Abstract
Purpose
Binge eating disorder (BED) is characterized by frequent and persistent overeating episodes of binge eating without compensatory behaviors. The aim was to evaluate regional gray matter volume (GMV) abnormalities and appetite-regulating hormone levels (NPY and Leptin) in obese subjects either with or without BED compared to healthy controls (HC).
Methods
Twenty-six obese patients with BED, 25 obese patients without BED and 27 healthy subjects as an age-matched control group with neuroimaging and appetite-regulating hormone levels were found eligible for regional GMV abnormalities. A structural magnetic resonance scan and timely blood samples were drawn to assess the appetite-regulating hormone levels.
Results
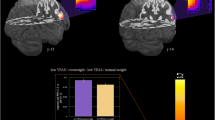
The BED obese patients had a greater GMVs of the right medial orbitofrontal cortex (OFC) and the left medial OFC compared to the non-BED obese patients. BED patients were characterized by greater GMV of the left medial OFC than HCs. Relative to the HCs, higher serum NPY levels were found in BED obese and non-BED obese groups. Serum leptin levels (pg/mL) had positively correlations with GMV in right medial OFC, left medial OFC, right lateral OFC, and left anterior cingulate cortex.
Conclusion
Among the reward processing network, which is largely associated with feeding behaviours in individuals with obesity and binge eating disorder, the OFC volumes was correlated with serum leptin concentrations. The results of our study may provide a rationale for exploring the link between regional grey matter volumes and appetite-related hormone levels in people with BED.
Level of evidence
Level III, case–control analytic study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Binge eating disorder (BED) is an eating disorder characterized by frequent and persistent overeating episodes of binge eating without compensatory behaviors (e.g., self-induced vomiting) according to the Diagnostic and Statistical Manual of Mental Disorders (DSM), 5th edition [1]. BED is more prevalent than other eating disorders with an estimated 1.9–2.8% of the general population and 1.6% of adolescents (13–18 years) [2,3,4]. In addition to being prevalent, BED in adolescence and adults is associated with significant eating-disorder psychopathology (i.e., dietary restraint, shape, weight, and eating concerns), general psychopathology (e.g., mood and anxiety symptoms), and impaired health-related quality of life independent from the existence of comorbid obesity [4,5,6]. Due to these relevant comorbidities, some researchers suggest that BED appears to represent a distinct familial phenotype in people with obesity [7]. Another exploratory study on eating disorders among adolescents found some risks, such as parental weight-related teasing and dieting, directly associated with binge eating and obesity [8]. Despite the high rates of comorbidity between BED and obesity, studies on the functional and neurobiological features of these eating disorders are scarce.
In recent decades, neuroimaging techniques have provided information on biological features between neurological processes and eating disorders. For example, models of binge-type eating in rodents emphasizes the importance of insular activation which regulate both binge eating and cue reactivity [9]. Previous studies have predominantly focused on the grey matter volume (GMV) and activities in individuals with BED compared to other eating disorders (e.g., bulimia nervosa) and healthy controls (HC) [10,11,12,13,14]. Schafer and colleagues reported increased GMV in the medial orbitofrontal cortex (OFC) and anterior cingulate cortex (ACC) in the BN and BED groups compared to HC participants reflecting possible alterations in food reward processing [11]. Schienle et al. conducted a functional Magnetic Resonance Imaging (fMRI) with BED, BN, and HC participants to investigate the neural correlates of visually induced reward-related activity to visually presented high-caloric food. Consistent with structural MRI findings, participants with BED who underwent fMRI while viewing food images, showed greater OFC activity than BN and HCs groups [12]. In another fMRI study examining neural response to visual food stimuli, patients with BED experienced significantly higher activation in the ventral striatum compared to HCs [13]. Interestingly, Balodis and colleagues who used non-food reward processing, with decreased activity in bilateral ventral striatal regions reported in BED patients compared to a BMI-matched non-BED group [14]. Lastly, one study used Single-Photon Emission Computed Tomography (SPECT), finding regional cerebral blood flow (rCBF) in BED participants, with significantly increased in left frontal and prefrontal regions [15].
Current evidence suggests that some neuropeptides (neuropeptide Y (NPY), peptide YY (PYY), and leptin) play an important role in appetite and weight regulation [16]. Leptin is produced by adipocytes and acts on central neural networks, especially in the hypothalamus [17]. The most important mechanism of leptin is to inhibit the release and expression of NPY from the arcuate nucleus, primarily involved in pituitary hormone regulation and increasing appetite [18]. Recent studies suggested NPY and leptin levels might be explain feeling hungry more frequently, which could give rise to binge eating; however, findings were incoherent regarding the association between NPY, leptin levels, and binge eating behavior [19,20,21,22].
Taken together, these neuroimaging and biochemical techniques provide limited information on whether variations serve as a cause or a consequence of disordered eating behaviors. Based on the current literature, most of the studies have investigated the associations of plasma leptin levels and cortical thickness in obesity and AN (either separately or together). It is not known to what extent the increases in GMVs reflect local interactions of the appetite-related hormone receptors in the brain and levels in blood serum. This is the “chicken or the egg” question faced by many professionals who treat people with eating disorders-it is difficult to determine which comes first. For this reason, some researchers have focused on neuroendocrine abnormalities of the hypothalamic–pituitary–adrenal axis which could be involved in the neuroendocrine control of bingeing behavior. To the best of our knowledge, this is the first study which aimed to evaluate regional GMV abnormalities and appetite-regulating hormone levels (NPY and leptin) in obese individuals either with or without BED compared to HCs. We hypothesized that obese patients with BED would have greater volume in the OFC, and the ACC compared to obese patients without BED and HCs, based on prior studies [10]. Also, we expected to find the relationship between appetite-regulating hormone levels and GMVs would be altered in BED.
Materials and methods
Participants
Fifty-one adolescents (12–18 years) with a body mass index (BMI) at or above the 95th percentile were recruited from outpatient units at the Department of Pediatrics and Department of Pediatric Endocrinology and Metabolism in Dokuz Eylul University Faculty of Medicine [23]. BED diagnosis was determined at admission by a clinical evaluation performed by trained psychiatrists, and according to the DSM-V criteria. The obesity sample was divided into two different diagnostic groups: obesity with BED (n = 26) and obesity without BED (n = 25). One obese BED outpatient was excluded because of refusing to participate. Exclusion criteria included the history of psychotic disorder or hospitalization for psychiatric illness, intellectual disability, psychotic symptoms, drug/alcohol abuse, bipolar disorder, neurological disorders or traumatic brain injury, claustrophobia, attention-deficit and hyperactivity disorder, left-handedness, presence/absence of a history of AN or BN, and antipsychotic medication. Seven participants (One obese BED, one obese non-BED, and five HC) were excluded from Magnetic Resonance Imaging (MRI) scanning because of technical problems (e.g., dental braces, metal implants, schedule incompatibilities). The population-based HC group (n = 27) was matched with the other two groups according to age, gender, and educational status. Inclusion criteria for the HC group were BMI ranging from > 25th to < 75th percentile, the absence of current or lifetime psychiatric illness, and chronic medical condition history. Other exclusion criteria were the same for the other groups.
The study was performed in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of The Dokuz Eylul University (Date: January 1, 2016, Decision Number: 2016/20-03, Protocol Number: 349).
Procedure
Written informed consents were obtained from all adolescents who were invited to the study and met inclusion criteria and their parents, respectively, and study procedures were fully explained before the participants were evaluated to be recruited in the study. The participants completed a sociodemographic data form before blood sampling and MRI scanning. Their height was measured with the Harpenden stadiometer with an accuracy of 0.1 cm, and body weight was measured using a scale (SECA, Hamburg, Germany) with a sensitivity of 0.1 kg while wearing light clothing. The BMI was calculated by dividing body weight (kg) by height squared (m2) for each patient. Obesity was defined as BMI > 95 percentile for the obese group, according to the criteria of the Centers for Disease Control and Prevention data [23].
K-SADS-PL: The diagnostic procedure and confirmation of comorbidities included a semi-structured interview with the adolescent using the Schedule for Affective Disorders and Schizophrenia for School-age Children, Present and Lifetime Version (K-SADS-PL; Kaufman et al.) [24]. The Turkish psychometric evaluations of the K-SADS-PL were conducted by Gokler and colleagues [25].
Eating Disorder Examination Questionnaire (EDE-Q): Eating behaviors were evaluated by the EDE-Q [26], which was developed to assess problematic eating behaviors and attitudes. In the Turkish psychometric evaluations [27], Cronbach’s alpha internal consistency reliability coefficient value was 0.93 and the test–retest reliability was 0.91.
Children Depression Inventory (CDI): Depressive symptomatology was assessed with the CDI, a self-rated and symptom-oriented 27-item questionnaire [28].
Blood sampling and biochemical assays
The blood specimens from fasting subjects were drawn via venipuncture from the antecubital vein (between 8 and 10 AM). After allowing 60 min for spontaneous blood clotting, the serums were separated from the blood cells by centrifugation at 900g for 10 min. The serums were diluted before analysis according to the manufacturer’s instructions.
Serum leptin levels were measured by a sandwich immunoassay with the Leptin Human Leptin PicoKineä ELISA kit (Cat no EK0437, Boster, US). According to the manufacturer’s declaration, the sensitivity of the method was 10 pg/mL, the measuring range was 62.5–4000 pg/mL, and the coefficient of variation (CV) < 5.9%.
Serum NPY levels were measured with the Human Neuropeptide Y (NPY) ELISA kit (Cat no CSB-E08168h, CUSABIO, China) by a sandwich immunoassay. According to the manufacturer's declaration, the sensitivity of the method was 0.105 pg/mL, the measurement range was 0.78–50 pg/mL, intra-assay coefficient of variation (CV) < 8.0%, and inter-assay CV < 10%.
MRI scanning
MRI was performed using a 1.5 T Philips Tesla Achieva MRI Scanner and SENSE head coil/8 channel. T1-weighted volumetric MRI studies were acquired with a spoiled gradient echo technique (time to echo, 3,7 ms; time to repeat, 25 ms; flip angle, 30°; field of view, 240 × 240 mm; 140 contiguous 1 mm section thickness).
Analysis were performed with Computational Anatomy Toolbox (CAT12) (http://www.neuro.uni-jena.de/cat/) within SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/) while running MATLAB (R2018b; MathWorks, Natick, MA, USA). The images were segmented into gray matter (GM), white matter (WM), and cerebrospinal fluid adding surface and thickness estimation for region-of-interest (ROI) analysis, and normalized to standard space based on the Montreal Neurological Institute template [29].
CAT12 allows for estimating tissue volumes within ROIs for each hemisphere separately. The estimated GM volumes (GMV) were extracted for ACC and lateral and middle OFC defined by the LONI Probabilistic Brain Atlas (LPBA40) [30] using CAT12 “estimate mean values inside the ROI function. All GMVs were normalized by the ratio between the volume of ROI and total intracranial volume, provided by the CAT12 report files, to correct for differences in individual brain size.
Statistical analysis
Data was analysed using SPSS version 23.0 (SPSS Inc., Chicago, IL, USA). Descriptive statistics included means and standard deviations; differences between groups were explored through chi-square (χ2) test. Group comparisons with all groups (obese BED versus obese non-BED versus HC) used non-parametric testing (Kruskal–Wallis) with one way ANOVA tests for post hoc comparisons after assessing normality of distribution and equality of variances using the Shapiro–Wilk test. Correlation analyses used Spearman coefficients between the results of the GMV with Appetite-Related Hormone Levels, EDE Q total score and BMI.
Results
Self-report data and BMI
Table 1 shows the demographic and clinical features of the study participants. A total of 77 adolescents (25 BED obese, 25 non-BED obese and 27 HCs). There was no statistical difference between groups regarding age and sex (p > 0.05). As predicted, BMI scores were lower in the HCs compared with the other two groups, which did not differ significantly from each other. Also, Table 1 summarizes the total and subscale scores of groups from EDE-Q and CDI. Significant differences have been found between three groups in terms of Restraint, Weight Concern and Shape Concern Subscales, EDE-Q- total scores and CDI scores (p < 0.05). We found that the Restraint, Weight Concern and Shape Concern subscale scores, EDE-Q- total scores of BED obese and non-BED obese groups higher than HC group. There were no differences in Eating Concern Subscale scores between three groups (p > 0.05).
Grey matter volumes and appetite-related hormone levels
The patients with BED and obesity had a greater GMVs of the right medial OFC and the left medial OFC compared to the non-BED obese participants (Table 2). Adolescents with BED and obesity were characterized by greater GMVs of the left medial OFC than HCs. Relative to the HCs, higher serum NPY levels were found in BED obese and non-BED obese groups. All other GMV (insula, ACC and lateral OFC) and Appetite-Related Hormone Level comparisons as well as the exploratory analyses were nonsignificant.
Correlations of GMV with appetite-related hormone levels, EDE Q total score and BMI in BED patients
Serum leptin levels (pg/mL) are positively correlated with GMV in right medial OFC, left medial OFC, right lateral OFC, and left ACC. There were no association between NPY levels, BMI and EDE-Q total score with GMVs, respectively (Table 3).
Discussion
This is the first study which aimed to investigate the regional GMV abnormalities and appetite-regulating hormone levels (NPY and leptin) in obese individuals either with or without BED compared to HCs. The present results showed that the left medial OFC volumes were greater in participants with a diagnosis of BED compared other groups, and greater right medial OFC volume compared to the non-BED obese group which is in line with our hypothesis.
Neuroimaging studies have demonstrated that one key cortical region for reward processing is the OFC which also implicated in motivation, goal-oriented behavior and further decision-making processes [31, 32]. For example, a prior study of children with obesity, greater OFC activation to food pictures has been identified in satiated obese compared to normal-weight children [33]. Furthermore, Schienle and colleagues (2009) found increased activation of OFC to images of high calorie foods in patients with BED [12]. Although structural MRI studies in patients with BED are limited, our study is consistent with previous cross-sectional study which evaluated regional GMV abnormalities, and found greater medial OFC volumes in patients with BED relative to healthy controls [11]. This is consistent with the clinical features of BED, which is characterized by loss of control over eating behaviors. These results may reflect a biobehavioral process which becomes even more rewarding in patients with BED than the normal satiation of hunger.
Structural imaging studies also have showed lower total GMVs and reduced regional GMVs in the striatal, prefrontal, and ACC [34, 35]. These areas are implicated in reward, emotion, and motivation; for example, ACC is ascribed a role in reward motivation and has been specifically linked to disinhibition with visual food stimuli [36,37,38]. A previous study demonstrated that the higher adolescent adiposity was most commonly associated with lower GMVs [39]. There have been inconsistent results in the literature whether the GMVs are larger or not in the obese children. The cause of these results may be related to the multifactorial etiology of the obesity and relatively small samples of the studies.
Second, and regarding to the main objective of this study, our findings suggest that serum leptin concentrations are associated with some regional GMVs (right medial OFC, left medial OFC, right lateral OFC, and left ACC). Leptin is produced mainly by adipocytes of the white adipose tissue and circulating concentrations correlate with the amount of body fat. Leptin plays key role within the CNS, particularly in hypothalamus to alter brain pathways via leptin receptors to influence food intake [40]. We didn’t find any significant difference between leptin levels between obese and HC groups. One possible explanation for this result may be related with acute changes in serum leptin concentrations. Also, the relationship between leptin and brain volumes has not been definitively clarified. In functional MRI studies, researchers observed greater OFC activation which associated with reward- and food-processing in a congenital leptin-deficient patient after acute and long-term leptin replacement [41]. In their follow study conducted after 12 and 24 months, a stabilization of the long-term effects in the OFC was observed with visual high- and low-caloric food pictures and non-food pictures [42]. BED patients have greater inhibitory control deficits [43]. In keeping with this notion, the OFC acts as a critical area that regulates behaviors by inhibiting the effect of a broad scope of sensations, feelings, thoughts, and actions [44]. Thus, we assumed that correlations between serum leptin concentrations and regional GMVs, especially OFC volumes were supported by an important pilot study which evaluated the concentrations of the appetite-regulating hormones and their association with neuropsychological performance in patients with eating disorders and HCs [45]. In their study, Wollenhaupta et al. described dysfunctional hormone secretion patterns in patients with eating disorders would be associated with impaired performance in the inhibitory control task. Although, our results may point towards a complex interactions related to direct or mediating effects of serum leptin concentrations on OFC volumes.
Furthermore, serum NPY concentrations of obese patients with or without BED were greater than HCs. NPY is a 36 amino acid peptide abundantly distributed throughout the central and peripheral nervous systems that plays a major role in food intake and obesity [46, 47]. The best possible effect of NPY in obesity, NPY may regulate orexigenic pathways and adipocyte metabolism via a family of 5 G-protein coupled receptors [48]. In adipocytes, NPY inhibits lipolysis and stimulates proliferation and differentiation of new adipocytes [49]. Taken together, both central and peripheral NPY levels are crucial for the risk of obesity.
Limitations
The present study had several limitations. First, we found volumetric alterations in the OFC and ACC which are associated with reward processing and inhibitory control, it remains unclear whether these alterations are a cause or a consequence of obesity. Cross-sectional imaging differences are correlational, and we cannot infer insight into longer-term behaviors. Secondly, MRI cannot assess cellular morphologic changes that may explain the possible neurobiological mechanisms in individuals with BED. In addition, we examined a modest sample size, and evaluation of GMVs and appetite-related hormone levels within a larger sample of adolescents with BED would better determine the robustness of our findings. Moreover, we used structural imaging to assess the regions of interest, which may not be able to show possible functional changes. Another limitation is that we used a single fasting blood measure of leptin. However, acute changes in serum leptin concentrations are related to many factors such as meals and fat stores.
Conclusion
The present study explored regional gray matter volume abnormalities and appetite-regulating hormone levels (NPY and leptin) in obese subjects either with or without BED compared to HCs. Among the reward processing network, which is largely associated with feeding behaviors in individuals with obesity and BED, the OFC volumes were correlated with serum leptin concentrations. The results of our study may provide a rationale for exploring the link between regional GMVs and appetite-related hormone levels in people with BED.
What is already known on this subject?
In recent decades, neuroimaging studies have focused their atention on changes in individuals with BED that may partially underlie altered reward sensitivity and food-related attentional biase. On the other hanf, some researchers have focused on neuroendocrine abnormalities which could be involved in the neuroendocrine control of bingeing behavior. However, relationship between altered regional GMVs and appetite-related hormone levels in BED psychopathology has been given less attention with limited exploration of this variable despite its high prevalence.
What this study adds
This study findings have revealed differences in gray-matter volumes in the right and left medial OFC in BED individuals. And also, we found that serum leptin levels (pg/mL) had positively correlations with GMV in right medial OFC, left medial OFC, right lateral OFC, and left ACC. The study has implications for underlying neuropathophysiology of BED.
References
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders, 5th edn. American Psychiatric Publishing, Arlington
Kessler RC, Berglund PA, Chiu WT et al (2013) The prevalence and correlates of binge eating disorder in the WHO World Mental Health Surveys. Biol Psychiatry 73:904–914. https://doi.org/10.1016/j.biopsych.2012.11.020
Hudson JI, Hiripi E, Pope HG et al (2007) The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry 61:348–358. https://doi.org/10.1016/j.biopsych.2006.03.040
Swanson SA, Crow SJ, Le Grange D et al (2011) Prevalence and correlates of eating disorders in adolescents. Results from the national comorbidity survey replication adolescent supplement. Arch Gen Psychiatry 68(7):714–723
Glasofer DR, Tanofsky-Kraff M, Eddy KT et al (2007) Binge eating in overweight treatment-seeking adolescents. J Pediatr Psychol 32(1):95–105. https://doi.org/10.1093/jpepsy/jsl012
Pasold TL, McCracken A, Ward-Begnoche WL (2014) Binge eating in obese adolescents: emotional and behavioral characteristics and impact on health-related quality of life. Clin Child Psychol Psychiatry 19(2):299–312. https://doi.org/10.1177/1359104513488605
Hudson JI, Lalonde JK, Berry JM et al (2006) Binge-eating disorder as a distinct familial phenotype in obese individuals. Arch Gen Psychiatry 63(3):313–319. https://doi.org/10.1001/archpsyc.63.3.313
Haines J, Kleinman KP, Rifas-Shiman SL et al (2010) Examination of shared risk and protective factors for overweight and disordered eating among adolescents. Arch Pediatr Adolesc Med 164(4):336–343. https://doi.org/10.1001/archpediatrics.2010.19
Price AE, Stutz SJ, Hommel JD et al (2019) Anterior insula activity regulates the associated behaviors of high fat food binge intake and cue reactivity in male rats. Appetite 133:231–239. https://doi.org/10.1016/j.appet.2018.11.011
Donnelly B, Touyz S, Hay P et al (2018) Neuroimaging in bulimia nervosa and binge eating disorder: a systematic review. J Eat Disord 6:3. https://doi.org/10.1186/s40337-018-0187-1
Schäfer A, Vaitl D, Schienle A (2010) Regional grey matter volume abnormalities in bulimia nervosa and binge-eating disorder. Neuroimage 50(2):639–643. https://doi.org/10.1016/j.neuroimage.2009.12.063
Schienle A, Schäfer A, Hermann A et al (2009) Binge-eating disorder: reward sensitivity and brain activation to images of food. Biol Psychiatry 65(8):654–661. https://doi.org/10.1016/j.biopsych.2008.09.028
Lee JE, Namkoong K, Jung YC (2017) Impaired prefrontal cognitive control over interference by food images in binge-eating disorder and bulimia nervosa. Neurosci Lett 651:95–101. https://doi.org/10.1016/j.neulet.2017.04.054
Balodis IM, Kober H, Worhunsky PD et al (2013) Monetary reward processing in obese individuals with and without binge eating disorder. Biol Psychiatry 73(9):877–886. https://doi.org/10.1016/j.biopsych.2013.01.014
Karhunen LJ, Vanninen EJ, Kuikka JT et al (2000) Regional cerebral blood flow during exposure to food in obese binge eating women. Psychiatry Res 99(1):29–42. https://doi.org/10.1016/s0925-4927(00)00053-6
Baranowska B, Wasilewska-Dziubińska E, Radzikowska M et al (1997) Neuropeptide Y, galanin, and leptin release in obese women and in women with anorexia nervosa. Metabolism 46(12):1384–1389. https://doi.org/10.1016/s0026-0495(97)90136-0
Zhang Y, Proenca R, Maffei M et al (1994) Positional cloning of the mouse obese gene and its human homologue. Nature 372(6505):425–432. https://doi.org/10.1038/372425a0
Wang Q, Bing C, Al-Barazanji K, Mossakowaska DE et al (1997) Interactions between leptin and hypothalamic neuropeptide Y neurons in the control of food intake and energy homeostasis in the rat. Diabetes 46(3):335–341. https://doi.org/10.2337/diab.46.3.335
Adami GF, Campostano A, Cella F et al (2002) Serum leptin concentration in obese patients with binge eating disorder. Int J Obes Relat Metab Disord 26(8):1125–1128. https://doi.org/10.1038/sj.ijo.0802010
Monteleone P, Di Lieto A, Tortorella A et al (2000) Circulating leptin in patients with anorexia nervosa, bulimia nervosa or binge-eating disorder: relationship to body weight, eating patterns, psychopathology and endocrine changes. Psychiatry Res 94(2):121–129. https://doi.org/10.1016/s0165-1781(00)00144-x
Miller R, Tanofsky-Kraff M, Shomaker LB et al (2014) Serum leptin and loss of control eating in children and adolescents. Int J Obes 38(3):397–403. https://doi.org/10.1038/ijo.2013.126
Smitka K, Papezova H, Vondra K et al (2011) A higher response of plasma neuropeptide Y, growth hormone, leptin levels and extracellular glycerol levels in subcutaneous abdominal adipose tissue to Acipimox during exercise in patients with bulimia nervosa: single-blind, randomized, microdialysis study. Nutr Metab 8(1):81. https://doi.org/10.1186/1743-7075-8-81
Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM et al (2002) 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11 246:1–190
Kaufman J, Birmaher B, Brent D et al (1997) Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36(7):980–988. https://doi.org/10.1097/00004583-199707000-00021
Gokler B, Unal F, Pehlivanturk B et al (2004) Reliability and validity of schedule for affective disorders and schizophrenia for school age children present and lifetime version-Turkish version (K-SADS-PL-T). Turk J Child Adolesc Ment Health 11(3):109–116
Fairburn CG, Cooper Z, O’Connor M (2008) Eating disorderexamination. In: Fairburn CG (ed) Cognitive behavior therapy and eating disorders. Guilford Press, New York, pp 309–313
Yucel B, Polat A, Ikiz T et al (2011) The Turkish version of the eating disorder examination questionnaire: reliability and validity in adolescents. Eur Eat Disord Rev 19:509–511. https://doi.org/10.1002/erv.1104
Kovacs M (1987) Rating scale to depression in school-aged children. Acta Paedopsychiatr 46:305–315
Kurth F, Gaser C, Luders E (2015) A 12-step user guide for analyzing voxel-wise gray matter asymmetries in statistical parametric mapping (SPM). Nat Protoc 10:293–304. https://doi.org/10.1038/nprot.2015.014
Shattuck DW, Mirza M, Adisetiyo V et al (2008) Construction of a 3D probabilistic atlas of human cortical structures. Neuroimage 39:1064–1080. https://doi.org/10.1016/j.neuroimage.2007.09.031
Rothkirch M, Schmack K, Schlagenhauf F et al (2012) Implicit motivational value and salience are processed in distinct areas of orbitofrontal cortex. NeuroImage 62(3):1717–1725. https://doi.org/10.1016/j.neuroimage.2012.06.016
Wallis JD (2007) Orbitofrontal cortex and its contribution to decision-making. Annu Rev Neurosci 30:31–56. https://doi.org/10.1146/annurev.neuro.30.051606.094334
Bruce AS, Holsen LM, Chambers RJ et al (2010) Obese children show hyperactivation to food pictures in brain networks linked to motivation, reward and cognitive control. Int J Obes 34:1494–1500. https://doi.org/10.1038/ijo.2010.84
Gerlach G, Herpertz S, Loeber S (2015) Personality traits and obesity: a systematic review. Obes Rev 16(1):32–63. https://doi.org/10.1111/obr.12235
Yokum S, Ng J, Stice E (2012) Relation of regional gray and white matter volumes to current BMI and future increases in BMI: a prospective MRI study. Int J Obes 36(5):656–664. https://doi.org/10.1038/ijo.2011.175
Haber SN (2011) Neuroanatomy of reward: a view from the ventral striatum. In: Gottfried JA (ed) Neurobiology of sensation and reward. CRC Press/Taylor & Francis, Boca Raton, Chapter 11
Martin LE, Holsen LM, Chambers RJ et al (2010) Neural mechanisms associated with food motivation in obese and healthy weight adults. Obesity (Silver Spring) 18(2):254–260. https://doi.org/10.1038/oby.2009.220
Volkow ND, Wang GJ, Tomasi D et al (2013) Obesity and addiction: neurobiological overlaps. Obes Rev 14(1):2–18. https://doi.org/10.1111/j.1467-789X.2012.01031.x
Alosco ML, Stanek KM, Galioto R et al (2014) Body mass index and brain structure in healthy children and adolescents. Int J Neurosci 124(1):49–55. https://doi.org/10.3109/00207454.2013.817408
Zhou Y, Rui L (2013) Leptin signaling and leptin resistance. Front Med 7(2):207–222. https://doi.org/10.1007/s11684-013-0263-5
Frank S, Heni M, Moss A et al (2011) Leptin therapy in a congenital leptin-deficient patient leads to acute and long-term changes in homeostatic, reward, and food-related brain areas. J Clin Endocrinol Metab 96(8):E1283–E1287. https://doi.org/10.1210/jc.2010-2713
Frank S, Heni M, Moss A et al (2013) Long-term stabilization effects of leptin on brain functions in a leptin-deficient patient. PLoS ONE 8(6):e65893. https://doi.org/10.1371/journal.pone.0065893
Giel KE, Teufel M, Junne F et al (2017) Food-related impulsivity in obesity and binge eating disorder-a systematic update of the evidence. Nutrients 9(11):1170. https://doi.org/10.3390/nu9111170
Hooker CI, Knight RT (2010) The role of lateral orbitofrontal cortex in the inhibitory control of emotion. In: Zald D, Rauch S (eds) The orbitofrontal cortex. Oxford University Press, New York, pp 307–324
Wollenhaupt C, Wilke L, Erim Y et al (2019) The association of leptin secretion with cognitive performance in patients with eating disorders. Psychiatry Res 276:269–277. https://doi.org/10.1016/j.psychres.2019.05.001
Yi M, Li H, Wu Z et al (2018) A Promising therapeutic target for metabolic diseases: neuropeptide Y receptors in humans. Cell Physiol Biochem 45(1):88–107. https://doi.org/10.1159/000486225
Kuo LE, Kitlinska JB, Tilan JU et al (2007) Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat Med 13(9):1120. https://doi.org/10.1038/nm1611
Singer K, Morris DL, Oatmen KE et al (2013) Neuropeptide Y is produced by adipose tissue macrophages and regulates obesity-induced inflammation. PLoS ONE 8(3):e57929. https://doi.org/10.1371/journal.pone.0057929
Kos K, Harte AL, James S et al (2007) Secretion of neuropeptide Y in human adipose tissue and its role in maintenance of adipose tissue mass. Am J Physiol Endocrinol Metab 293(5):E1335–E1340. https://doi.org/10.1152/ajpendo.00333.2007
Acknowledgements
This study was supported by a grant from the Dokuz Eylul University Deparment Of Scientific Research Projects (2017.KB.SAG.051).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Turan, S., Sarioglu, F.C., Erbas, I.M. et al. Altered regional grey matter volume and appetite-related hormone levels in adolescent obesity with or without binge-eating disorder. Eat Weight Disord 26, 2555–2562 (2021). https://doi.org/10.1007/s40519-021-01117-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40519-021-01117-4