaturase and elongase, two enzymes involved in highly unsaturated fatty acids biosynthesis pathways during follicle maturation in zebrafish. Reprod Biol Endocrinol 6:56–56. https://doi.org/10.1186/1477-7827-6-56
Aller EEJG, Abete I, Astrup A, Martinez JA, van Baak MA (2011) Starches, sugars and obesity. Nutrients 3:341–369. https://doi.org/10.3390/nu3030341
Raben A, Tagliabue A, Christensen NJ, Madsen J, Holst JJ, Astrup A (1994) Resistant starch: the effect on postprandial glycemia, hormonal response, and satiety. Am J Clin Nutr 60:544–551. https://doi.org/10.1093/ajcn/60.4.544
Glimcher LH, Lee A-H (2009) From sugar to fat: How the transcription factor XBP1 regulates hepatic lipogenesis. Ann NY Acad Sci 1173:E2. https://doi.org/10.1111/j.1749-6632.2009.04956.x
Dias J, Rueda-Jasso R, Panserat S, Conceição LED, Gomes EF, Dinis MT (2004) Effect of dietary carbohydrate-to-lipid ratios on growth, lipid deposition and metabolic hepatic enzymes in juvenile Senegalese sole (Solea senegalensis, Kaup). Aquac Res 35:1122–1130. https://doi.org/10.1111/j.1365-2109.2004.01135.x
Ble-Castillo JL, Aparicio-Trapala MA, Juárez-Rojop IE, Torres-Lopez JE, Mendez JD, Aguilar-Mariscal H, Olvera-Hernández V, Palma-Cordova LC, Diaz-Zagoya JC (2012) Differential effects of high-carbohydrate and high-fat diet composition on metabolic control and insulin resistance in normal rats. Int J Environ Res Public Health 9:1663–1676. https://doi.org/10.3390/ijerph9051663
Polakof S, Panserat S, Soengas JL, Moon TW (2012) Glucose metabolism in fish: a review. J Comp Physiol B 182:1015–1045. https://doi.org/10.1007/s00360-012-0658-7
Parlee SD, MacDougald OA (2014) Maternal nutrition and risk of obesity in offspring: The Trojan horse of developmental plasticity. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 1842:495–506. https://doi.org/10.1016/j.bbadis.2013.07.007
Wurtman J, Wurtman RJCOR (2018) The trajectory from mood to obesity. Curr Obes Rep 7:1–5. https://doi.org/10.1007/s13679-017-0291-6
Cachat JM, Canavello PR, Elegante MF, Bartels BK, Elkhayat SI, Hart PC, Tien AK, Tien DH, Beeson E, Mohnot S, Laffoon AL, Stewart AM, Gaikwad S, Wong K, Haymore W, Kalueff AV (2011) Modeling stress and anxiety in zebrafish. In: Kalueff AV, Cachat JM (eds) Zebrafish models in neurobehavioral research. Humana Press, Totowa, pp 73–88. https://doi.org/10.1007/1978-1001-60761-60922-60762_60763
Dutheil S, Ota KT, Wohleb ES, Rasmussen K, Duman RS (2016) High-fat diet induced anxiety and anhedonia: impact on brain homeostasis and inflammation. Neuropsychopharmacology 41:1874–1887. https://doi.org/10.1038/npp.2015.357
Gainey SJ, Kwakwa KA, Bray JK, Pillote MM, Tir VL, Towers AE, Freund GG (2016) Short-term high-fat diet (HFD) induced anxiety-like behaviors and cognitive impairment are improved with treatment by glyburide. Front Behav Neurosci 10:156–156. https://doi.org/10.3389/fnbeh.2016.00156
Meguro S, Hosoi S, Hasumura T (2019) High-fat diet impairs cognitive function of zebrafish. Sci Rep 9:17063. https://doi.org/10.1038/s41598-019-53634-z
Angelidis A, Solis E, Lautenbach F, van der Does W, Putman P (2019) I’m going to fail! Acute cognitive performance anxiety increases threat-interference and impairs WM performance. PLoS ONE 14:e0210824. https://doi.org/10.1371/journal.pone.0210824
Ehrenreich MJ (2006) A case of the re-emergence of panic and anxiety symptoms after initiation of a high-protein very low carbohydrate diet. Psychosomatics 47:178–179. https://doi.org/10.1176/appi.psy.47.2.178
Santos CJ, Ferreira AVM, Oliveira AL, Oliveira MC, Gomes JS, Aguiar DC (2018) Carbohydrate-enriched diet predispose to anxiety and depression-like behavior after stress in mice. Nutr Neuros 21:33–39. https://doi.org/10.1080/1028415X.2016.1213529
Freeman LR, Haley-Zitlin V, Rosenberger DS, Granholm A-CJNN (2014) Damaging effects of a high-fat diet to the brain and cognition: a review of proposed mechanisms. Nutr Neurosc 17:241–251. https://doi.org/10.1179/1476830513Y.0000000092
Huang D, Shenoy A, Cui J, Huang W, Liu PK (2000) In situ detection of AP sites and DNA strand breaks bearing 3’-phosphate termini in ischemic mouse brain. Faseb J 14:407–417. https://doi.org/10.1096/fasebj.14.2.407
Donmez-Altuntas H, Sahin F, Bayram F, Bitgen N, Mert M, Guclu K, Hamurcu Z, Arıbas S, Gundogan K, Diri H (2014) Evaluation of chromosomal damage, cytostasis, cytotoxicity, oxidative DNA damage and their association with body-mass index in obese subjects. Mutat Res/Genet Toxicol Environ Mutagen 771:30–36. https://doi.org/10.1016/j.mrgentox.2014.06.006
Elwakkad A, Hassan NE, Sibaii H, el Zayat S (2011) Relationship between obesity and 8-hydroxy-2-deoxy. J Med Sci 11:231–235. https://doi.org/10.3923/jms.2011.231.235
Tomiga Y, Yoshimura S, Ra S-G, Takahashi Y, Goto R, Kugimoto I, Uehara Y, Kawanaka K, Higaki Y (2019) Anxiety-like behaviors and hippocampal nNOS in response to diet-induced obesity combined with exercise. J Physiol Sci 69:711–722. https://doi.org/10.1007/s12576-019-00686-5
Tomiga Y, Yoshimura S, Ito A, Nakashima S, Kawanaka K, Uehara Y, Tanaka H, Higaki Y (2017) Exercise training rescues high fat diet-induced neuronal nitric oxide synthase expression in the hippocampus and cerebral cortex of mice. Nitric Oxide 66:71–77. https://doi.org/10.1016/j.niox.2017.03.002
Muranyi M, Ding C, He Q, Lin Y, Li P-A (2006) Streptozotocin-induced diabetes causes astrocyte death after ischemia and reperfusion injury. Diabetes 55:349–355. https://doi.org/10.2337/diabetes.55.02.06.db05-0654
Middeldorp J, Hol EM (2011) GFAP in health and disease. Prog Neurobiol 93:421–443. https://doi.org/10.1016/j.pneurobio.2011.01.005
Cansell C, Stobbe K, Le Thuc O, Mosser C, Ben-Fradj S, Leredde J, Lebeaupin C, Debayle D, Fleuriot L, Brau F, Devaux N, Benani A, Audinat E, Blondeau N, Nahon J, Rovère C (2019) Fat food exacerbates post-prandial hypothalamic inflammation involving GFAP+ cells and microglia. bioRxiv. https://doi.org/10.1101/835967
Tsai S-F, Wu H-T, Chen P-C, Chen Y-W, Yu M, Wang T-F, Wu S-Y, Tzeng S-F, Kuo Y-M (2018) High-fat diet suppresses the astrocytic process arborization and downregulates the glial glutamate transporters in the hippocampus of mice. Brain Res 1700:66–77. https://doi.org/10.1016/j.brainres.2018.07.017
Xia M, Abazyan S, Jouroukhin Y, Pletnikov M (2016) Behavioral sequelae of astrocyte dysfunction: focus on animal models of schizophrenia. Schizophr Res 176:72–82. https://doi.org/10.1016/j.schres.2014.10.044
Dunning KR, Anastasi MR, Zhang VJ, Russell DL, Robker RL (2014) Regulation of fatty acid oxidation in mouse cumulus-oocyte complexes during maturation and modulation by PPAR agonists. PLoS ONE. https://doi.org/10.1371/journal.pone.0087327
Weinstock PH, Levak-Frank S, Hudgins LC, Radner H, Friedman JM, Zechner R, Breslow JL (1997) Lipoprotein lipase controls fatty acid entry into adipose tissue, but fat mass is preserved by endogenous synthesis in mice deficient in adipose tissue lipoprotein lipase. Proc Natl Acad Sci 94:10261–10266. https://doi.org/10.1073/pnas.94.19.10261
Peeters A, Baes M (2010) Role of PPARα in hepatic carbohydrate metabolism. PPAR Res 2010:572405. https://doi.org/10.1155/2010/572405
den Broeder MJ, Moester MJB, Kamstra JH, Cenijn PH, Davidoiu V, Kamminga LM, Ariese F, de Boer JF, Legler J (2017) Altered adipogenesis in zebrafish larvae following high fat diet and chemical exposure is visualised by stimulated raman scattering microscopy. Int J Mol Sci. https://doi.org/10.3390/ijms18040894
Guo M, Li C, Lei Y, Xu S, Zhao D, Lu XY (2017) Role of the adipose PPARγ-adiponectin axis in susceptibility to stress and depression/anxiety-related behaviors. Mol Psychiatry 22:1056–1068. https://doi.org/10.1038/mp.2016.225
Gaspar JC, Okine BN, Llorente-Berzal A, Roche M, Finn DP (2020) Pharmacological Blockade of PPAR isoforms increases conditioned fear responding in the presence of nociceptive tone. Molecules (Basel, Switzerland) 25:1007. https://doi.org/10.3390/molecules25041007
Ozmen S, Seker A, Demirci E (2019) Ghrelin and leptin levels in children with anxiety disorders. J Pediatr Endocrinol Metab 32:1043–1047. https://doi.org/10.1515/jpem-2019-0229
Shiozaki K, Kawabe M, Karasuyama K, Kurachi T, Hayashi A, Ataka K, Iwai H, Takeno H, Hayasaka O, Kotani T, Komatsu M, Inui A (2020) Neuropeptide Y deficiency induces anxiety-like behaviours in zebrafish (Danio rerio). Sci Rep-Uk 10:5913. https://doi.org/10.1038/s41598-020-62699-0
Chigr F, Rachidi F, Tardivel C, Najimi M, Moyse E (2014) Modulation of orexigenic and anorexigenic peptides gene expression in the rat DVC and hypothalamus by acute immobilization stress. Front Cell Neurosci 8:198. https://doi.org/10.3389/fncel.2014.00198
Spencer SJ, Xu L, Clarke MA, Lemus M, Reichenbach A, Geenen B, Kozicz T, Andrews ZB (2012) Ghrelin regulates the hypothalamic-pituitary-adrenal axis and restricts anxiety after acute stress. Biol Psychiat 72:457–465. https://doi.org/10.1016/j.biopsych.2012.03.010
Wang W, Liu S-L, Li K, Chen Y, Jiang B, Li Y-K, Xiao J-L, Yang S, Chen T, Chen J-G, Li J-G, Wang F, Leptin, (2015) A potential anxiolytic by facilitation of fear extinction. CNS Neurosci Ther 21:425–434. https://doi.org/10.1111/cns.12375
Dietrich MO, Zimmer MR, Bober J, Horvath TL (2015) Hypothalamic agrp neurons drive stereotypic behaviors beyond feeding. Cell 160:1222–1232
Shankar K, Harrell A, Liu X, Gilchrist JM, Ronis MJ, Badger TM (2008) Maternal obesity at conception programs obesity in the offspring. Am J Physiol-Regul Integr Compar Physiol 294:R528–R538. https://doi.org/10.1152/ajpregu.00316.2007
Guo F, Jen K-LC (1995) High-fat feeding during pregnancy and lactation affects offspring metabolism in rats. Physiol Behav 57:681–686. https://doi.org/10.1016/0031-9384(94)00342-4
Acknowledgement
This project was supported by The Scientific and Technological Research Council of Turkey (TUBİTAK, Project no: 218O058) and by Atatürk University Scientific Research Project (AtaUniBAP, Project no: FBG-2019-6929), thus the authors thank TUBITAK and Atatürk University for this financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All procedures were performed as accordance with the approved Institutional Ethical Rules of Atatürk University, and fallowed OECD guidelines (TG 305 and 236).
Informed consent
For this type of study, no informed consent is required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper includes part of master thesis of Medine TÜRKOĞLU.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Türkoğlu, M., Baran, A., Sulukan, E. et al. The potential effect mechanism of high-fat and high-carbohydrate diet-induced obesity on anxiety and offspring of zebrafish. Eat Weight Disord 27, 163–177 (2022). https://doi.org/10.1007/s40519-021-01140-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40519-021-01140-5