Abstract
Purpose
Anorexia nervosa (AN) affects approximately 2.9% of females and has the highest mortality rate among all psychiatric disorders. Despite several advances, the neurobiology of this disorder is still not well understood. Several studies have reported abnormalities in the white matter, but it is not know if these are disease-related or secondary to undernutrition. This study aimed to further our understanding of white matter pathology using diffusion-weighted imaging in underweight adolescents with AN, and to examine changes occurring after short-term weight restoration.
Methods
Analyses were conducted on diffusion-weighted imaging from 24 female adolescents with AN and 17 age- and gender-matched healthy controls (HC), aged 14–19 years. Groups were compared on fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD) and radial diffusivity (RD) using tract-based spatial statistics analysis and DTI measures were correlated with eating disorder examination questionnaire (EDE-Q) subscales and body mass index (BMI). Preliminary repeated-measure analyses were also conducted on eight participants after short-term weight restoration (median 41 days).
Results
Widespread increases in MD of up to 9% were found in underweight AN relative to HC, particularly in the corpus callosum. This was associated with both increased AD and RD, suggestive of dys- or de-myelination. There were no significant group differences in FA, and no significant correlations between DTI measures, BMI or EDE-Q subscale score. Weight restoration therapy significantly reduced MD, to levels significantly lower than HC, but did not consistently alter FA across individuals.
Conclusions
White matter microstructure is significantly altered in female adolescents with AN, with preliminary longitudinal data suggesting that it may be reversible with short-term weight restoration.
Level of evidence
Level III: evidence obtained from well-designed cohort or case–control analytic studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anorexia nervosa (AN) is a serious psychiatric and physical health disorder, characterized by severe food restriction, perception of being overweight despite emaciation, and an intense fear of weight gain (Amercian Psychiatric Association, 2013). AN affects approximately 2.9% of females and has the highest mortality rate among all psychiatric disorders [2]. However, despite a recent increase in studies investigating the neurobiology of AN, its underlying pathophysiology is still poorly understood, as are the contributions of psychological factors, such as cognitions relating to body image, versus physiological factors, such as nutrition.
Several studies have reported widespread decreases in gray and white matter volumes during underweight phases of AN [3, 4], with a meta-analysis revealing global reductions of 5.6% and 3.8% in gray and white matter, respectively [5]. Some have found associations between gray matter volume, AN symptoms and predisposing factors, such as a rigid eating pattern and body image distortion [6, 7], and state-based physiological factors such as body mass index (BMI) [3]. While alterations have been seen to partially reverse with weight gain, there are conflicting data on whether they resolve completely [7,8,9].
Diffusion tensor imaging (DTI) is a method that allows us to move beyond basic volumetrics and to assess white matter integrity within the brain [10]. It allows for the assessment of microstructural properties of white matter based on water diffusion within the brain, using two key measures of interest: fractional anisotropy (FA) and mean diffusivity (MD). FA is a scalar index of the amount of diffusion at every voxel, reflecting the direction and degree of water diffusion. MD is an index of overall diffusivity, irrespective of direction. MD is usually higher in damaged tissue due to increased free diffusion, while FA decreases due to the loss of coherence in the preferred diffusion direction [11]. These measures are aggregates of radial diffusivity (RD) and axial diffusivity (AD), which reflect perpendicular and parallel diffusivity directions within white matter tracts, respectively. Increased RD is interpreted as dysmyelination or demyelination [12], while decreased AD is thought to indicate axonal injury [13].
Several studies have used DTI to examine potential white matter pathology associated with AN. A recent meta-analysis identified decreased FA in the posterior areas of the corpus callosum, the left superior longitudinal fasciculus, and the left precentral gyrus, as well as increased FA in the right cortico-spinal projections, and lingual gyrus in AN relative to controls [14]. A systematic review by our research group identified these regions and also lower FA in the fornix, cingulum and several thalamic regions, and higher MD in the fornix and superior longitudinal fasciculus in AN [15]. These studies also found positive correlations between body mass index during underweight AN and FA, which may indicate an association between white matter integrity and acute malnutrition. The majority of studies have examined FA alone, or only looked at MD within regions with significant FA differences. Although FA and MD are calculated on the same eigenvariates and are typically inverse, they do measure different aspects of white matter microstructure and are most informative when both utilized [16]. Those who did examine MD mostly found increased MD in underweight AN relative to HC [17,18,19,20], although a few studies have found no differences [21,22,23] or reduced MD [24].
To shed light on whether white matter differences observed in underweight AN are due to disorder-related pathology or are secondary to acute undernutrition, three studies conducted longitudinal studies to examine alterations after partial weight restoration. At a whole-brain level, Cha et al. [25] found no differences between HC and the AN group in either state, nor any changes in FA from underweight AN to weight-restored AN. Vogel et al. [24] reported widespread increased FA and decreased MD in underweight AN relative to HC, which was normalized after weight restoration treatment, while von Schwanenflug et al. [26] found reduced FA in the body of the corpus callosum in underweight AN which normalized after weight restoration. Despite the inconsistencies reported during underweight AN, these longitudinal studies suggest that white matter alterations may be state-dependent and reversible with weight restoration treatment.
The primary aim of this study was to further our understanding of trait-based and state-dependent variations in white matter (patho-)physiology in adolescents with AN. Adolescence is a critical period of neurodevelopment and is also the peak time of anorexia nervosa onset [27]. By examining individuals who are closer to their disorder onset, we can expect fewer confounding factors relating to chronicity, and potentially identify pathophysiology that is pertinent to early intervention. We used DTI to examine FA and MD in female adolescents with AN who presented to a hospital setting with acute starvation, relative to age-matched healthy controls (HC). We also examined the relationship between altered white matter microstructural parameters, BMI, and cognitive and behavioral features as measured by the Eating Disorders Examination Questionnaire (EDE-Q) [28]. Further, in a small exploratory longitudinal analysis, we examined change in white matter microstructural properties after short-term weight restoration. We hypothesized that patients would exhibit reduced FA and increased MD in underweight AN relative to HC, and that individual differences in these measures would be associated with both psychological and physiological measures. We also anticipated partial normalization of white matter microstructure upon weight rehabilitation.
Methods
Participants
Twenty-six underweight adolescent girls hospitalized with AN and 19 HC, all aged 14–19 years, underwent a magnetic resonance imaging (MRI) scan. Two AN participants were excluded due to incomplete scan data. Three HC participants were excluded due to an abnormal neurology finding, a history of eating disorder, and to aid in matching groups on their mean age. This left 24 AN and 17 HC participants for analyses. AN participants were recruited at the Children’s Hospital at Westmead (Sydney, Australia) and HC participants were recruited through public advertisements in the same catchment area (Greater Western Sydney). Participant characteristics are presented in Table 1.
AN participants were diagnosed according to the diagnostic and statistical manual of mental disorders criteria (DSM-5) [1], and had AN for less than 3 years. They were recruited in the first week of their admission to a specialist inpatient pediatric eating disorder program at the hospital. Underweight AN participants underwent MRI scans after being medically stabilized with normal biochemistry levels [29]. Psychiatric comorbidities, other than psychotic conditions, were not excluded as this would not have represented a typical AN sample [30]. The following comorbid conditions were identified in the AN group: major depressive disorder (n = 12), dysthymia (n = 2), hypomania (n = 1), panic disorder (n = 1), social phobia (n = 7), obsessive compulsive disorder (n = 3), general anxiety disorder (n = 5), alcohol dependence (n = 1).
HCs were of a normal weight range for age, height and gender and did not have a current or past history of an eating disorder or any major psychiatric disorder, as assessed with the mini structured clinical interview for the DSM [31]. All participants were 14–19 years of age, English speaking, had no history of significant brain injury or neurological conditions, and no contraindications for MRI (e.g. claustrophobia, metallic implants).
Informed written consent was obtained from all participants and from parents of those under the age of 16. The study protocol (HREC/13/SCHN/385) received approval by the Human Research Ethics Committees of Children’s Hospital at Westmead, Western Sydney University and Westmead Hospital, and was carried out in accordance with the Declaration of Helsinki.
Questionnaires
AN and HC participants completed the Eating Disorder Examination Questionnaire (EDE-Q) [28] to assess general eating disorder symptomatology. It contains subscales of restraint, eating concern, shape concern and weight concern. It provides subscale scores reflecting the severity of eating disorder behaviors. Subscale scores and a global score are derived from 22 items addressing attitudinal aspects of eating-disorder psychopathology. Australian community normative data for the EDE-Q have been published [32]. The scale has acceptable internal consistency, test–retest reliability and temporal stability.
Longitudinal cohort
Treatment included refeeding, individual psychological support and preparation for the outpatient family-based therapy [33], with family meetings and psychoeducation. Girls with AN were refed initially with 24–72 h of continuous nasogastric feeds which ceased with day-time medical stability (heart rate > 50 bpm, blood pressure > 80/40 mmHg, temperature > 36°C, no cardiac arrhythmias and normal biochemistry). This was followed by a combination of nocturnal feeds and an oral meal plan for a total intake of 2400–3000 kcal/day. The nasogastric feeding duration was determined by markers of medical instability [29]. They were managed on a specialist eating disorder unit with a lenient behavioral program which included hospital-based school attendance, a daily adolescent group program and a second daily physiotherapy program. There were daily medical and psychiatric reviews and three supportive psychotherapy sessions each week [29]. When AN participants improved to at least 85% of their Expected Body Weight (%EBW) [33], they were invited to complete a second MRI scan. Ten AN participants completed the follow-up research scan; however, only data from eight individuals could be included due to two return participants having incomplete baseline scan data.
MRI acquisition
Diffusion-weighted images (DWI) were collected on a 3 T MRI (GE HDxt Twinspeed magnet system) using an eight-channel head coil. DWI acquisition parameters included 55 axial contiguous 2.5 mm slices; 1.5 mm × 1.5 mm resolution; 128 × 128 matrix; repetition time = 1700 ms; echo time = 95 ms; frequency direction = right/left. For each slice, diffusion gradients were applied along 32 directions (b = 1000 s/mm2) and 4 b0 images were collected.
Pre-processing
DWI data were processed and analyzed using FMRIB Software Library (FSL) v6.0 [34]. Non-brain tissue was removed using FSL’s brain extraction tool (BET) and diffusion-weighted images were corrected for subject motion and eddy current-induced distortions using ‘eddy’ as implemented in FSL [35]. Data were corrected for B-matrix rotation following subject motion. Diffusion tensors were fitted to the data by linear regression using a least-square minimization approach (DTIFIT; FMRIB Diffusion Toolbox). FA, MD, AD (λ1) and RD((λ2 + λ3)/2) maps were estimated using the eigenvalues associated with the fitted tensor model.
Tract-based spatial statistics
Tract-based spatial statistics (TBSS) was used to test for between-group differences in white matter microstructural measures. Each participant’s FA images were aligned to a common target in MNI 152 standard space (FMRIB58_FA template; https://fsl.fmrib.ox.ac.uk/fsl/fsl6.0/tbss/FMRIB58_FA.html) using nonlinear registration, and resampled to a 1 × 1 × 1 mm3 voxel size. These normalization parameters were subsequently applied to the other diffusivity parameters maps (MD, RD, AD). FA images were averaged to create a mean FA map and a mean FA skeleton, which represents the center of all tracts common to the group. The mean skeleton map was thresholded to FA > 0.2 to exclude any peripheral areas with high variability and/or partial volume effects. FA maps of each individual were then projected onto the mean FA skeleton. This same procedure was also applied to the MD, RD and AD maps.
Statistical analyses
Group comparisons were carried out using non-parametric permutation-based statistics using “randomize” in FSL [36], with 5,000 permutations run for each contrast. Threshold-free cluster enhancement (TFCE) [37] was used to obtain clusters and the threshold for significance was set at family-wise error-corrected p value (pFWE) of < 0.05.
Group differences in FA and MD were tested between underweight AN and HC using two-sample t tests, with age as a covariate of no interest. Paired t tests were used to compare weight-restored AN with underweight AN, with time between scans as a covariate of no interest. Finally, two-sample t tests were used to compared weight-restored AN with HC.
For any analyses with a significant group difference in FA or MD, values from significant voxels within the region were averaged as a summary measure of the diffusion properties in each participant. To better characterize microstructural changes underlying the between‐group differences in FA and MD, AD and RD were also examined within significant clusters. Post hoc two‐sample t tests compared the groups on each component measure within these regions‐of‐interest. The John Hopkins University (JHU) White Matter Labels Atlas were used for anatomical localization of results.
Correlational analyses
Correlation analyses were performed separately for AN and HC groups using randomize in FSL to examine associations between FA and MD, and BMI and EDE-Q subscale scores, while covarying for age.
Post hoc comparisons
To explore effects of common comorbidities, two-sample t tests we performed between individuals with AN with comorbid depression versus those without comorbid depression for MD and FA.
Results
Underweight AN v HC
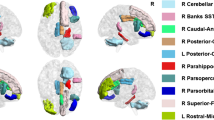
Group comparisons revealed a significant increase in MD in underweight AN relative to HC in a single white matter cluster of 8797 voxels, peak MNI coordinates: − 16, 12, 36 (L superior corona radiata). It was comprised of 18 white matter regions identified with the John Hopkins university atlas (Table 2). These regions included the corpus callosum, association fibers, namely the left sagittal stratum and external capsule, projection fibers such as the bilateral corona radiata (anterior, superior and posterior portions), left internal capsule (anterior, posterior and retrolenticular) and limbic fibers such as the bilateral cingulum and left fornix (Fig. 1). Post hoc examination of the component measures revealed that within this significant MD cluster, there was significantly increased RD and AD in underweight AN relative to HC (Fig. 2).
TBSS results comparing MD, AD and RD in underweight adolescents with anorexia nervosa (AN) versus age-matched healthy controls (HC). Significant tracts (p < .05) with underweight AN > HC are shown in orange. MNI coordinates, x = − 1, y = − 25. Underlying white matter skeleton shown in green. MD mean diffusivity, AD axial diffusivity, RD radial diffusivity
There were no significant differences between groups in FA. Mean FA and MD were extracted from the whole-brain white matter skeleton for each individual and are presented in Fig. 1 to provide an overview of individual differences.
The AN sample consisted of 23 with restrictive subtype (ANr) and 1 with purging subtype (ANbp). Results did not change when the individual with ANbp was excluded from analyses.
Correlational analyses with symptom measures
There were no significant correlations between any MD or FA cluster and BMI or EDE-Q subscale score, for either the AN or HC group after controlling for age (uncorrected for multiple comparisons).
Longitudinal analyses (underweight AN vs weight-restored AN)
Eight clinical participants completed a follow up assessment after they had completed short-term weight restoration. The second MRI scan was conducted on average 71 days (range 13–294 days; ± 92 SD; 41 median) after the initial MRI scan.
After weight restoration, this subsample had a significant increase in BMI (mean change 3.14 ± 1.04, p = 0.003) and decrease in EDE-Global scores (mean change 1.36 ± 0.91, p = 0.004) relative to at their scan whilst underweight. On average, patients gained 1.63 kg per week of treatment (range 0.25–4.2 kg per week).
There was a significant reduction in MD after weight restoration in a single white matter cluster of 104,171 voxels. These widespread changes occurred in all 46 JHU-atlas regions of interest (see Table 3 for mean values and percent change). All results remained significant when covarying for differing lengths of treatment period between individuals and rate of weight gain. There were, however, no significant changes in FA after weight restoration. Figure 3 shows the average whole-brain MD and FA for each individual pre- and post-treatment (underweight AN vs weight-restored AN), relative to the treatment duration. Change in BMI and EDE scores after weight restoration and rate of weight gain did not correlate with the magnitude of change in MD or FA either globally or in any of the regions of interest.
Weight-restored AN v HC
There was significantly lower MD in the weight-restored AN group relative to HC in a single white matter cluster of 102,447 voxels. See supplementary Table 1 for a full list of regions with significant difference in MD. There were no significance differences in FA between the weight-restored AN group and HC.
Post hoc exploration of the effects of comorbid depression
There were no significant differences between individuals with AN and comorbid depression (n = 12) versus those without comorbid depression (n = 12) in any WM microstructural measure.
Discussion
Widespread increases in MD of up to 9% were found in underweight AN relative to HC, which was associated with both increased AD and RD, suggestive of dys- or de-myelination. However, there were no significant differences between the underweight AN and HC groups in FA, and no significant correlations between DTI measures and BMI or EDE-Q subscales. In a small, preliminary analysis, weight restoration therapy reduced MD, to levels significantly lower than age-matched HC, but did not consistently alter FA across individuals. These data show that white matter microstructure is significantly altered in female adolescents with AN, with preliminary longitudinal data suggesting that it may be reversible with short-term weight restoration.
Pattern of microstructural alterations
Similar to previous studies, we found increases in MD, AD and RD in underweight AN relative to HC [17, 19, 20]. While there were no significant group differences in FA between underweight AN and HC, Fig. 1 highlights a trend for lower FA in underweight AN relative to HC. This pattern of alterations in DTI measures is thought to reflect higher extracellular volume fraction and lower membrane density due to chronic myelin and axon loss, potentially after widespread inflammation [38]. Demyelination of white matter fibers disrupts the restricted diffusion in the perpendicular direction, causing an increase in RD, and cellular debris is subsequently cleared by microglia, causing an increase in AD [39]. In a neurodevelopmental context, however, it is possible that this pattern of findings reflects a critical disruption to the rapid myelination process that occurs throughout adolescence via a lack of glial cell proliferation and subsequent insufficient production of myelin [40].
Regional findings
Consistent with the recent meta-analysis by Barona et al. [14], the most significant difference in underweight AN relative to HC was found in the corpus callosum. The corpus callosum is critical for the interhemispheric integration of cognitive, emotional and perceptual functions, all of which are implicated in AN symptomology [41, 42]. Other key alterations were found within the internal and external capsule and corona radiata, which are critical for information flow between cortical and subcortical regions responsible for reward processing, cognitive flexibility and goal-directed behavior [43]. The uncinate fasciculus also contains reciprocal connections between the ventrolateral prefrontal cortex and amygdala, regions that are also integral to decision-making and reinforcement learning [43, 44]. Impairment of these processes could contribute to the impaired response inhibition and behavioral avoidance often seen in AN [4].
The bilateral cingulum bundles were also found to have significantly reduced white matter integrity in underweight AN. These diffuse tracts interconnect core components of the default mode network (DMN), which is activated during introspection and suppressed during cognitive functions [45]. Cingulum integrity is positively associated with individual differences in cognitive abilities and functional activation of the DMN [46]. While there are mixed findings regarding the role of the DMN in the neuropathology of AN [47, 48], it is possible that DMN dysregulation plays a role in self- and body-focused ruminations.
Multiple association fibers, and in particular, the left superior longitudinal fasciculus, exhibited lower white matter integrity in underweight AN relative to HC in the current study. This long-range fiber connects frontal to parietotemporal and occipital areas and allows for the integration of information from non-adjacent regions [44]. Previous studies have implicated poor connectivity between these regions as being a contributing factor in distorted perception of body size, a putative endophenotype of AN [47]. Impairment within long-range tracts more generally may also contribute to deficits in global processing while local processing remains intact. This may be expressed as weak central coherence, which some have proposed as an endophenotype of AN [49].
Collectively, altered white matter diffusivity across such widespread regions likely contributes to the altered functional connectivity reported in the cognitive control network and visual and homeostatic integration [50]. Despite this, we were unable to find any significant correlations between measures of white matter microstructural integrity and symptom severity (as measured by the EDE-Q), or BMI.
Longitudinal findings
In our exploratory longitudinal sample, short-term weight restoration significantly reduced MD in almost all of the white matter skeleton. Figure 3 demonstrates that this was a consistent direction of findings across all eight individuals, the degree of which was not related to the duration of treatment (range 13–289 days). Alternatively, changes in FA after weight restoration were inconsistent, with five individuals showing the expected increase in FA, while three showed no change or decreases. Previous longitudinal studies of short-term weight restoration have reported no change in FA [25], reduced FA [24] and increased FA [26]; therefore, this inconsistency is not unprecedented and highlights that there are likely multiple factors contributing to individual changes in microstructural properties after short-term weight restoration.
The magnitude of change in MD observed was striking, with some regions seeing reductions of up to 120%, compared to the initial group difference from HC of 3–9%. It is possible that this ‘overshoot’ in MD reduction may relate to the relatively rapid realimentation that occurred with this specific treatment protocol (e.g. 1.63 kg/week versus 0.4 kg/week [25]). Adolescence is a critical period for neurodevelopment, with increasing myelination allowing for better neural integration and the subsequent improvement in higher level cognitive skills [51]. It is possible that reintroduction of dietary substrates that are precursors to myelin production may provide short-term compensatory neuroplasticity.
It is important to note that although differences in age were accounted for statistically in all analyses, this is not reflected in the magnitude of change percentages. The HC and AN post-treatment groups were marginally older than the AN pre-treatment group (~ 10 months); therefore, the reported magnitude of change for MD may be slightly inflated due to normal developmental changes.
Our post hoc analyses did not find any significant WM microstructural differences between those with and without comorbid depression. It is likely, however, that the analysis was inadequately powered to detect small to moderate differences. Replication of this analysis in a larger sample is necessary to test this meaningfully and to allow for adequate statistical power to investigate the factors modulating microstructural plasticity.
On the basis of our preliminary results and evidence from previous studies, we tentatively suggest that weight-recovery treatment of young people with early onset of AN (less than 3 years of illness) and the neuroplasticity in adolescence could be key factors relevant to brain recovery. This is important for providing hope to young people with AN and their families that the effects of acute starvation on the adolescent brain may be reversible. While we did not find linear correlations between change in EDE-Q measures and microstructural properties, it is still possible that changes in white matter microstructure contribute to changes in symptoms, even if they are not the key driver. Improved neural transmission and communication through core tracts such as the corpus callosum likely facilitate improved general cognition, which may enhance the efficacy of cognitive therapies. This complex relationship requires further exploration.
Limitations
Our findings should be considered in light of the following limitations. First, our sample size was relatively modest and replication is required. In particular, our longitudinal sample was small due to many participants being unwilling to continue engaging in research after hospitalization, and it was limited to short-term weight restoration. We did not collect comparative longitudinal data in HC to account for normative aging data, however accounted for time between scans statistically. Finally, although including psychiatric comorbidities represents a more typical AN sample [30], the range of included comorbidities has the potential to influence the results. Despite these limitations, our results were consistent with some previous literature and provided additional information to build upon in future research.
Conclusion
In conclusion, widespread alterations in white matter diffusivity are found in underweight female adolescents with AN, which preliminary longitudinal data suggests may be predominantly due to the effects of malnutrition. Our exploratory data found significant reversal, and in fact, overcompensation of white matter deficits was observed after short-term weight restoration. Replication in a larger sample is required before any firm conclusions can be made and future research on longer term outcomes in longitudinal samples is required to provide critical information regarding the effects of acute starvation on neurodevelopmental trajectories.
What is already known on this subject?
Inconsistent findings of altered FA and MD are reported in adolescents with AN. Most studies examine FA alone or MD in limited regions, thus providing an incomplete view of white matter microstructure.
What your study adds?
There is widespread dys- or de-myelination in underweight AN, identified via increased MD, AD and RD. Realimentation in a small subsample reduced MD but did not consistently alter FA.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Code used to perform preprocessing of DTI data in fsl available upon request.
References
Association AP (2013) Diagnostic and statistical manual of mental disorders (DSM-5®). Am Psychiatric Pub
Arcelus J, Mitchell AJ, Wales J, Nielsen S (2011) Mortality rates in patients with anorexia nervosa and other eating disorders: a meta-analysis of 36 studies. Arch Gen Psychiatry 68(7):724–731
Phillipou A, Rossell SL, Gurvich C, Castle DJ, Abel LA, Nibbs RG, Hughes ME (2018) Differences in regional grey matter volumes in currently ill patients with anorexia nervosa. Eur J Neurosci 47(2):177–183
Treasure J, Zipfel S, Micali N, Wade T, Stice E, Claudino A, Schmidt U, Frank GK, Bulik CM, Wentz E (2015) Anorexia nervosa. Nat Rev Dis Primers 1(1):15074. https://doi.org/10.1038/nrdp.2015.74
Seitz J, Herpertz-Dahlmann B, Konrad K (2016) Brain morphological changes in adolescent and adult patients with anorexia nervosa. Journal of Neural Transmission 123(8):949–959. https://doi.org/10.1007/s00702-016-1567-9
Frank GK, Shott ME, Hagman JO, Mittal VA (2013) Alterations in brain structures related to taste reward circuitry in ill and recovered anorexia nervosa and in bulimia nervosa. Am J Psychiatry 170(10):1152–1160
Martin Monzon B, Henderson LA, Madden S, Macefield VG, Touyz S, Kohn MR, Clarke S, Foroughi N, Hay P (2017) Grey matter volume in adolescents with anorexia nervosa and associated eating disorder symptoms. Eur J Neurosci 46(7):2297–2307
Mainz V, Schulte-Rüther M, Fink GR, Herpertz-Dahlmann B, Konrad K (2012) Structural brain abnormalities in adolescent anorexia nervosa before and after weight recovery and associated hormonal changes. Psychosom Med 74(6):574–582
Castro-Fornieles J, Bargalló N, Lázaro L, Andrés S, Falcon C, Plana MT, Junqué C (2009) A cross-sectional and follow-up voxel-based morphometric MRI study in adolescent anorexia nervosa. J Psychiatr Res 43(3):331–340
Mori S, Zhang J (2006) Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron 51(5):527–539
Soares J, Marques P, Alves V, Sousa N (2013) A hitchhiker’s guide to diffusion tensor imaging. Front Neurosci 7:31
Song S-K, Sun S-W, Ramsbottom MJ, Chang C, Russell J, Cross AH (2002) Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 17(3):1429–1436
Sun SW, Liang HF, Trinkaus K, Cross AH, Armstrong RC, Song SK (2006) Noninvasive detection of cuprizone induced axonal damage and demyelination in the mouse corpus callosum. Magn Reson Med 55(2):302–308
Barona M, Brown M, Clark C, Frangou S, White T, Micali N (2019) White matter alterations in anorexia nervosa: evidence from a voxel-based meta-analysis. Neurosci Biobehav Rev 100:285–295. https://doi.org/10.1016/j.neubiorev.2019.03.002
Martin Monzon B, Hay P, Foroughi N, Touyz S (2016) White matter alterations in anorexia nervosa: a systematic review of diffusion tensor imaging studies. World J Psychiatry 6(1):177
Alexander AL, Lee JE, Lazar M, Field AS (2007) Diffusion tensor imaging of the brain. Neurotherapeutics 4(3):316–329
Via E, Zalesky A, Sánchez I, Forcano L, Harrison BJ, Pujol J, Fernández-Aranda F, Menchón JM, Soriano-Mas C, Cardoner N (2014) Disruption of brain white matter microstructure in women with anorexia nervosa. J Psychiatry Neurosci 39(6):367
Nagahara Y, Nakamae T, Nishizawa S, Mizuhara Y, Moritoki Y, Wada Y, Sakai Y, Yamashita T, Narumoto J, Miyata J (2014) A tract-based spatial statistics study in anorexia nervosa: abnormality in the fornix and the cerebellum. Prog Neuropsychopharmacol Biol Psychiatry 51:72–77
Miles AE, Kaplan AS, French L, Voineskos AN (2019) White matter microstructure in women with acute and remitted anorexia nervosa: an exploratory neuroimaging study. Brain Imaging Behav. https://doi.org/10.1007/s11682-019-00193-6
Phillipou A, Carruthers SP, Di Biase MA, Zalesky A, Abel LA, Castle DJ, Gurvich C, Rossell SL (2018) White matter microstructure in anorexia nervosa. Hum Brain Mapp 39(11):4385–4392. https://doi.org/10.1002/hbm.24279
Olivo G, Swenne I, Zhukovsky C, Tuunainen AK, Saaid A, Salonen-Ros H, Larsson EM, Brooks SJ, Schiöth HB (2019) Preserved white matter microstructure in adolescent patients with atypical anorexia nervosa. Int J Eat Disord 52(2):166–174
Gaudio S, Quattrocchi CC, Piervincenzi C, Zobel BB, Montecchi FR, Dakanalis A, Riva G, Carducci F (2017) White matter abnormalities in treatment-naive adolescents at the earliest stages of anorexia nervosa: a diffusion tensor imaging study. Psychiatry Res Neuroimaging 266:138–145. https://doi.org/10.1016/j.pscychresns.2017.06.011
Pfuhl G, King JA, Geisler D, Roschinski B, Ritschel F, Seidel M, Bernardoni F, Müller DK, White T, Roessner V (2016) Preserved white matter microstructure in young patients with anorexia nervosa? Hum Brain Mapp 37(11):4069–4083
Vogel K, Timmers I, Kumar V, Nickl-Jockschat T, Bastiani M, Roebroek A, Herpertz-Dahlmann B, Konrad K, Goebel R, Seitz J (2016) White matter microstructural changes in adolescent anorexia nervosa including an exploratory longitudinal study. Neuroimage Clin 11:614–621. https://doi.org/10.1016/j.nicl.2016.04.002
Cha J, Ide JS, Bowman FD, Simpson HB, Posner J, Steinglass JE (2016) Abnormal reward circuitry in anorexia nervosa: a longitudinal, multimodal MRI study. Hum Brain Mapp 37(11):3835–3846
von Schwanenflug N, Müller DK, King JA, Ritschel F, Bernardoni F, Mohammadi S, Geisler D, Roessner V, Biemann R, Marxen M (2019) Dynamic changes in white matter microstructure in anorexia nervosa: findings from a longitudinal study. Psychol Med 49(9):1555–1564
Zipfel S, Giel KE, Bulik CM, Hay P, Schmidt U (2015) Anorexia nervosa: aetiology, assessment, and treatment. Lancet Psychiatry 2(12):1099–1111
Beglin SJ, Fairburn CG (1992) Evaluation of a new instrument for the detection of eating disorders in community samples. Psychiatry Res 44(3):191–201
Madden S, Miskovic-Wheatley J, Wallis A, Kohn M, Lock J, Le Grange D, Jo B, Clarke S, Rhodes P, Hay P (2015) A randomized controlled trial of in-patient treatment for anorexia nervosa in medically unstable adolescents. Psychol Med 45(2):415–427
Udo T, Grilo CM (2019) Psychiatric and medical correlates of DSM-5 eating disorders in a nationally representative sample of adults in the United States. Int J Eat Disord 52(1):42–50
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC (1998) The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59(Suppl 20):22–33
Mond JM, Hay PJ, Rodgers B, Owen C (2006) Eating disorder examination questionnaire (EDE-Q): norms for young adult women. Behav Res Ther 44(1):53–62
Lock J, Le Grange D (2015) Treatment manual for anorexia nervosa: a family-based approach. Guilford Publications
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23:S208–S219
Andersson JL, Sotiropoulos SN (2016) An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 125:1063–1078
Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE (2014) Permutation inference for the general linear model. Neuroimage 92:381–397
Smith SM, Nichols TE (2009) Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44(1):83–98
Sen PN, Basser PJ (2005) A model for diffusion in white matter in the brain. Biophys J 89(5):2927–2938
Sun S-W, Liang H-F, Cross AH, Song S-K (2008) Evolving Wallerian degeneration after transient retinal ischemia in mice characterized by diffusion tensor imaging. Neuroimage 40(1):1–10
Lebel C, Beaulieu C (2011) Longitudinal development of human brain wiring continues from childhood into adulthood. J Neurosci 31(30):10937–10947
Papathanasiou A, Messinis L, Zampakis P, Papathanasopoulos P (2017) Corpus callosum atrophy as a marker of clinically meaningful cognitive decline in secondary progressive multiple sclerosis. Impact on employment status. J Clin Neurosci 43:170–175
Hofer S, Frahm J (2006) Topography of the human corpus callosum revisited—comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage 32(3):989–994
Griffiths KR, Morris RW, Balleine BW (2014) Translational studies of goal-directed action as a framework for classifying deficits across psychiatric disorders. Front Syst Neurosci 8:101
Schmahmann JD, Schmahmann J, Pandya D (2009) Fiber pathways of the brain. OUP USA,
van den Heuvel M, Mandl R, Luigjes J, Pol HH (2008) Microstructural organization of the cingulum tract and the level of default mode functional connectivity. J Neurosci 28(43):10844–10851
Bathelt J, Johnson A, Zhang M, Astle DE (2019) The cingulum as a marker of individual differences in neurocognitive development. Sci Rep 9(1):1–16
Phillipou A, Abel LA, Castle DJ, Hughes ME, Nibbs RG, Gurvich C, Rossell SL (2016) Resting state functional connectivity in anorexia nervosa. Psychiatry Res Neuroimaging 251:45–52
Boehm I, Geisler D, King JA, Ritschel F, Seidel M, Deza Araujo Y, Petermann J, Lohmeier H, Weiss J, Walter M (2014) Increased resting state functional connectivity in the fronto-parietal and default mode network in anorexia nervosa. Front Behav Neurosci 8:346
Lopez C, Tchanturia K, Stahl D, Treasure J (2009) Weak central coherence in eating disorders: a step towards looking for an endophenotype of eating disorders. J Clin Exp Neuropsychol 31(1):117–125
Gaudio S, Wiemerslage L, Brooks SJ, Schiöth HB (2016) A systematic review of resting-state functional-MRI studies in anorexia nervosa: evidence for functional connectivity impairment in cognitive control and visuospatial and body-signal integration. Neurosci Biobehav Rev 71:578–589
Nagy Z, Westerberg H, Klingberg T (2004) Maturation of white matter is associated with the development of cognitive functions during childhood. J Cogn Neurosci 16(7):1227–1233
Acknowledgements
The authors would like to thank all the participants for their involvement in the study. We thank Sheryl Foster and Dr Anthony Peduto of the Radiology Department of Westmead Hospital for their invaluable support.
Funding
This work was supported by a Seeding grant from the School of Medicine, Western Sydney University, to PH. KG is supported by a NHMRC Early Career Fellowship (GNT1122842) and BMM was supported by a Western Sydney University Postgraduate Research Award.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Professor Touyz has received royalties from Shire/Takeda for commissioned reports and speaking engagements. He is also the Chair of the Australian Clinical Advisory Board for Binge Eating Disorder. He has also received research grant funding as well as travel grants. He has also received royalties from Taylor and Francis, Hogrefe and Huber and McGraw Hill for books/book chapters. Professor Touyz is a mental health adviser to the Commonwealth Department of Veteran Affairs. He has received honoraria from Weight Watchers (WW). Professor Hay receives/has received sessional fees and lecture fees from the Australian Medical Council, Therapeutic Guidelines publication, and New South Wales Institute of Psychiatry and royalties/honoraria from Hogrefe and Huber, McGraw Hill Education, and Blackwell Scientific Publications, Biomed Central and PlosMedicine and she has received research grants from the NHMRC and ARC. She is Chair of the National Eating Disorders Collaboration Steering Committee in Australia (2012) and Member of the ICD-11 Working Group for Eating Disorders (2012–2018) and was Chair Clinical Practice Guidelines Project Working Group (Eating Disorders) of RANZCP (2012–2015). In the past 5 years she has prepared a report under contract for Shire Pharmaceuticals) and received honoraria for education of psychiatrists from Shire Pharmaceuticals (Takeda group). All views in this paper are her own. Associate Professor Madden has received research grants from the NHMRC. He is on the Steering Committee of the National Eating Disorders Collaboration in Australia (2012-) and was a member of the Clinical Practice Guidelines Project Working Group (Eating Disorders) of RANZCP (2012–2015). All other Authors declare that there are no conflicts of interest.
Ethics approval
The study protocol (HREC/13/SCHN/385) received approval by the Human Research Ethics Committees of Children’s Hospital at Westmead, Western Sydney University and Westmead Hospital, and was carried out in accordance with the Declaration of Helsinki.
Consent to participate
Informed written consent was obtained from all participants and from parents of those under the age of 16.
Consent for publication
Informed written consent was obtained from all participants for their de-identified data to be published in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Griffiths, K.R., Martin Monzon, B., Madden, S. et al. White matter microstructural differences in underweight adolescents with anorexia nervosa and a preliminary longitudinal investigation of change following short-term weight restoration. Eat Weight Disord 26, 1903–1914 (2021). https://doi.org/10.1007/s40519-020-01041-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40519-020-01041-z