Abstract
Purpose
The Laparoscopic Roux-en-Y Gastric Bypass (LRYGB) is an effective weight loss procedure. The gastro-jejunal (GJ) anastomosis required can be performed on the anterior or posterior gastric pouch wall. No studies have compared these variants in terms of efficacy and onset of dumping syndrome (DS) and weight regain (WR). We aimed at assessing the prevalence of DS in relation to the site of anastomosis together with identifying prognostic factors of DS and WR.
Methods
Patients who had undergone LRYGB with anterior (AGJ) or posterior (PGJ) anastomosis in 2010–2019 were retrospectively analyzed. We collected demographic data, medical history and the prevalence of DS evaluated through the Sigstad Score, together with WR data.
Results
213 patients were enrolled, of which 51.6% had an AGJ and 48.4% had a PGJ. The mean follow-up time was 81 ± 18 and 27 ± 13 months in the AGJ and PGJ group, respectively (p < 0.0001). Excess weight loss was 77.59% and 94.13% in patients with AGJ and PGJ, respectively (p < 0.001). WR rate was 16% and 4% in the AGJ and PGJ population, respectively (p < 0.001). DS prevalence was 38% and 76% in the AGJ and the PGJ population, respectively (p < 0.0001). The site of anastomosis was identified as an independent predictor of DS (OR5.15; 95% CI 2.82–9.41; p < 0.0001) and WR (OR5.31; 95% CI 2.32–12.15; p < 0.0001). Obesity-related complications significantly improved after surgery independent of the anastomosis site.
Conclusion
LRYGB is effective in determining long-term weight loss and improvement of complications. AGJ is associated with lower prevalence of DS but more frequent WR. The anastomosis site is a factor to be considered when performing LRYGB.
Level of evidence
Level V, cross-sectional descriptive study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is nowadays a major health burden, with its prevalence as high as 40% in the US [1], but that of Italy rapidly reaching the same pace having now hit 10% of the general population [2]. The obesity pandemic has inevitably led to an increase in prevalence of many complications of weight excess, some of which are well acknowledged, such as Type 2 Diabetes (T2D), obstructive sleep apnea syndrome (OSAS), non-alcoholic fatty liver disease NAFLD, and cardiovascular disease [3,4,5,6], and others are emerging and currently being investigated [7, 8]. Several strategies have been proposed for the treatment of weight excess and its complications, ranging from dietary regimens [9,10,11,12,13,14,15], to food supplements [16, 17], pharmacological treatments [18,19,20], physical exercise [21], and psychological approaches [22]. Most of these are safe [19, 23, 24], although some have risen concern [25, 26], but despite leading to improvements in the majority of cases, the major issue is long-term maintenance. To date, obesity and metabolic surgery is the treatment that most commonly leads to long term success [27].
The Laparoscopic Roux-en-Y Gastric By-pass (LRYGB) has become one of the most performed surgical procedures for morbid obesity accounting for 30% of all procedures, only second to sleeve gastrectomy [28]. Despite its well-established efficacy, LRYGB is not devoid of weight regain in the years following surgery. Moreover, adverse events can occur in up to 25% of the cases, including leaks, bleeding, anastomotic ulcers or strictures, gastro-gastric fistulas, internal hernias, vitamin deficiency and Dumping Syndrome (DS) [29,30,31]. Noteworthy, the prevalence of DS following LRYGB varies between 15 and 70% [32,33,34,35,36,37], with a wide range of presentations. The symptoms usually occur within the first hour following a meal (early DS) and include vasomotor symptoms such as palpitations, profuse sweating, dizziness, flushing, hypotension, and gastrointestinal symptoms, including diarrhea, bloating, nausea, or abdominal pain. The mechanisms underlying the onset of DS following LRYGB seem to be a higher speed of gastric emptying leading to autonomic responses, osmotic effects and possibly an increased release of gastrointestinal hormones such as glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) [38,39,40]. Late DS occurs one to three hours following a meal and is primarily characterized by hypoglycemia due to excess insulin secretion that leadsto confusion, hunger, syncope, tremor, irritability etc.[41]. In its most severe cases, DS may lead to car accidents and even death, on top of poor quality of life and possibly malnutrition, often linked to a limitation in the dietary intake [42].
The gastro-jejunal (GJ) anastomosis, being one of the most prominent factors in the mechanisms of gastric pouch emptying, represents an important player in the development of DS [32, 43]. It may be performed in different locations of the gastric pouch, including the anterior (AGJ) or the posterior (PGJ) wall of the pouch, possibly influencing the rate of DS onset. In fact, the AGJ might exert an antigravity effect possibly delaying emptying compared to the PGJ, which could conversely lead to a more rapid emptying.
To date, no studies have investigated the clinical impact of the GJ anastomosis site on the gastric pouch wall. We therefore aimed at analyzing the relationship between the site of GJ anastomosis and parameters of efficacy in terms of DS together with the development of WR in patients undergoing a LRYGB.
Methods
Study design
A cross-sectional study was conducted in patients with morbid obesity undergoing LRYGB at Belcolle Hospital and Campus Bio-medico University of Rome from January 2010 to January 2019, by means of a phone interview. Inclusion criteria were as follows: age > 18 years; patients with diagnosed obesity (pre-operative Body mass Index (BMI) > 35 kg/m2) candidate to obesity and metabolic surgery and treated with LRYGB. Patients were excluded if any of the following conditions was present: previous upper gastro-intestinal surgery (other than LRYGB); occurrence of post-operative complications which had required a re-intervention; inability to provide all necessary information; lack of informed consent.
The ethics committee of Campus Bio-Medico University of Rome approved this study and its development (prot. 9/20). All the procedures performed in the study complied with the ethical standards of the institutional and/or national research committee and the Helsinki Declaration of 1964 and its subsequent amendments or comparable ethical standards. Written informed consent was obtained from all participants prior to enrollment.
Surgical technique
All surgical procedures had been performed by the same experienced surgeon (VB). The RYGB procedure was performed laparoscopically as previously described [44, 45]. Briefly, a small sub-cardial gastric pouch of 15-25 mL of volume was created, and an enterotomy performed at 120 cm from where the ligament of Treitz was, realizing a jejunal limb. Then, a gastro-jejunal latero-lateral antecolic 3-cm large anastomosis was realized with a mechanical linear stapler at the anterior (AGJ) or posterior (PGJ) wall of the pouch. At 100 cm from the GJ anastomosis in the alimentary tract, an enterotomy was then performed to make a second jejuno-jenunal anastomosis between the two limbs realizing a roux-en-Y. All patients included in the analysis had been managed according to ERAS (Enhanced recovery after surgery) post-operative care. Mobilization, liquid assumption and hospital discharge were scheduled at 3, 5 and 48 h from surgery in case of no complications. Urinary catheter, abdominal drainage or nasogastric tube were not routinely positioned. Surgical and nutritional follow up occurred at 1, 3, 6 and 12 months after surgery, as per national guidelines [46].
Data collection and variables
Partial data regarding the variables of interest were retrieved through the hospital electronic records. In order to complete the collection, the study involved the administration of a structured phone interview conducted by a qualified medical doctor from August to October 2019. Weight, BMI, obesity-related complications, and smoking habits were assessed both at the time of the interview and at the time of surgery, together with the prevalence of DS according to the Sigstad Scoring system at the time of the interview [47]. Briefly, this score was first proposed in 1970 and aids DS diagnosis by allocating points to several suggestive symptoms. A score less than 4 excludes the diagnosis, while one of 7 and above suggests it [47]. Noteworthy, the time of symptoms occurrence is not investigated by the score, but it was inquired about during the phone interview. Excess Weight Loss (EWL) was defined as the percentage of excess weight lost after surgery [(pre-operative weight – nadir post-operative weight)/(pre-operative weight − ideal weight)]. Total Weight Loss (TWL) was defined as the total percentage of weight lost after surgery [(pre-operative weight – nadir post − operative weight)/(pre-operative weight)]. Δ Weight (Kg) was defined as the difference between the weight at the time of the surgery and the weight at the time of the interview; Δ BMI was defined as the difference between that at the time of the surgery and that at the time of the interview). Weight regain (WR) was defined as the recovery of at least 20% of the weight lost following surgery. The nadir of postoperative weight loss was considered for this calculation independent of when it occurred.
Statistical analysis
Based on a preliminary pilot study of 20 patients, the mean ± SD value of the Sigstad score in the patient population was 7.9 ± 4.5. Assuming a power of 0.80 and alpha of 0.05, 196 patients were considered appropriate to highlight an expected difference of 7 points in the Sigstad score. The number of patients was further increased to 213 to prevent possible missing data.
The Statistical Package for Social Sciences (SPSS), v.20 (Armonk, NY: IBM Corp), was used for statistical analysis. Results are presented as mean ± standard deviation (SD) for continuous variables and as frequency (absolute number and percentage) for categorical variables. Normality was assessed with the Kolmogorov–Smirnov test. Differences between groups were analyzed with the Student t test for continuous variables and Chi-Square test/Fisher's exact test for categorical variables. Univariate and multivariate logistic regression models were performed to analyze the relationship between the presence of DS and WR as the dependent variables of two different analysis and clinical and anthropometrical variables as independent variables. Univariate analysis was performed by converting continuous variables into dummy dichotomic variables based on median values, while continuous variables were used for multivariate analyses. To build a multivariate logistic regression models with DS and WR as the dependent variables of two different analysis, we used a forward stepwise approach. To build a multivariate logistic regression model with presence of dumping syndrome and weight regain as the dependent variables in two different analysis, we used a forward stepwise approach including all statistically significant variables of the univariate analysis as regressors in one single model. The forward stepwise selection method does not provide ORs and 95% CI for the variables not retained in the model because they do not significantly improve prediction. Therefore, only the variables with statistically significant results were added in the table in the multivariate model, reporting their OR and 95% CI, [R2]. For the forward stepwise analysis, a P-IN = 0.05 and a P-OUT = 0.10 were used. The effect estimate is reported as Nagelkerke's R2, which informs on how much the model explains the variance of the dependent variable. The ORs represent the mean change in the dependent variable per one unit of change in the independent variable while holding other predictors in the model constant. The results were considered statistically significant when p < 0.05.
Results
Two hundred and thirteen patients were enrolled, of which 179 (84%) were female. Their general characteristics at the time of surgery and at that of the phone interview are summarized in Table 1. Briefly, at the time of surgery their age was between 21 and 67 years (mean age 45 ± 10), body weight was 122.54 ± 21.35 kg and BMI of 44.15 ± 5.61 kg/m2. Smoking history was present in 78 patients (36.6%). In regard to obesity-related complications, 27.7% had T2D, 40.8% were pharmacologically treated for hypertension, 25.4% had dyslipidemia, and 28.2% had OSAS.
Analyzing the post-operative variations of the anthropometric indexes, at an average follow-up of 55 months, the patients presented a weight reduction of 44.18 ± 15.46 kg with a % EWL of 85.67 ± 25.3 and a % TWL of 35.71 ± 9.7. The minimum weight reached was on average 73.25 kg, while the average minimum BMI was 26.42. At the time of the interview, 42 patients (19.7%) presented weight regain. In regard to obesity-related complications, the patients reported of having T2D, hypertension, dyslipidemia or OSAS in 3.8, 8.5, 4.7 and 2.3% of cases, respectively. At the time of the interview, the study population presented a weight of 78.35 ± 16.13 kg with an average BMI of 28.25. The prevalence of DS at the time of the interview as assessed by a Sigstad score ≥ 7 was 56.3%, with a score of 9.59 ± 7.64 (Table 1). Our study population reported of having experienced symptoms suggestive of DS within 6 months after the surgery and that these symptoms always occurred within the first hour after the meal consumption, suggesting an early rather than late DS.
There were no differences in terms of gender, age at the time of the interview or preoperative weight between the two groups, nor there were significant differences in terms of preoperative complications such as T2D, hypertension, dyslipidemia, OSAS, presenting respectively in 13.6%, 20%, 12.7% and 15% of patients with AGJ and in 14%, 21%, 13% and 14% of patients with PGJ. However, the preoperative BMI was significantly higher in the AGJ group compared to PGJ (45.32 ± 5.6 kg/m2 and 42.90 ± 5.36 kg/m2, respectively, p = 0.04) (Table 2).
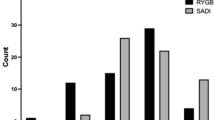
The minimum post-intervention BMI was higher in the AGJ group compare to the PGJ one (27.09 ± 4.45 kg/m2 and 25.69 ± 3.95 kg/m2, respectively, p = 0.011), in line with lower % EWL and % TWL in the AGJ group compared to PGJ (p < 0.0001). A significant difference was also observed at the time of the interview (BMI 29.68 ± 5.08 and 26.71 ± 4.17, respectively p < 0.001), with the AGJ group reporting weight regain more frequently (16% vs. 4%, p < 0.001) (Table 2).
A univariate analysis showed that the remission rate of obesity-related complications such as T2D, hypertension, dyslipidemia and OSAS did not differ between the two study groups, with both showing significant improvements following surgery (Table 3).
The presence of DS as assessed by the Sigstad Score was 38% in the AGJ group and 76% in the PGJ group (p < 0.0001), with the score being significantly lower in the AGJ group compared to the PJG one (6.89 ± 6.57 and 12.48 ± 7.67, respectively, p < 0.0001). Univariate association with the presence of DS at the time of the interview were assessed for all baseline clinical characteristics together with the time of follow-up since surgery and the minimum BMI reached after surgery. The minimum BMI reached after surgery, time elapsed since surgery, female gender and PGJ were found to have a significant univariate association with DS (Table 4). Multivariate analysis showed that also when correcting for all the significant variables at the univariate analysis, PGJ and female gender were significant predictors of DS (OR 5.15; 95% CI 2.82–9.409; p = 0.0001 and OR 2.54; 95% CI 1.125–5.775; p = 0.025 respectively, Table 4) explaining 21% of the clinical outcome DS (R2 0.21) (Table 4).
Univariate association with the presence of WR at the time of the interview were assessed for all baseline clinical characteristics together with the time of follow-up since surgery and the minimum BMI reached after surgery. The minimum BMI reached after surgery, time elapsed since surgery, and AGJ were found to have a significant univariate association with WR (Table 5). When the Sigstad score was converted into a dummy dichotomic variable based on its median value of 9, its univariate association with WR almost reached significance with a p = 0.052. Multivariate analysis showed that when correcting for all the significant variables at the univariate analysis, AGJ was the only significant predictor of WR (OR 5.31; 95% CI 2.323–12.15; p = 0.0001) (Table 5) explaining 13.7% of the clinical outcome WR (R2 0.137) (Table 5).
Discussion
The incidence of obesity and obesity-related pathologies has increased of 75% from 1980 and seems to affect childhood and adolescence more and more [48]. Consequently, obesity and metabolic surgery has gained popularity in the last decade, representing one of the most solid and preferable therapeutic options. The LRYGB is one of the most performed procedures in obesity and metabolic surgery, even more so in patients with T2D [28], and represents a mixed type surgery as it combines different mechanisms: a positive appetite-controlling gastro-intestinal hormonal response to food ingestion, a reduction in size of the stomach, and a variable grade of absorption reduction in relation with the length of the Roux-en-Y limbs [49].
The effectiveness of the procedure in leading to the remission of weight excess complications was not influenced by the technical variants of the anastomosis packaging. Interestingly, greater weight loss and much less frequent weight regain were observed when the anastomosis had been performed on the posterior wall. Noteworthy, the significant difference in terms of follow-up between the two groups could have hindered the comparison between the two techniques in terms of weight loss and regain, as it is described that patients undergoing obesity and metabolic surgery tend to slowly and progressively recover the lost weight over time. However, the anastomosis site was maintained as independent predictor of WR, upon inclusion of the time elapsed since surgery in the regression model.
The location of the anastomosis was also strongly related to the onset of DS. In our analysis, the general prevalence of DS was 56.33%, similar to what reported in the literature [33]. Interestingly, that of patients of the AGJ group was 38%, while the PGJ group had one of 76%, with a statistically significant difference (p < 0.0001). It is possible to hypothesize that such a striking difference might be related to a slower emptying of the gastric pouch, attributable to the anti-gravity position of the anastomosis on the anterior wall.
DS has always been considered a complication of LRYGB surgery as described for the first time in patients undergoing surgery with different objectives than the treatment of obesity, such as total gastrectomy for neoplastic disease or Billroth II type gastrectomy for ulcers. Clinical studies have shown that patients occasionally consider DS to be a positive protective mechanism against excessive consumption of foods with a high glycemic index, since the discomfort that derives from the intake of calorie dense foods limits their intake, facilitating weight loss [37]. However, DS among patients undergoing LRYGB may have an impact on food choices [33] and therefore, in some cases, on maintaining weight loss after LRYGB. We could postulate that the higher prevalence of DS observed with the PGJ anastomosis might have led to better weight maintenance possibly due to the modification of eating behaviors to avoid the unpleasant symptoms of DS, a hypothesis partially supported by the almost significant association between the Sigstad score and the presence of WR. As there could to be a causal link between the anastomosis site and the presence of DS, investigating the association of both the site and the presence DS with WR was not feasible, and mechanistic studies are needed to shed further light on the topic on which we cannot be conclusive on.
Our study presents several limitations. First, the means of data collection, including retrieval from electronic records and phone interviews, may have led to possible bias: symptoms were self-reported by the patient as well as anthropometric data, often with reference to distant times of which the patient can lose or alter the memory. It is therefore possible that some of the information might have been lost by the time of the phone interview. Second, we did not assess the prevalence of DS in the immediate post-operative period, as the mean time from surgery was 55 months, providing an incomplete picture regarding the clinical course of the disease following surgery. Third, we had limited knowledge regarding the possible confounding factors that might have occurred, such as pharmacological or dietary interventions that might have been implemented by the patient after surgery. This is particularly important for the outcome of WR, as this is of multifactorial origin and many other aspects that were not taken into account could have concurred in leading to the observed results. Fourth, the cross-sectional design of the study did not allow for cause-effect assessments. Fifth, the score used to identify the DS focuses on early symptoms. We may therefore infer that the site of anastomosis is associated to the onset of early DS, and we are instead unable to report an association with late DS. Of note, patients reported of having symptoms within the first hour after the meal, and within six months of surgery. Sixth, an indirect means of evaluation of DS was used, whose sensitivity and specificity in patients subjected to obesity and metabolic surgery is currently unknown. Seventh, EWL was defined as the nadir reached following surgery independent of the time of occurrence. All patients had undergone a minimum follow up time of 12 months, an interval within which the nadir of weight loss is usually hit. However, we cannot exclude Querythat some patients might have been still losing weight by the time of the last in person follow up or phone interview. Finally, the significantly different time elapsed since surgery of the two groups identified based of GJ anastomosis site might have influenced the comparison. However, when adjusting by follow-up time, analyses retained their significance, suggesting that this factor might be of limited relevance.
Our study also features some strengths. First, this is the first research conducted to investigate the pathophysiological implications of the GJ anastomosis site, and despite its many limitations may lay the path for ad hoc designed prospective studies. Second, a relatively large population was enrolled, allowing for a high study power. Third, the follow-up exceeding 4 years following surgery allowed us to both investigate the long-term efficacy and safety of the surgical procedure. Fourth, the same experienced surgeon performed all procedures, de facto excluding a possible operator-dependent bias.
Conclusion
The choice of the site (anterior or posterior wall of the gastric pouch) of the gastro-jejunal anastomosis seems to be correlated with the development of DS and may also correlate with the effectiveness of the procedure in terms of weight regain. Therefore, the findings emerged from the present study suggest that the choice of the GJ anastomosis site should be taken into account, among other technical and clinical pre-operative factors, when performing a LRYGB, as what was simply considered a variant of a well-established surgical technique may indeed have a functional and clinical implication.
What is already known on this subject?
LRYGB is effective but linked with WR and DS. The LRYGB gastro-jejunal anastomosis can be on the anterior or posterior gastric pouch wall, whose difference is of unknown clinical significance.
What this study adds?
The anterior gastro-jejunal anastomosis is associated with lower dumping syndrome prevalence but more frequent weight regain, showing clinical implications for the two variants evaluated.
Availability of data
All data will be made available upon reasonable request to the corresponding author.
References
World Health Organization. Mean body mass index trends among adults, crude (kg/m2) estimates by country. https://apps.who.int/gho/data/view.main.BMIMEANADULT. Accessed 2020
Watanabe M, Risi R, De Giorgi F, Tuccinardi D, Mariani S, Basciani S, Lubrano C, Lenzi A, Gnessi L (2020) Obesity treatment within the Italian national healthcare system tertiary care centers: what can we learn? Eat Weight Disord. https://doi.org/10.1007/s40519-020-00936-1
Mariani S, Fiore D, Varone L, Basciani S, Persichetti A, Watanabe M, Saponara M, Spera G, Moretti C, Gnessi L (2012) Obstructive sleep apnea and bone mineral density in obese patients. Diabet Metab Synd Obes Targets Therapy 5:395–401. https://doi.org/10.2147/DMSO.S37761
Lonardo A, Mantovani A, Lugari S, Targher G (2020) Epidemiology and pathophysiology of the association between NAFLD and metabolically healthy or metabolically unhealthy obesity. Ann Hepatol. https://doi.org/10.1016/j.aohep.2020.03.001
Dwivedi AK, Dubey P, Cistola DP, Reddy SY (2020) Association between obesity and cardiovascular outcomes: updated evidence from meta-analysis studies. Curr Cardiol Rep 22(4):25. https://doi.org/10.1007/s11886-020-1273-y
Okamura T, Hashimoto Y, Hamaguchi M, Obora A, Kojima T, Fukui M (2019) Ectopic fat obesity presents the greatest risk for incident type 2 diabetes: a population-based longitudinal study. Int J Obes (Lond) 43(1):139–148. https://doi.org/10.1038/s41366-018-0076-3
Palermo A, Tuccinardi D, Defeudis G, Watanabe M, D’Onofrio L, Lauria AP, Napoli N, Pozzilli P, Manfrini S (2016) BMI and BMD: the potential interplay between obesity and bone fragility. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph13060544
Arboleda S, Vargas M, Losada S, Pinto A (2019) Review of obesity and periodontitis: an epidemiological view. Br Dent J 227(3):235–239. https://doi.org/10.1038/s41415-019-0611-1
Basciani S, Camajani E, Contini S, Persichetti A, Risi R, Bertoldi L, Strigari L, Prossomariti G, Watanabe M, Mariani S, Lubrano C, Genco A, Spera G, Gnessi L (2020) Very-low-calorie ketogenic diets with whey, vegetable or animal protein in patients with obesity: a randomized pilot study. J Clin Endocrinol Metab. https://doi.org/10.1210/clinem/dgaa336
Basciani S, Costantini D, Contini S, Persichetti A, Watanabe M, Mariani S, Lubrano C, Spera G, Lenzi A, Gnessi L (2015) Safety and efficacy of a multiphase dietetic protocol with meal replacements including a step with very low calorie diet. Endocrine 48(3):863–870. https://doi.org/10.1007/s12020-014-0355-2
Farr OM, Tuccinardi D, Upadhyay J, Oussaada SM, Mantzoros CS (2018) Walnut consumption increases activation of the insula to highly desirable food cues: A randomized, double-blind, placebo-controlled, cross-over fMRI study. Diabetes Obes Metab 20(1):173–177. https://doi.org/10.1111/dom.13060
Filippou CD, Tsioufis CP, Thomopoulos CG, Mihas CC, Dimitriadis KS, Sotiropoulou LI, Chrysochoou CA, Nihoyannopoulos PI, Tousoulis DM (2020) Dietary approaches to stop hypertension (DASH) diet and blood pressure reduction in adults with and without hypertension: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. https://doi.org/10.1093/advances/nmaa041
Watanabe M, Tozzi R, Risi R, Tuccinardi D, Mariani S, Basciani S, Spera G, Lubrano C, Gnessi L (2020) Beneficial effects of the ketogenic diet on nonalcoholic fatty liver disease: a comprehensive review of the literature. Obes Rev. https://doi.org/10.1111/obr.13024
Watanabe M, Risi R, Camajani E, Contini S, Persichetti A, Tuccinardi D, Ernesti I, Mariani S, Lubrano C, Genco A, Spera G, Gnessi L, Basciani S (2020) Baseline HOMA IR and circulating FGF21 levels predict NAFLD improvement in patients undergoing a low carbohydrate dietary intervention for weight loss: a prospective observational pilot study. Nutrients. https://doi.org/10.3390/nu12072141
Tuccinardi D, Farr OM, Upadhyay J, Oussaada SM, Klapa MI, Candela M, Rampelli S, Lehoux S, Lazaro I, Sala-Vila A, Brigidi P, Cummings RD, Mantzoros CS (2019) Mechanisms underlying the cardiometabolic protective effect of walnut consumption in obese people: a cross-over, randomized, double-blind, controlled inpatient physiology study. Diabetes Obes Metab 21(9):2086–2095. https://doi.org/10.1111/dom.13773
Balaji M, Ganjayi MS, Hanuma Kumar GE, Parim BN, Mopuri R, Dasari S (2016) A review on possible therapeutic targets to contain obesity: the role of phytochemicals. Obes Res Clin Pract 10(4):363–380. https://doi.org/10.1016/j.orcp.2015.12.004
Watanabe M, Gangitano E, Francomano D, Addessi E, Toscano R, Costantini D, Tuccinardi D, Mariani S, Basciani S, Spera G, Gnessi L, Lubrano C (2018) Mangosteen extract shows a potent insulin sensitizing effect in obese female patients: a prospective randomized controlled pilot study. Nutrients. https://doi.org/10.3390/nu10050586
Sumida Y, Yoneda M (2018) Current and future pharmacological therapies for NAFLD/NASH. J Gastroenterol 53(3):362–376. https://doi.org/10.1007/s00535-017-1415-1
Srivastava G, Apovian CM (2018) Current pharmacotherapy for obesity. Nat Rev Endocrinol 14(1):12–24. https://doi.org/10.1038/nrendo.2017.122
Tuccinardi D, Farr OM, Upadhyay J, Oussaada SM, Mathew H, Paschou SA, Perakakis N, Koniaris A, Kelesidis T, Mantzoros CS (2019) Lorcaserin treatment decreases body weight and reduces cardiometabolic risk factors in obese adults: a 6-month, randomized, placebo-controlled, double-blind clinical trial. Diabetes Obes Metab 21(6):1487–1492. https://doi.org/10.1111/dom.13655
Wiklund P (2016) The role of physical activity and exercise in obesity and weight management: time for critical appraisal. J Sport Health Sci 5(2):151–154. https://doi.org/10.1016/j.jshs.2016.04.001
Castelnuovo G, Pietrabissa G, Manzoni GM, Cattivelli R, Rossi A, Novelli M, Varallo G, Molinari E (2017) Cognitive behavioral therapy to aid weight loss in obese patients: current perspectives. Psychol Res Behav Manag 10:165–173. https://doi.org/10.2147/PRBM.S113278
Bruci A, Tuccinardi D, Tozzi R, Balena A, Santucci S, Frontani R, Mariani S, Basciani S, Spera G, Gnessi L, Lubrano C, Watanabe M (2020) Very low-calorie ketogenic diet: a safe and effective tool for weight loss in patients with obesity and mild kidney failure. Nutrients. https://doi.org/10.3390/nu12020333
Watanabe M, Tuccinardi D, Ernesti I, Basciani S, Mariani S, Genco A, Manfrini S, Lubrano C, Gnessi L (2020) Scientific evidence underlying contraindications to the ketogenic diet: an update. Obes Rev. https://doi.org/10.1111/obr.13053
FDA requests the withdrawal of the weight-loss drug Belviq, Belviq XR (lorcaserin) from the market. (2020). https://www.fda.gov/drugs/drug-safety-and-availability/fda-requests-withdrawal-weight-loss-drug-belviq-belviq-xr-lorcaserin-market. Accessed 6.2020
Brancheau D, Patel B, Zughaib M (2015) Do cinnamon supplements cause acute hepatitis? Am J Case Rep 16:250–254. https://doi.org/10.12659/AJCR.892804
Courcoulas AP, Yanovski SZ, Bonds D, Eggerman TL, Horlick M, Staten MA, Arterburn DE (2014) Long-term outcomes of bariatric surgery: a National Institutes of Health symposium. JAMA Surg 149(12):1323–1329. https://doi.org/10.1001/jamasurg.2014.2440
Angrisani L, Santonicola A, Iovino P, Vitiello A, Higa K, Himpens J, Buchwald H, Scopinaro N (2018) IFSO Worldwide Survey 2016: Primary, Endoluminal, and Revisional Procedures. Obes Surg 28(12):3783–3794. https://doi.org/10.1007/s11695-018-3450-2
Seeras K, Acho RJ, Lopez PP (2020) Roux-en-Y gastric bypass chronic complications. In: StatPearls. StatPearls Publishing, Treasure Island, FL. https://www.ncbi.nlm.nih.gov/books/NBK519489/
Palermo M, Acquafresca PA, Rogula T, Duza GE, Serra E (2015) Late surgical complications after gastric by-pass: a literature review. Arq Bras Cir Dig 28(2):139–143. https://doi.org/10.1590/S0102-67202015000200014
Cai JX, Schweitzer MA, Kumbhari V (2016) Endoscopic management of bariatric surgery complications. Surg Laparosc Endosc Percutan Tech 26(2):93–101. https://doi.org/10.1097/SLE.0000000000000230
Tack J, Arts J, Caenepeel P, De Wulf D, Bisschops R (2009) Pathophysiology, diagnosis and management of postoperative dumping syndrome. Nat Rev Gastroenterol Hepatol 6(10):583–590. https://doi.org/10.1038/nrgastro.2009.148
Ukleja A (2005) Dumping syndrome: pathophysiology and treatment. Nutr Clin Pract 20(5):517–525. https://doi.org/10.1177/0115426505020005517
Hepburn DA, Deary IJ, Frier BM, Patrick AW, Quinn JD, Fisher BM (1991) Symptoms of acute insulin-induced hypoglycemia in humans with and without IDDM. Factor Anal Approach Diab Care 14(11):949–957. https://doi.org/10.2337/diacare.14.11.949
de Zwaan M, Hilbert A, Swan-Kremeier L, Simonich H, Lancaster K, Howell LM, Monson T, Crosby RD, Mitchell JE (2010) Comprehensive interview assessment of eating behavior 18–35 months after gastric bypass surgery for morbid obesity. Surg Obes Relat Dis 6(1):79–85. https://doi.org/10.1016/j.soard.2009.08.011
Kalarchian MA, Marcus MD, Courcoulas AP, Cheng Y, Levine MD (2014) Self-report of gastrointestinal side effects after bariatric surgery. Surg Obes Relat Dis 10(6):1202–1207. https://doi.org/10.1016/j.soard.2014.08.007
Mallory GN, Macgregor AM, Rand CS (1996) The influence of dumping on weight loss after gastric restrictive surgery for morbid obesity. Obes Surg 6(6):474–478. https://doi.org/10.1381/096089296765556368
Goldfine AB, Mun EC, Devine E, Bernier R, Baz-Hecht M, Jones DB, Schneider BE, Holst JJ, Patti ME (2007) Patients with neuroglycopenia after gastric bypass surgery have exaggerated incretin and insulin secretory responses to a mixed meal. J Clin Endocrinol Metab 92(12):4678–4685. https://doi.org/10.1210/jc.2007-0918
Tzovaras G, Papamargaritis D, Sioka E, Zachari E, Baloyiannis I, Zacharoulis D, Koukoulis G (2012) Symptoms suggestive of dumping syndrome after provocation in patients after laparoscopic sleeve gastrectomy. Obes Surg 22(1):23–28. https://doi.org/10.1007/s11695-011-0461-7
Morinigo R, Moize V, Musri M, Lacy AM, Navarro S, Marin JL, Delgado S, Casamitjana R, Vidal J (2006) Glucagon-like peptide-1, peptide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab 91(5):1735–1740. https://doi.org/10.1210/jc.2005-0904
Scarpellini E, Arts J, Karamanolis G, Laurenius A, Siquini W, Suzuki H, Ukleja A, Van Beek A, Vanuytsel T, Bor S, Ceppa E, Di Lorenzo C, Emous M, Hammer H, Hellstrom P, Laville M, Lundell L, Masclee A, Ritz P, Tack J (2020) International consensus on the diagnosis and management of dumping syndrome. Nat Rev Endocrinol 16(8):448–466. https://doi.org/10.1038/s41574-020-0357-5
Gys B, Plaeke P, Lamme B, Lafullarde T, Komen N, Beunis A, Hubens G (2019) Heterogeneity in the definition and clinical characteristics of dumping syndrome: a review of the literature. Obes Surg 29(6):1984–1989. https://doi.org/10.1007/s11695-019-03818-3
Tsai C, Steffen R, Kessler U, Merki H, Zehetner J (2020) Short-term outcomes of endoscopic gastro-jejunal revisions for treatment of dumping syndrome after Roux-En-Y gastric bypass. Surg Endosc 34(8):3626–3632. https://doi.org/10.1007/s00464-019-07137-7
Schlottmann F, Buxhoeveden R (2018) Laparoscopic Roux-en-Y Gastric Bypass: surgical technique and tips for success. J Laparoendosc Adv Surg Tech A 28(8):938–943. https://doi.org/10.1089/lap.2018.0393
Nurczyk K, Herbella FA, Patti MG (2020) Roux-en-Y gastric bypass for obesity: how we do it. J Laparoendosc Adv Surg Tech A 30(6):623–626. https://doi.org/10.1089/lap.2020.0156
Di Lorenzo N, Foschi D (2016) Linee guida di chirurgia dell’obesità - Società Italiana di Chirurgia dell’Obesità e delle malattie metaboliche. https://www.sicob.org/00_materiali/linee_guida_2016.pdf. Accessed 15 Mar 2020
Padoin AV, Galvao Neto M, Moretto M, Barancelli F, Schroer CE, Mottin CC (2009) Obese patients with type 2 diabetes submitted to banded gastric bypass: greater incidence of dumping syndrome. Obes Surg 19(11):1481–1484. https://doi.org/10.1007/s11695-009-9943-2
Flegal KM (2005) Epidemiologic aspects of overweight and obesity in the United States. Physiol Behav 86(5):599–602. https://doi.org/10.1016/j.physbeh.2005.08.050
Beckman LM, Beckman TR, Earthman CP (2010) Changes in gastrointestinal hormones and leptin after Roux-en-Y gastric bypass procedure: a review. J Am Diet Assoc 110(4):571–584. https://doi.org/10.1016/j.jada.2009.12.023
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
RDA: conceptualization, investigation, data curation, methodology, writing-original draft. MW: writing-original draft, writing-review and editing. IFG: investigation, data curation. SM: validation, writing-review and editing. DT: conceptualization, validation, data curation, formal analysis, writing-review and editing. VB: conceptualization, validation, supervision, writing-review and editing, project administration.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest (financial or non-financial).
Ethics approval
The ethics committee of Campus Bio-Medico University of Rome approved this study and its development (prot. 9/20). All the procedures performed in the study complied with the ethical standards of the institutional and/or national research committee and the Helsinki Declaration of 1964 and its subsequent amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from all participants prior to enrollment.
Code availability
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
D’Alessio, R., Watanabe, M., Gallo, I.F. et al. The gastro-jejunal anastomosis site influences dumping syndrome and weight regain in patients with obesity undergoing Laparoscopic Roux-en-Y Gastric Bypass. Eat Weight Disord 26, 1871–1880 (2021). https://doi.org/10.1007/s40519-020-01030-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40519-020-01030-2