Abstract
Background
Evidence shows the role of polymorphisms in rs17782313 MC4R gene with increased risk of obesity in Asians adult. In the current report, we investigated the interaction between rs17782313 MC4R gene and major dietary patterns on α-melanocyte stimulating hormone (α-MSH), Agouti-related peptide (AgRP), serum lipids and blood pressure among obese individuals.
Methods
This cross-sectional study was performed in 288 obese adults between 20 and 50 years of age. Anthropometric measurements and biochemical assays were conducted with standard methods. To evaluate appetite, the Visual Analogue Scale (VAS) was used. Dietary patterns were obtained by principal component analysis (PCA). Genotyping of rs17782313 was assessed by restriction fragment length polymorphism (PCR–RFLP) method.
Results
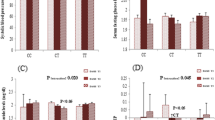
Three major dietary patterns were extracted: Prudent Dietary Pattern (PDP), Legume Dietary Pattern (LDP) and Mixed Dietary Pattern (MDP). Higher PDP score was associated with reduced SBP and insulin concentration while highest MDP score was associated with lower TG concentration (P < 0.05). Significant interactions were observed between higher adherence to PDP and rs17782313 CC genotype on increased SBP (PInteraction = 0.04), serum insulin (PInteraction = 0.05) and AgRP (PInteraction = 0.03) and also between higher adherence to MDP and CC genotype of rs17782313 on reduced serum TG (P = 0.03).
Conclusions
The findings of the current study showed that being on CC genotype of rs17782313 polymorphism made obese individuals more prone to have higher SBP, insulin and AgRP even in highest adherence to PDP. However, adherence to MDP could attenuate the risky effects of being on CC genotype of rs17782313 by reducing serum TG concentrations.
Level of evidence
Level V, cross-sectional descriptive study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity, defined as body mass index (BMI) greater than 30 kg/m2, occurs when energy intake is more than what body requires [1]. Obesity has a growing prevalent worldwide and is considered as a major global health problem and an indispensable risk factor for chronic metabolic diseases [2]. It has been estimated that more than 50% of the Iranian adult are obese or overweight, with a higher proportion of women [3].Obesity is a result of interaction between changeable (e.g. life-style and dietary factors) and unchangeable risk factors (e.g. genotype) [4].
Numerous genes are involved in the energy balance, thermogenesis, hormonal signaling and obesity state [5]. Genome wide association studies (GWASs) have demonstrated several genes associated with obesity including fat mass and obesity associated (FTO), G protein beta 3 (GNB3) [6], melanocortin 4 receptor (MC4R) [7], adiponectin, C1Q [8], leptin [9] and SH2B1 [10] genes. MC4R gene is mainly expressed in the brain regions including areas related to the striatum; modulating melanocortin and dopamine-rich signaling, and also in hypothalamus; the main regulation center of satiety [11, 12]. Moreover, it is also expressed in other metabolically active tissues including pancreas [13] and adipose tissue [14]. MC4R activity is modulated by the effects of agonist α-melanocyte stimulating hormone (α-MSH) and antagonist Agouti-related peptide (AgRP) [14]. MC4R mutations are considered as one of the most common causes of monogenic obesity [15]. Several researches have demonstrated that some single-nucleotide polymorphisms (SNPs) near MC4R (rs17782313, rs17700144, rs571312, and rs2331841) are strongly associated with obesity in different age groups; individuals carrying the minor allele of these variants had a higher risk of being obese [16,17,18,19].
The two well-known MC4R gene SNPs, rs17782313 and rs17700633, have been associated with increased risk of obesity and insulin resistance [20]. Polymorphism of rs17782313 located on chromosome 18q 21.32 at 188-kb downstream of the MC4R gene, has been shown to be strongly correlated with weight adjustment and has a modulating role in MC4R function [21]. According to the previous reports, heterozygous or homozygous carriers of C allele for rs17782313 variant are in greater risk of increased BMI [22,23,24,25], greater preference for the high fat intake, raised snacking behavior [21] and reduced satiety responsiveness [12, 26].
Recently, dietary patterns have attracted a particular attention because of their associations with health and disease. Dietary patterns representing the food preferences of individuals, are influenced by genetic, environmental, cultural, economic and lifestyle factors [27]. Since nutrients in a usual human diet are consumed in combination of each other and not isolate, dietary patterns comprises a real image of usual dietary intake while studies about single nutrients will not have enough information regarding the factual dietary habits of an individual [28]. Numerous studies have been performed regarding the association between obesity-susceptible variants and dietary factors [29,30,31,32]. Both dietary patterns and the genetic map of human is highly influenced by the geographical area and the race of individuals [33]. Since, there was no study evaluating the interaction of rs17782313 gene polymorphism with dietary patterns on metabolic risk factors among Iranian obese individuals; therefore in the current study we aimed to evaluate the interactions of rs17782313 polymorphism with dietary patterns extracted by principal component analysis (PCA) on AgRP, α-MSH, lipid profile and markers of glucose homeostasis among Iranian obese individuals.
Materials and methods
Study population
In the current cross-sectional study, 288 obese adults, ages between 20 and 50 years were enrolled. Participants were apparently healthy volunteers invited by announcements or posters. Obesity was defined as BMI between 30 and 40 kg/m2 and exclusion criteria were: having cardiovascular disease, kidney failure and diabetes, pregnancy, lactation or menopause, being on weight loss regimens or weight reduction more than two kilograms at least 3 months prior to participation in the study, any use of multi-vitamin or mineral supplements, laxative (more than twice a week), medications effective for weight loss (hormonal drugs, antidepressants), diuretics, antibiotic glucocorticoids and any physical or mental disabilities possibly affect the basal metabolic rate. The participants were enrolled after signing written informed consent. The data of the current research has been extracted from two registered protocols of the Tabriz University of Medical Sciences and their protocol have been approved by research undersecretary of Tabriz University of Medical Sciences (identifier: IR.TBZMED.REC.1397.237 and IR.TBZMED.REC.1398.460). The work is obtained from M.S. thesis of Mohaddeseh Mohammadi.
Anthropometric and physical activity measurements
Anthropometric measurements included height, weight, BMI, waist circumference (WC), hip circumference (HC) and waist-to-hip ratio (WHR). Height was measured to the nearest 0.1 cm, by using a stadiometer, while the subjects were in a relaxed position and not wearing shoes. Weight was measured to the nearest 100 g by using digital scale (SECA, Hamburg, Germany) while participants were wearing light clothing and barefoot. BMI was calculated as weight (kg)/height (m)2. The WC was measured by a flexible non-elastic measuring tape in the standing position with a precision of 0.1, midway between the last rib and the ileac crest. Measurement of the HC was also carried out at the widest part of the hips by an un-stretchable meter with an accuracy of 0.1 cm. WHR was calculated as waist circumference divided by hip circumference. Body composition measurements (fat mass and fat free mass) were performed by bioelectrical impedance analysis technology (Tanita, BC-418 MA, Tokyo, Japan).
Biochemical assessment
Systolic and diastolic blood pressure (SBP, DBP) was evaluated in a sitting position using the mercury sphygmomanometer and then average of the two measurements was considered as the blood pressure.
Between 7.30 and 9.00 A.M. blood samples were collected from individuals who had been fasting 12 h. Using a centrifuge, the plasma and serum samples were isolated (4500 rpm, for 10 min at 4 °C) and then frozen at 80 °C until assay. The blood was collected into tubes with ethylene diamine tetra acetic acitable 2d (anticoagulant) for genetic evaluations. Fasting serum glucose (FSG), total cholesterol (TC), triglyceride (TG), and high–density lipoprotein (HDL) were determined by a kit (Pars Azmoon, Tehran, Iran). LDL concentration was calculated according to the Friedewald equation. According to the manufacturer’s instructions, α- MSH and AgRP concentrations were evaluated using the Enzyme-Linked Immuno-Sorbent Assay (ELISA) kits (Bioassay Technology Laboratory, Shanghai Korean Biotech, Shanghai City, China). The minimum levels of detection of α- MSH and AgRP were 5.07 ng/l and 1.03 pg/ml, respectively. Serum insulin level was measured with similar ELIZA kit. Homeostatic model assessment for insulin resistance (HOMA-IR) was calculated according to the formula: fasting insulin (μ IU/ml) × fasting glucose (mmol/l) /22.5.
Appetite and dietary intakes assessments
The Visual Analogue Scale (VAS) was used for appetite assessments, and completed by the experienced expert. VAS score was calculated by placing a vertical mark on a line of 100 mm with opposite phrases (like “I’m not hungry at all” and “I have not been so hungry”) at each end of this line. This questionnaire includes questions about hunger, satiety, fullness, desire to eat sweet/salty/fatty foods and prospective food consumption [34]. Quantification of appetite assessment was performed by measuring the distance from the left side of the line to the mark.
Dietary data was collected using a 132-item semi-quantitative food-frequency questionnaire (FFQ) that was validated and adopted for use in the Iranian population [35]. A trained interviewer asked to participants to report the portion sizes of the consumed foods on a daily, weekly, or monthly basis during the last year. The reported portion sizes were converted to grams by using household measures. Nutrient intake was analyzed using the Iranian Food Composition Table (FCT). To identify dietary patterns, we classified food items into 25 predefined food groups based on the similarity of nutrients (Table 1).
Physical activity assessment
To measure physical activity (PA), a self-administered short form of the International Physical Activity Questionnaire (IPAQ) was used and data were expressed as metabolic equivalent-hours per week [36]. This questionnaire consists of 7 questions to estimate time spent sitting, walking and performing physical activities (moderate to vigorous) over the last week.
Genotyping
Genomic DNA extraction was performed using standard salting-out method [37]. The rs17782313 polymorphism was genotyped by using Polymerase Chain Reaction-Restriction Fragment Length Polymorphism (PCR–RFLP) (Fig. 1). The PCR amplification of DNA fragment containing rs17782313 polymorphism was carried out by a pair of primers: 5′ AAG TTC TAC CTA CCA TGT TCT TGG 3′ as forward primer and 5′ TTC CCC CTG AAG CTT TTC TTGTCA TTT TGA T 3′ as reverse primer (Macro-gene, Korea). PCR reaction was performed in a mixed solution of 200 ng of DNA, 1 μl of each primer, 8.5 μl of distilled water, 15μ of 1 × PCR master-mix (Amplicon; Germany) with a final volume of 25 μl. The amplification protocol contained: primary denaturation stage at 95 °C for 2 min, followed by 35 cycles of denaturation at 95 °C for 30 s, anealing at 58 °C for 30 s, 30 s of extension at 72 °C, and a final extension at 72 °C for 5 min. Digestion was carreid out with 5 μl of each PCR product, 0.5 μl of BclI 10 U/μl (Fermentas, Germany) and 1.5 μl of 10 × restriction G-buffer. The over-night incubation was performed at 58 °C and finally, the reaction was stopped at 0 °C for 5 min. Then electrophoresis was performed on a gel containing 2% agarose (Boehringer Manheim GmbH, Mannheim, Germany) and was visualized on a Gel Doc-system (U.V.P Company, Cambridge, UK), to help safe stain (Pars Tous, Iran). For rs7782313 polymorphism, the uncut fragment was 137 bp (C allele) and digestion products were 30 bp and 107 bp (G allele).
Statistical analysis
To generate major dietary patterns in the present study, principal component analysis with orthogonal rotation procedure was used. Factors were retained for further analysis based on their natural interpretation, eigenvalues more than 1.0, and Scree test [38]. Accordingly after the third factor, the eigenvalues of the factors declined and remained similar to each other. So, these derived factors were considered as major dietary patterns and were labeled according to our interpretation of the data and also previous literature. A factor score for each pattern was obtained by summing of food intakes weighed by their factor loading and each subject then was given a factor score for each identified pattern [39]. We categorized subjects based on tertile of dietary pattern scores. All of the statistical analyses were carried out by statistical package for social science (SPSS) version 23. Normality of data distribution was examined by the Kolmogorov–Smirnov test. Between-group comparison of continuous variables was performed by one-way ANOVA following Tukey’s post hoc analysis. The analysis of co-variance (ANCOVA) was used for adjustment of confounders including age, gender, basal metabolic rate (BMR), and physical activity. The interaction between rs17782313 polymorphism genotypes and tertiles of dietary patterns on biochemical risk factors was assessed by General Linear Model (GLM). The sample size was calculated by using G-power software based on a power of 80%, α-error = 0.05 and correlation coefficient (r) of 0.25. Totally, the final sample size of 288 was considered for the current project.
Results
The participants in this study were 288 adults including 51.1% men and 48.9% women. Mean of age was 37.88 (7.83) and the mean of BMI was 34.72 (3.88). The minor C allele frequency of rs17782313 was 37%. Three main dietary patterns were extracted: Prudent Dietary Pattern (high in vegetables, mayonnaise, beverages, sweets and desserts, red or processed meat, pizza, snacks and low in high-fat dairy and poultry), Legume Dietary Pattern (high in legumes, fruits, spices, vegetable oils, nuts, fish and whole grains and low in sweets and desserts, red or processed meat, French fries, baked potato and refined grains) and Mixed Dietary Patterns (high in refined grains, tea and coffee, hydrogenated fats, French fries, egg, baked potato, salt and pickles and low in high fat dairy, beverages, organ meats, fish, nuts and vegetable oils), which together explained 27% of total variance in dietary intakes. The factor-loading matrixes for the major dietary patterns are presented in Table 1. General characteristics of study participants according to dietary patterns tertiels are shown in Table 2. Participants in the lowest tertile of the Legume Dietary Pattern (LDP) were significantly older (P = 0.02) and had significantly lower appetite (P < 0.001) than those in the highest category. On the other hand, compared with those in the bottom tertile, those in the top tertile of Mixed Dietary Patterns (MDP) were significantly less physically active (P = 0.04), and significantly had higher VAS score and percent of fat mass (P < 0.001). Conversely, higher scores of Prudent Dietary Pattern (PDP) were also in association with reduced waist circumference and increased physical activity levels. No significant difference was found in other general characteristics of participants across tertiles of dietary patterns. Table 3 presents metabolic parameters of participants by different dietary patterns tertiles. Higher PDP score were accompanied with lower SBP (P < 0.001), insulin (P = 0.04) and AgRP (P = 0.02) concentrations and also lower HOMA-IR values (P = 0.04). Even after adjusting for potential confounding variables, these associations remained statistically significant (P < 0.05 for all of the mentioned parameters). Moreover, higher MDP score was associated with lower TG concentration (P = 0.03). This significant result remained unchanged after adjustment for potential confounders (P = 0.03). There was no statistical significant difference regarding metabolic parameters across rs17782313 genotypes (Table 4).
The significant interaction effects between rs17782313 genotypes and dietary patterns on metabolic parameters using GLM method are shown in Fig. 2. There were significant interactions between PDP and rs17782313 SNP on SBP (pInteraction = 0.04), insulin (pInteraction = 0.05) and AgRP (pInteraction = 0.03) concentrations. Furthermore, rs17782313 genotypes and MDP interacted in changing in the serum TG levels (pInteraction = 0.03). In other word, those in CC genotype of rs17782313 were more likely to have higher SBP, insulin and AgRP concentrations compared with other genotype groups (i.e. TT and TC) when their adherence to PDP was high. As well as, when adherence to the MDP was low, those with CC genotype of rs17782313 were more likely to have higher TG concentrations than other genotypes.
Discussion
In this present cross-sectional study, three major dietary patterns were identified using principal components factor analysis: prudent, legume and mixed dietary patterns. Numerous studies in Iran have identified several dietary patterns including western, healthy and traditional Iranian dietary patterns in different populations [4, 38, 40]. These researches have indicated adverse effects of western and unhealthy dietary patterns on obesity, metabolic syndrome, inflammation and other cardiovascular risk factors [38, 41]. In current study, adherence to PDP (characterized by high intake of vegetables, mayonnaise, beverages, sweets and desserts, red or processed meat, pizza, snacks and low intake of high-fat dairy and poultry) was associated with low SBP, insulin, HOMA-IR and AgRP. The associations observed in this regard are similar to previous findings in relation to PDP and cardio-metabolic risk. In a cross-sectional study in an Iranian population [42], prudent PDP characterized by high consumption of peas, honey, nuts, juice, dry fruits, vegetable oil and liver and organic meat was positively associated with increased HDL-C and lower glucose levels. These observed desirable associations might be due to high loading factors of healthy foods such as vegetables in this dietary pattern. A similar association was reported by Yu et al. and Nkondjock et al. and they found an inverse association between the healthy dietary pattern (high in vegetables, fruits, fish, eggs, milk and nuts) and the risk of hypertension [43, 44]. On the other hand, since Iranian people traditionally consume more full-fat dairy than low fat dairy products, low-loading factors of dairy products in PDP might neutralize the adverse effects of harmful foods such as sweets and desserts, red or processed meat and pizza were loaded in this pattern. In this regard, lack of protective effects of high fat dairy products intake in relation to metabolic syndrome have shown in several investigations [45]. As well as, vegetarian dietary pattern was reported to be related to lower insulin resistance in a population of Asian Indians [46]. The high fiber, phytochemicals and antioxidant content of fruits and vegetables may mediate beneficial effects of this dietary pattern on the cardiovascular risk factors [47,48,49]. Moreover, we observed an inverse relation between MDP and serum concentrations of TG. It seems that beneficial effects of MDP on TG could be due to its high content of tea and coffee and lower consumption of harmful food like organ meats and high fat dairy products. Growing evidence from observational studies has reported the beneficial effects of tea and coffee consumption on human health [50, 51]. Previous meta-analysis of observational studies showed that intake of ≥ 3 cups of green or black tea a day has protective effects on CVD and myocardial infarction [52]. In an another study by Fujita et al. black tea users had significantly lower serum TG concentrations [53]. Caffeine and other bioactive compounds like polyphenols are commonly found in tea and coffee may mediate beneficial effects of these drinks on the risk markers through anti-inflammatory, anti-oxidant [54] or anti-thrombotic properties [55]. Furthermore, it is suggested that these phytochemicals exert their protective effects on CVD by improving endothelial function and glucose metabolism and also oxidative stress inhibition [55, 56]. Accordingly, low-loading factor of high cholesterol and saturated and trans fatty acids foods (organ meats and high fat dairy products) in MDP might attenuate the adverse effects of the high-loaded items like refined grain and French fries. There is considerable evidence that saturated fat and cholesterol are frequently related to unfavorable health outcomes [57, 58]. For instance, saturated fatty acids, which are associated with higher total cholesterol and low density lipoprotein levels, are known as independent predictor of CVDs [59]. Moreover, experimental investigations using high fat meals have suggested that high fat content of diet might change expression and function of MC4R gene through effect on DNA methylation status [60]. So, low intake of unhealthy constitutes beside high coffee and tea consumption in this pattern may contribute to this findings. Although we did not detect any association between metabolic parameters and rs17782313 genotypes, several evidences have reported higher risk of obesity and its-related metabolic factors in carriers of MC4R rs17782313 minor allele (C) [10, 18, 60]. For instance, Yang et al. and Qi et al. demonstrated that rs17782313 polymorphism is associated with a higher risk of metabolic syndrome and type 2 diabetes, respectively [61, 62].
Noticeably, we found interaction effects between rs17782313 genotypes and dietary patterns in changing metabolic parameters. We detected significant interactions between PDP and rs17782313 SNP on SBP, insulin and AgRP levels. Moreover, adherence to MDP interacts with the MC4R rs17782313 variant to influence on TG levels. In other word, those in CC genotype of rs17782313 were more likely to have higher TG concentrations compared with other genotype groups (i.e. TT and TC) when their adherence to MDP was low. Since experimental studies in mice and rats have indicated that a high-fat diet could enhance MC4R gene expression and lead to weight gain [63], reported interactions between MC4R gene and dietary pattern is not surprising. Despite the reports about the interactions between this SNP and lifestyle factors such as dietary patterns are scarce; Azorín et al. reported that adherence to Mediterranean diet might reduce the risk of diabetes in carriers of the minor allele of MC4R rs17782313 [64]. Similar interactions between dietary intake and MC4R SNPs were found in another study, in which higher intakes of saturated fatty acid and western dietary pattern were associated with higher risk of metabolic syndrome among A allele carriers of the rs12970134 MC4R gene [32]. The Precise and underlying mechanisms behind these interactions remain unclear and further studies are needed to explain these observed interactions. However, some of the studies have suggested an association between rs17782313 variant and dietary intakes. According to report of Park et al., C allele carriers of rs17782313 compared to TT genotype, had a higher intake of processed foods and a lower intake of fruits [32, 65].
Other finding of our study was that we found a positive association between LDP and appetite; those in upper tertile of this pattern had higher mean of VAS score. Even though previous investigations have shown beneficial effect of legume-based diets on short-term satiety and energy intake, there was evidence that suggested legume intake might not reduce appetite when meals were controlled for available carbohydrate [66]. In present study, high loading factor of fruits in LDP without any energy restriction, which is commonly observed in obese subjects, might attenuate satiety effects of legumes in this pattern. Despite the growing evidence supports main role common variants near MC4R in relation to obesity and its-related metabolic traits, exact mechanisms behind this action are still not clear. Functional studies have shown that MC4R may contribute to glucose production and utilization, insulin sensitivity [13, 14, 67] and secretion and also lipid metabolism [68]. Thus, alterations in MC4R could lead to insulin resistance and type2 diabetes independent of hyperphagia and obesity. There is evidence that suggest that the effects of rs17782313 on MC4R gene function may be mediated by interference in expression and translation regulation [69,70,71,72]. So, it’s not surprising that MC4R rs17782313 variant may through effect on appetite regulation and energy homeostasis be associated with weight change and obesity-related diseases [21, 60, 61, 73,74,75]. Figure 3 presents the mechanistic pathways of these associations.
To the best of our knowledge, this is the first study to investigate the specific interaction of rs17782313 MC4R gene with dietary pattern in changing metabolic risk factors and hypothalamic hormones. However, there are several potential limitations that should be taken into account. The cross-sectional design of our study makes causal inferences impossible and further studies with longitudinal designs are required to confirm and better understanding of the findings. Moreover, since we just included participants from Tabriz, a city in North-west of Iran, our finding cannot be extrapolated to all Iranian population.
In conclusion, our results indicated significant interactions between dietary patterns and rs17782313 MC4R gene in relation to cardio-metabolic risk factors and hypothalamic-pituitary axis hormones; higher adherence to MDP by CC homozygote carriers was associated with lower TG concentrations. On the other hand, we observed significant gene-PDP interactions in relation to SBP, insulin, HOMA-IR and AgRP. Further studies are warranted to confirm these results.
What is already known on this subject?
Previous studies revealed the role of polymorphisms in rs17782313 MC4R gene with increased risk of obesity and insulin resistance. It has been shown that the polymorphisms in this gene has been is strongly correlated with weight adjustment. While, no study is available evaluating the association between rs17782313 MC4R genotypes with dietary patterns or eating behaviors in obese individuals.
What does this study add?
The current study, for the first time, evaluated the rs17782313 MC4R gene polymorphisms with the dietary patterns and the interaction of its genotype with dietary patterns to affect metabolic risk factors and hypothalamus-pituitary axis hormones in obesity. This is the novelty of the current study.
References
Thorleifsson G, Walters GB, Gudbjartsson DF, Steinthorsdottir V, Sulem P, Helgadottir A, Styrkarsdottir U, Gretarsdottir S, Thorlacius S, Jonsdottir I, Jonsdottir T, Olafsdottir EJ, Olafsdottir GH, Jonsson T, Jonsson F, Borch-Johnsen K, Hansen T, Andersen G, Jorgensen T, Lauritzen T, Aben KK, Verbeek AL, Roeleveld N, Kampman E, Yanek LR, Becker LC, Tryggvadottir L, Rafnar T, Becker DM, Gulcher J, Kiemeney LA, Pedersen O, Kong A, Thorsteinsdottir U, Stefansson K (2009) Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat Genet 41(1):18–24. https://doi.org/10.1038/ng.274
Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH (2006) Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 113(6):898–918. https://doi.org/10.1161/circulationaha.106.171016
Mirzazadeh A, Sadeghirad B, Haghdoust A, Bahreyni F, Rezazadeh KM (2009) The prevalence of obesity in Iran in recent decade; a systematic review and meta-analysis study. Iran J Public Health 38:1–11
Esmaillzadeh A, Azadbakht L (2008) Major dietary patterns in relation to general obesity and central adiposity among Iranian women. J Nutr 138(2):358–363. https://doi.org/10.1093/jn/138.2.358
Marcadenti A, Fuchs FD, Matte U, Sperb F, Moreira LB, Fuchs SC (2013) Effects of FTO RS9939906 and MC4R RS17782313 on obesity, type 2 diabetes mellitus and blood pressure in patients with hypertension. Cardiovasc diabetol 12:103. https://doi.org/10.1186/1475-2840-12-103
Moselhy SS, Alhetari YA, Iyer A, Huwait EA, Al-Ghamdi MA, Al-Ghamdi S, Balamash KS, Basuni AA, Alama MN, Kumosani TA, Yaghmoor SS (2017) Analysis of SNPs of MC4R, GNB3 and FTO gene polymorphism in obese Saudi subjects. Afr Health Sci 17(4):1059–1069. https://doi.org/10.4314/ahs.v17i4.14
Grant SF, Bradfield JP, Zhang H, Wang K, Kim CE, Annaiah K, Santa E, Glessner JT, Thomas K, Garris M, Frackelton EC, Otieno FG, Shaner JL, Smith RM, Imielinski M, Chiavacci RM, Li M, Berkowitz RI, Hakonarson H (2009) Investigation of the locus near MC4R with childhood obesity in Americans of European and African ancestry. Obesity (Silver Spring, Md) 17(7):1461–1465. https://doi.org/10.1038/oby.2009.53
Lu JF, Zhou Y, Huang GH, Jiang HX, Hu BL, Qin SY (2014) Association of ADIPOQ polymorphisms with obesity risk: a meta-analysis. Hum Immunol 75(10):1062–1068. https://doi.org/10.1016/j.humimm.2014.09.004
Zhang L, Yuan LH, Xiao Y, Lu MY, Zhang LJ, Wang Y (2014) Association of leptin gene-2548 G/A polymorphism with obesity: a meta-analysis. Ann Nutr Metab 64(2):127–136. https://doi.org/10.1159/000363392
McCaffery JM, Papandonatos GD, Peter I, Huggins GS, Raynor HA, Delahanty LM, Cheskin LJ, Balasubramanyam A, Wagenknecht LE, Wing RR (2012) Obesity susceptibility loci and dietary intake in the look AHEAD trial. Am J Clin Nutr 95(6):1477–1486. https://doi.org/10.3945/ajcn.111.026955
Horstmann A, Kovacs P, Kabisch S, Boettcher Y, Schloegl H, Tonjes A, Stumvoll M, Pleger B, Villringer A (2013) Common genetic variation near MC4R has a sex-specific impact on human brain structure and eating behavior. PLoS One 8(9):e74362. https://doi.org/10.1371/journal.pone.0074362
Sun Y, Sun J, Wu J, Yang M (2016) Combined effects of FTO rs9939609 and MC4R rs17782313 on elevated nocturnal blood pressure in the Chinese Han population. Cardiovasc J Afr 27(1):21–24. https://doi.org/10.5830/cvja-2015-064
Mansour M, White D, Wernette C, Dennis J, Tao YX, Collins R, Parker L, Morrison E (2010) Pancreatic neuronal melanocortin-4 receptor modulates serum insulin levels independent of leptin receptor. Endocrine 37(1):220–230. https://doi.org/10.1007/s12020-009-9289-5
Tao YX (2010) The melanocortin-4 receptor: physiology, pharmacology, and pathophysiology. Endocr Rev 31(4):506–543. https://doi.org/10.1210/er.2009-0037
Larsen LH, Echwald SM, Sorensen TI, Andersen T, Wulff BS, Pedersen O (2005) Prevalence of mutations and functional analyses of melanocortin 4 receptor variants identified among 750 men with juvenile-onset obesity. J Clin Endocrinol Metab 90(1):219–224. https://doi.org/10.1210/jc.2004-0497
den Hoed M, Ekelund U, Brage S, Grontved A, Zhao JH, Sharp SJ, Ong KK, Wareham NJ, Loos RJ (2010) Genetic susceptibility to obesity and related traits in childhood and adolescence: influence of loci identified by genome-wide association studies. Diabetes 59(11):2980–2988. https://doi.org/10.2337/db10-0370
Loos RJ, Lindgren CM, Li S, Wheeler E, Zhao JH, Prokopenko I, Inouye M, Freathy RM, Attwood AP, Beckmann JS, Berndt SI, Jacobs KB, Chanock SJ, Hayes RB, Bergmann S, Bennett AJ, Bingham SA, Bochud M, Brown M, Cauchi S, Connell JM, Cooper C, Smith GD, Day I, Dina C, De S, Dermitzakis ET, Doney AS, Elliott KS, Elliott P, Evans DM, Sadaf Farooqi I, Froguel P, Ghori J, Groves CJ, Gwilliam R, Hadley D, Hall AS, Hattersley AT, Hebebrand J, Heid IM, Lamina C, Gieger C, Illig T, Meitinger T, Wichmann HE, Herrera B, Hinney A, Hunt SE, Jarvelin MR, Johnson T, Jolley JD, Karpe F, Keniry A, Khaw KT, Luben RN, Mangino M, Marchini J, McArdle WL, McGinnis R, Meyre D, Munroe PB, Morris AD, Ness AR, Neville MJ, Nica AC, Ong KK, O’Rahilly S, Owen KR, Palmer CN, Papadakis K, Potter S, Pouta A, Qi L, Randall JC, Rayner NW, Ring SM, Sandhu MS, Scherag A, Sims MA, Song K, Soranzo N, Speliotes EK, Syddall HE, Teichmann SA, Timpson NJ, Tobias JH, Uda M, Vogel CI, Wallace C, Waterworth DM, Weedon MN, Willer CJ, Wraight Yuan X, Zeggini E, Hirschhorn JN, Strachan DP, Ouwehand WH, Caulfield MJ, Samani NJ, Frayling TM, Vollenweider P, Waeber G, Mooser V, Deloukas P, McCarthy MI, Wareham NJ, Barroso I, Jacobs KB, Chanock SJ, Hayes RB, Lamina C, Gieger C, Illig T, Meitinger T, Wichmann HE, Kraft P, Hankinson SE, Hunter DJ, Hu FB, Lyon HN, Voight BF, Ridderstrale M, Groop L, Scheet P, Sanna S, Abecasis GR, Albai G, Nagaraja R, Schlessinger D, Jackson AU, Tuomilehto J, Collins FS, Boehnke M, Mohlke KL (2008) Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat Genet 40(6):768–775. https://doi.org/10.1038/ng.140
Scherag A, Dina C, Hinney A, Vatin V, Scherag S, Vogel CI, Muller TD, Grallert H, Wichmann HE, Balkau B, Heude B, Jarvelin MR, Hartikainen AL, Levy-Marchal C, Weill J, Delplanque J, Korner A, Kiess W, Kovacs P, Rayner NW, Prokopenko I, McCarthy MI, Schafer H, Jarick I, Boeing H, Fisher E, Reinehr T, Heinrich J, Rzehak P, Berdel D, Borte M, Biebermann H, Krude H, Rosskopf D, Rimmbach C, Rief W, Fromme T, Klingenspor M, Schurmann A, Schulz N, Nothen MM, Muhleisen TW, Erbel R, Jockel KH, Moebus S, Boes T, Illig T, Froguel P, Hebebrand J, Meyre D (2010) Two new Loci for body-weight regulation identified in a joint analysis of genome-wide association studies for early-onset extreme obesity in French and german study groups. PLoS Genet 6(4):e1000916. https://doi.org/10.1371/journal.pgen.1000916
Okada Y, Sim X, Go MJ, Wu JY, Gu D, Takeuchi F, Takahashi A, Maeda S, Tsunoda T, Chen P, Lim SC, Wong TY, Liu J, Young TL, Aung T, Seielstad M, Teo YY, Kim YJ, Lee JY, Han BG, Kang D, Chen CH, Tsai FJ, Chang LC, Fann SJ, Mei H, Rao DC, Hixson JE, Chen S, Katsuya T, Isono M, Ogihara T, Chambers JC, Zhang W, Kooner JS, Albrecht E, Yamamoto K, Kubo M, Nakamura Y, Kamatani N, Kato N, He J, Chen YT, Cho YS, Tai ES, Tanaka T (2012) Meta-analysis identifies multiple loci associated with kidney function-related traits in east Asian populations. Nat Genet 44(8):904–909. https://doi.org/10.1038/ng.2352
Chambers JC, Elliott P, Zabaneh D, Zhang W, Li Y, Froguel P, Balding D, Scott J, Kooner JS (2008) Common genetic variation near MC4R is associated with waist circumference and insulin resistance. Nat Genet 40(6):716–718. https://doi.org/10.1038/ng.156
Stutzmann F, Cauchi S, Durand E, Calvacanti-Proenca C, Pigeyre M, Hartikainen AL, Sovio U, Tichet J, Marre M, Weill J, Balkau B, Potoczna N, Laitinen J, Elliott P, Jarvelin MR, Meyre D, Horber F, Froguel P (2005) Common genetic variation near MC4R is associated with eating behaviour patterns in European populations. Int J Obes 33(3):373–378. https://doi.org/10.1038/ijo.2008.279
Hong J, Shi J, Qi L, Cui B, Gu W, Zhang Y, Li L, Xu M, Wang L, Zhai Y, Miao L, Wang R, Bi Y, Wang W, Ning G (2013) Genetic susceptibility, birth weight and obesity risk in young Chinese. Int J Obes 37(5):673–677. https://doi.org/10.1038/ijo.2012.87
Kochetova OV, Korytina GF, Akhmadishina LZ, Semenov EE, Viktorova TV (2015) Association of polymorphic variants of FTO and MC4R genes with obesity in a Tatar population. Genetika 51(2):248–255
Hong KW, Oh B (2012) Recapitulation of genome-wide association studies on body mass index in the Korean population. Int J Obes 36(8):1127–1130. https://doi.org/10.1038/ijo.2011.202
Sull JW, Lee M, Jee SH (2013) Replication of genetic effects of MC4R polymorphisms on body mass index in a Korean population. Endocrine 44(3):675–679. https://doi.org/10.1007/s12020-013-9909-y
Liu G, Zhu H, Lagou V, Gutin B, Barbeau P, Treiber FA, Dong Y, Snieder H (2010) Common variants near melanocortin 4 receptor are associated with general and visceral adiposity in European- and African-American youth. J Pediatr 156(4):598.e591–605.e591. https://doi.org/10.1016/j.jpeds.2009.10.037
Maskarinec G, Novotny R, Tasaki K (2000) Dietary patterns are associated with body mass index in multiethnic women. J Nutr 130(12):3068–3072. https://doi.org/10.1093/jn/130.12.3068
Hebebrand J, Volckmar AL, Knoll N, Hinney A (2010) Chipping away the ‘missing heritability’: GIANT steps forward in the molecular elucidation of obesity—but still lots to go. Obes Facts 3(5):294–303. https://doi.org/10.1159/000321537
Koochakpoor G, Mirmiran P, Daneshpour MS, Hosseini-Esfahani F, Sedaghati-Khayat B, Hosseini SA, Azizi F (2018) Dietary factors influence the association of cyclin D2 polymorphism rs11063069 with the risk of metabolic syndrome. Nutr Res (New York, NY) 52:48–56. https://doi.org/10.1016/j.nutres.2017.12.006
Mollahosseini M, Rahimi MH, Yekaninejad MS, Maghbooli Z, Mirzaei K (2019) Dietary patterns interact with chromosome 9p21 rs1333048 polymorphism on the risk of obesity and cardiovascular risk factors in apparently healthy Tehrani adults. Eur J Nutr 2:1–9. https://doi.org/10.1007/s00394-018-1872-1
Otto MC, Padhye NS, Bertoni AG, Jacobs DR Jr, Mozaffarian D (2015) Everything in moderation-dietary diversity and quality, central obesity and risk of diabetes. PLoS One 10(10):e0141341. https://doi.org/10.1371/journal.pone.0141341
Koochakpoor G, Daneshpour MS, Mirmiran P, Hosseini SA, Hosseini-Esfahani F, Sedaghatikhayat B, Azizi F (2016) The effect of interaction between Melanocortin-4 receptor polymorphism and dietary factors on the risk of metabolic syndrome. Nutr Metab 13:35. https://doi.org/10.1186/s12986-016-0092-z
Xi B, Takeuchi F, Chandak GR, Kato N, Pan HW, Zhou DH, Pan HY, Mi J (2012) Common polymorphism near the MC4R gene is associated with type 2 diabetes: data from a meta-analysis of 123,373 individuals. Diabetologia 55(10):2660–2666. https://doi.org/10.1007/s00125-012-2655-5
Flint A, Raben A, Blundell JE, Astrup A (2000) Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord 24(1):38–48. https://doi.org/10.1038/sj.ijo.0801083
Ebrahimi-Mameghani M, Behroozi-Fared-Mogaddam A, Asghari-Jafarabadi M (2014) Assessing the reliability and reproducibility of food frequency questionnaire and identify major dietary patterns in overweight and obese adults in Tabriz, Iran. J Mazandaran Univ Med Sci 23:45–57
Booth M (2000) Assessment of physical activity: an international perspective. Res Q Exerc Sport 71(2 Suppl):S114–S120
Truett GE, Heeger P, Mynatt RL, Truett AA, Walker JA and Warman ML (2000) Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT). BioTechniques 29(1):52–54. https://doi.org/10.2144/00291bm09
Esmaillzadeh A, Kimiagar M, Mehrabi Y, Azadbakht L, Hu FB, Willett WC (2007) Dietary patterns, insulin resistance, and prevalence of the metabolic syndrome in women. Am J Clin Nutr 85(3):910–918. https://doi.org/10.1093/ajcn/85.3.910
Kim J-O, Mueller CW (1978) Factor analysis: statistical methods and practical issues. trans, vol., vol 14, edn. sage
Esmaillzadeh A, Azadbakht L (2011) Dietary energy density and the metabolic syndrome among Iranian women. Eur J Clin Nutr 65(5):598–605. https://doi.org/10.1038/ejcn.2010.284
Esmaillzadeh A, Kimiagar M, Mehrabi Y, Azadbakht L, Hu FB, Willett WC (2007) Dietary patterns and markers of systemic inflammation among Iranian women. J Nutr 137(4):992–998. https://doi.org/10.1093/jn/137.4.992
Amini M, Esmaillzadeh A, Shafaeizadeh S, Behrooz J, Zare M (2010) Relationship between major dietary patterns and metabolic syndrome among individuals with impaired glucose tolerance. Nutrition (Burbank, Los Angeles County, Calif) 26(10):986–992. https://doi.org/10.1016/j.nut.2010.03.006
Yu G, Fu H, Huang W, Zhang N, Deng D, Li G, Lei H (2018) A dietary pattern of higher fish, egg, milk, nut, vegetable and fruit, and lower salt intake correlates with the prevalence and control of hypertension. Am J Hypertens 31(6):679–686. https://doi.org/10.1093/ajh/hpy005
Nkondjock A, Bizome E (2010) Dietary patterns associated with hypertension prevalence in the Cameroon defence forces. Eur J Clin Nutr 64(9):1014–1021. https://doi.org/10.1038/ejcn.2010.109
Nikniaz L, Nikniaz Z, Sadeghi-Bazargani H, Abdollahi HM and Farhangi MA (2019) Association between major dietary patterns and metabolic syndrome components: a population-based study from north-west of Iran. Int J Diabetes Dev C 1–9
Gadgil MD, Anderson CA, Kandula NR, Kanaya AM (2014) Dietary patterns in Asian Indians in the United States: an analysis of the metabolic syndrome and atherosclerosis in South Asians Living in America study. J Acad Nutr Diet 114(2):238–243. https://doi.org/10.1016/j.jand.2013.09.021
Touyz RM, Schiffrin EL (2004) Reactive oxygen species in vascular biology: implications in hypertension. Histochem Cell Biol 122(4):339–352. https://doi.org/10.1007/s00418-004-0696-7
Erdman JW Jr, Balentine D, Arab L, Beecher G, Dwyer JT, Folts J, Harnly J, Hollman P, Keen CL, Mazza G, Messina M, Scalbert A, Vita J, Williamson G, Burrowes J (2007) Flavonoids and heart health: proceedings of the ILSI North America Flavonoids Workshop, May 31–June 1, 2005, Washington, DC. J Nutr 137(3 Suppl 1):718s–737s. https://doi.org/10.1093/jn/137.3.718S
Touyz RM (2003) Reactive oxygen species in vascular biology: role in arterial hypertension. Expert Rev Cardiovasc Ther 1(1):91–106. https://doi.org/10.1586/14779072.1.1.91
Di Lorenzo A, Curti V, Tenore GC, Nabavi SM, Daglia M (2017) Effects of tea and coffee consumption on cardiovascular diseases and relative risk factors: an update. Curr Pharm Des 23(17):2474–2487. https://doi.org/10.2174/1381612823666170215145855
Mineharu Y, Koizumi A, Wada Y, Iso H, Watanabe Y, Date C, Yamamoto A, Kikuchi S, Inaba Y, Toyoshima H, Kondo T, Tamakoshi A (2011) Coffee, green tea, black tea and oolong tea consumption and risk of mortality from cardiovascular disease in Japanese men and women. J Epidemiol Community Health 65(3):230–240. https://doi.org/10.1136/jech.2009.097311
Wang ZM, Zhou B, Wang YS, Gong QY, Wang QM, Yan JJ, Gao W, Wang LS (2011) Black and green tea consumption and the risk of coronary artery disease: a meta-analysis. Am J Clin Nutr 93(3):506–515. https://doi.org/10.3945/ajcn.110.005363
Fujita H, Yamagami T (2008) Antihypercholesterolemic effect of Chinese black tea extract in human subjects with borderline hypercholesterolemia. Nutr Res (New York, NY) 28(7):450–456. https://doi.org/10.1016/j.nutres.2008.04.005
Gardner EJ, Ruxton CH, Leeds AR (2007) Black tea–helpful or harmful? A review of the evidence. Eur J Clin Nutr 61(1):3–18. https://doi.org/10.1038/sj.ejcn.1602489
Quinones M, Miguel M, Aleixandre A (2013) Beneficial effects of polyphenols on cardiovascular disease. Pharmacol Res 68(1):125–131. https://doi.org/10.1016/j.phrs.2012.10.018
Gaeini Z, Bahadoran Z, Mirmiran P, Azizi F (2019) Tea, coffee, caffeine intake and the risk of cardio-metabolic outcomes: findings from a population with low coffee and high tea consumption. Nutr Metab 16:28. https://doi.org/10.1186/s12986-019-0355-6
Jakobsen MU, O’Reilly EJ, Heitmann BL, Pereira MA, Balter K, Fraser GE, Goldbourt U, Hallmans G, Knekt P, Liu S, Pietinen P, Spiegelman D, Stevens J, Virtamo J, Willett WC, Ascherio A (2009) Major types of dietary fat and risk of coronary heart disease: a pooled analysis of 11 cohort studies. Am J Clin Nutr 89(5):1425–1432. https://doi.org/10.3945/ajcn.2008.27124
Mozaffarian D, Micha R, Wallace S (2010) Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLoS Med 7(3):e1000252. https://doi.org/10.1371/journal.pmed.1000252
Widiker S, Karst S, Wagener A, Brockmann GA (2010) High-fat diet leads to a decreased methylation of the Mc4r gene in the obese BFMI and the lean B6 mouse lines. J Appl Genet 51(2):193–197. https://doi.org/10.1007/bf03195727
Xi B, Chandak GR, Shen Y, Wang Q, Zhou D (2012) Association between common polymorphism near the MC4R gene and obesity risk: a systematic review and meta-analysis. PLoS One 7(9):e45731. https://doi.org/10.1371/journal.pone.0045731
Qi L, Kraft P, Hunter DJ, Hu FB (2008) The common obesity variant near MC4R gene is associated with higher intakes of total energy and dietary fat, weight change and diabetes risk in women. Hum Mol Genet 17(22):3502–3508. https://doi.org/10.1093/hmg/ddn242
Yang CW, Li CI, Liu CS, Bau DT, Lin CH, Lin WY, Li TC, Lin CC (2013) The joint effect of cigarette smoking and polymorphisms on LRP5, LEPR, near MC4R and SH2B1 genes on metabolic syndrome susceptibility in Taiwan. Mol Biol Rep 40(1):525–533. https://doi.org/10.1007/s11033-012-2089-7
Butler AA, Cone RD (2003) Knockout studies defining different roles for melanocortin receptors in energy homeostasis. Ann N Y Acad Sci 994:240–245. https://doi.org/10.1111/j.1749-6632.2003.tb03186.x
Ortega-Azorin C, Sorli JV, Asensio EM, Coltell O, Martinez-Gonzalez MA, Salas-Salvado J, Covas MI, Aros F, Lapetra J, Serra-Majem L, Gomez-Gracia E, Fiol M, Saez-Tormo G, Pinto X, Munoz MA, Ros E, Ordovas JM, Estruch R, Corella D (2012) Associations of the FTO rs9939609 and the MC4R rs17782313 polymorphisms with type 2 diabetes are modulated by diet, being higher when adherence to the Mediterranean diet pattern is low. Cardiovasc Diabetol 11:137. https://doi.org/10.1186/1475-2840-11-137
Park S, Daily JW, Zhang X, Jin HS, Lee HJ, Lee YH (2016) Interactions with the MC4R rs17782313 variant, mental stress and energy intake and the risk of obesity in Genome Epidemiology Study. Nutr Metab 13:38. https://doi.org/10.1186/s12986-016-0096-8
McCrory MA, Hamaker BR, Lovejoy JC, Eichelsdoerfer PE (2010) Pulse consumption, satiety, and weight management. Adv Nutr 1(1):17–30. https://doi.org/10.3945/an.110.1006
Loos RJ (2011) The genetic epidemiology of melanocortin 4 receptor variants. Eur J Pharmacol 660(1):156–164. https://doi.org/10.1016/j.ejphar.2011.01.033
Katsuura-Kamano S, Uemura H, Arisawa K, Yamaguchi M, Hamajima N, Wakai K, Okada R, Suzuki S, Taguchi N, Kita Y, Ohnaka K, Kairupan TS, Matsui D, Oze I, Mikami H, Kubo M, Tanaka H (2014) A polymorphism near MC4R gene (rs17782313) is associated with serum triglyceride levels in the general Japanese population: the J-MICC Study. Endocrine 47(1):81–89. https://doi.org/10.1007/s12020-014-0306-y
Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB (2005) Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 123(3):493–505. https://doi.org/10.1016/j.cell.2005.08.035
Fan W, Ellacott KL, Halatchev IG, Takahashi K, Yu P, Cone RD (2004) Cholecystokinin-mediated suppression of feeding involves the brainstem melanocortin system. Nat Neurosci 7(4):335–336. https://doi.org/10.1038/nn1214
Gautron L, Lee C, Funahashi H, Friedman J, Lee S, Elmquist J (2010) Melanocortin-4 receptor expression in a vago-vagal circuitry involved in postprandial functions. J Com Neurol 518(1):6–24. https://doi.org/10.1002/cne.22221
Wan S, Browning KN, Coleman FH, Sutton G, Zheng H, Butler A, Berthoud HR, Travagli RA (2008) Presynaptic melanocortin-4 receptors on vagal afferent fibers modulate the excitability of rat nucleus tractus solitarius neurons. J Neurosci 28(19):4957–4966. https://doi.org/10.1523/jneurosci.5398-07.2008
Acosta A, Camilleri M, Shin A, Carlson P, Burton D, O’Neill J, Eckert D, Zinsmeister AR (2014) Association of melanocortin 4 receptor gene variation with satiation and gastric emptying in overweight and obese adults. Genes Nutr 9(2):384. https://doi.org/10.1007/s12263-014-0384-8
Beckers S, Zegers D, de Freitas F, Mertens IL, Van Gaal LF, Van Hul W (2011) Association study of MC4R with complex obesity and replication of the rs17782313 association signal. Mol Genet Metab 103(1):71–75. https://doi.org/10.1016/j.ymgme.2011.01.007
Vogel CI, Boes T, Reinehr T, Roth CL, Scherag S, Scherag A, Hebebrand J, Hinney A (2011) Common variants near MC4R: exploring gender effects in overweight and obese children and adolescents participating in a lifestyle intervention. Obes Facts 4(1):67–75. https://doi.org/10.1159/000324557
Acknowledgements
The authors thank the participants of the current study. The data of the current study has been extracted from two registered protocol of the Tabriz University of Medical Sciences and their protocol has been approved by research undersecretary of Tabriz University of Medical Sciences (identifier: IR.TBZMED.REC.1397.237 and IR.TBZMED.REC.1398.460). The work is obtained from M.S. thesis of Mohaddeseh Mohammadi.
Funding
This study was supported by funding from Tabriz University of Medical Sciences (Grant number: IR.TBZMED.REC.1397.237 and IR.TBZMED.REC.1398.460).
Author information
Authors and Affiliations
Contributions
MM contributed to data collection and primary manuscript writing. MK assisted with data collection and also critically revised the manuscript and approved the version to be published. HK performed the genotyping analysis. MA designed the study and served as a supervisor for this research.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethics approval
Study protocol was approved by the ethics committee of Tabriz University of Medical Sciences (Registration code: IR.TBZMED.REC.1397.237 and IR.TBZMED.REC.1398.460) and all participants provided and signed written informed consent.
Informed consent
Informed written consents were obtained from all the candidates who were willing to participate in the study and they were ensured that they could withdraw from the study at any time they wish.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mohammadi, M., Khodarahmi, M., Kahroba, H. et al. Dietary patterns interact with the variations of 18q21.23 rs17782313 locus on regulation of hypothalamic-pituitary axis hormones and cardio-metabolic risk factors in obesity. Eat Weight Disord 25, 1447–1459 (2020). https://doi.org/10.1007/s40519-020-00855-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40519-020-00855-1