Abstract
Purpose
Asprosin is a centrally acting appetite-promoting hormone and promotes glucose production in the liver. This study is the first to investigate the difference in asprosin in the plasma between anorexia nervosa (AN) and healthy controls, and to explore the relationship between asprosin changes and plasma glucose levels and AN symptoms.
Methods
Plasma asprosin and glucose concentrations were detected in AN patients (n = 46) and healthy control subjects (n = 47). Eating Disorder Inventory-2 (EDI-2) was used to assess subjects’ eating disorder symptoms and related personality traits. The patient’s concomitant levels of depression and anxiety were also measured using the beck depression inventory and beck anxiety inventory, respectively.
Results
Results indicate that AN patients had a higher asprosin concentration in their plasma compared to healthy controls (p = 0.033). Among AN patients, plasma asprosin levels correlated positively with EDI-2 interoceptive awareness subscale score (p = 0.030) and negatively with duration of illness (p = 0.036). Multiple linear regression analyses showed that increases in asprosin levels (p = 0.029), glucose levels (p = 0.024) and body mass index (p = 0.003) were associated with an increase of the score of EDI-2 bulimia subscale.
Conclusions
Our findings suggest that the increase in plasma asprosin concentration in patients with AN may be a compensation for the body’s energy shortage, and asprosin may be involved in the development of bulimia and lack of interoceptive awareness in AN patients.
Level of evidence
Level III, case–control analytic study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anorexia nervosa (AN) is a serious psychiatric illness characterized by self-induced weight loss, aversion to fatness or weight gain, and disturbance in the way one experiences his or her body shape or weight. Weight loss and malnutrition can result in various complications in patients with AN, with a mortality rate of nearly 6% per decade [1]. Anorexia means a decrease in appetite, but how the appetite of AN changes has not been fully elucidated. Therefore, the appetite regulation mechanism of AN has become a research hotspot in recent years [2].
Recent studies have shown that AN is closely related to appetite dysregulation [3,4,5,6,7,8]. For example, studies have found that activation of the hypothalamic–pituitary–adrenal axis to be associated with appetite suppression in AN patients [6]. Dwyer et al. [9] suggested that defects in the starvation response in AN patients could make their bodies unable to activate the secretion of neurotransmitters of promoting appetite and food-seeking behaviors, thereby trapping these patients in a vicious circle of continuous catabolic state. Other studies have focused on orexigenic and anorexigenic hormones in an attempt to reveal the etiology of AN [10,11,12,13,14,15]. Leptin, an anorexigenic hormone, has been found to be reduced in patients with AN [10]; and hypoleptinemia can precipitate amenorrhea in AN patients [16]. A genome-wide association study suggested that mutation-mediated leptin signaling dysfunction may play a role in AN [17]. On the other hand, the orexigenic hormone ghrelin is increased in the symptomatic AN patients and can return to normal levels after weight recovery [11]. In addition, ghrelin receptor agonists or exogenous ghrelin may be useful in treating AN by stimulating appetite and relieving gastrointestinal symptoms [18,19,20]. Furthermore, appetite hormone obestatin [10, 21,22,23], Peptide YY [3, 12, 24], insulin-like growth factor I [13, 25, 26], insulin [15, 27], and glucose [14] have also been shown to be involved in the pathophysiology of AN.
Asprosin, a newly discovered fasting-induced glucogenic protein hormone [28], is secreted by white adipose tissue and promotes hepatic glucose production via the olfactory receptor OLFR734 activated cAMP signaling pathway [29]. Asprosin can cross the blood–brain barrier and function as a centrally acting appetite-promoting hormone by stimulating the orexigenic agouti-related neuropeptide (AgRP) neurons via a cAMP-dependent pathway and by inhibiting the anorexigenic neurons proopiomelanocortin (POMC) neurons in a GABA-dependent manner [30, 31]. In mice and humans, lower asprosin levels can lead to decreased fat mass and body weight as well as hypophagic behaviors, whereas neutralization of asprosin in the blood with a monoclonal antibody can reduce appetite and body weight in obese mice [30]. Researches have demonstrated that plasma asprosin levels rise concurrently with weight gain in obese adults [32, 33]. Although Long et al. [34] found that the level of asprosin in obese children was lower than that of normal-weight children, which may be due to the fact that obese children were still in the compensatory stage of metabolic balance, these studies have shown that asprosin plays an important role in regulating appetite and body weight. Furthermore, asprosin can also raise blood glucose during fasting. According to the theory proposed by Jean Mayer, blood glucose concentration changes can regulate satiety and feeding behavior [35]. Elevated blood glucose increases satiety and reduces feeding behavior, while a drop in blood glucose seems to reduce satiety and lead to feeding behavior [36]. Studies have also shown that lower postprandial glucose in patients with AN may be associated with delayed gastric emptying [14, 37]. With the refeeding of AN patients, although gastric emptying is improved, postprandial glucose is still low [14]. Besides, exogenous orexigenic hormone ghrelin infusion can significantly increase blood glucose levels in AN patients [38].
Taken together, we hypothesized that asprosin may be associated with appetite or weight and blood glucose in AN patients. In this exploratory study, we compared plasma asprosin and glucose concentrations between AN patients and healthy controls (HC). We also examined correlations between plasma asprosin levels and various aspects of AN symptomology in an attempt to further investigate its role in the development of AN.
Methods
Subjects
Forty-six anorexia nervosa outpatients were recruited from Shanghai Mental Health Center between July 2018 and June 2019. All patients met the following inclusion criteria: (1) female and Han Chinese; (2) met the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5, American Psychiatric Association, 2013) criteria for AN and not conform to other diagnoses at the time of inclusion, as confirmed by 2 senior psychiatrists; (3) have not taken psychiatric drugs since the onset of illness; (4) no history of another psychiatric disorder or chronic somatic disease; (5) not currently pregnant or breastfeeding; (6) capable of completing all tests and informed consent. These patients included AN-restricting-type subtypes (n = 28) and AN-binge-eating/purging-type subtypes (n = 18).
We recruited 47 healthy controls (HC) among colleges and health care workers through advertising between March and June 2019. Except that they did not meet the diagnosis of anorexia nervosa in DSM-5, the inclusion criteria were consistent with those in the AN group.
Clinical assessment
On the day of blood sampling, patients completed general information questionnaires and clinical psychological evaluations with the help of an evaluator. The Chinese version of the Eating Disorder Inventory-2 (EDI-2) was used to evaluate the severity of symptoms and personality traits related to eating disorders (EDs) in participants. The EDI-2 is a self-report questionnaire with 91 items and 11 subscales, including 3 main ED psychopathology subscales (drive for thinness, bulimia and body dissatisfaction) and 8 subscales measuring ED-related psychological constructs (ineffectiveness, perfectionism, interpersonal distrust, interoceptive awareness, maturity fears, asceticism, impulse regulation, and social insecurity). With each item, the EDI-2 requires respondents to estimate the frequency at which a given symptom, behavior or thought appears. The current study used transformed scores for statistical analysis, with “always”, “usually”, “often”, “sometimes”, “rarely or never” given a score of 3, 2, 1, 0, 0, 0, respectively. At present, EDI-1 has good clinical validity and its test–retest reliability in China [39, 40]. However, EDI-1 has only 64 items and can not measure asceticism, impulse regulation, and social insecurity; so, these three subscales are not included in our subsequent analysis of EDI-2.
Beck Depression Inventory, Version 2 (BDI-II) is the most commonly used self-reporting depression scale, developed by American scholar Beck in 1967. The BDI-II contains 21 items with a 4-point scale from 0 (no symptom) to 3 (severe symptom), which has good reliability and validity in Chinese patients with depression [41, 42].
The Chinese version of the beck anxiety inventory (BAI) is used to describe the degree of anxiety in the participants and composed of 21 items, which has high internal consistency. The BAI asked participants to respond to questionnaires with not at all, mild, moderate or severe, with values of, respectively, 0, 1, 2, and 3. The participants evaluated each item according to the situation of nearly a week. The higher the total score, the more serious the degree of anxiety [43, 44].
During the scale assessment, 4 patients missed some items, which resulted in a sample size of 44 for the BDI, BAI, drive for thinness, bulimia, interpersonal distrust subscales, 43 for the body dissatisfaction, perfectionism, maturity fears component subscales, and 42 for the ineffectiveness component subscales.
Plasma asprosin and glucose measurement
Plasma samples were collected in the morning (07:00 am–9:00 am) after at least 10 h of fasting. The collected blood samples were processed by the laboratory of the Shanghai Mental Health Center and stored at − 80 °C. Plasma asprosin levels were determined in duplicate with a custom-designed sandwich enzyme-linked immunosorbent assay (ELISA). Coating antibody, detection antibody, standard protein, and streptavidin-HRP are from a commercially available Asprosin (human) Matched Pair Detection Set (catalog number AG-46B-0011-KI01) purchased from Adipogen Ltd., San Diego, USA. We used the BioTNT (Cat. No. EL-CPX-5, Shanghai, China) company’s ELISA Construction Pack to assist in the detection of plasma asprosin concentration. The intra-assay coefficient of variation (CV) was < 5%, inter-assay CV < 7%. The detection range for plasma asprosin levels is between 0 and 5000 pg/ml. Plasma glucose levels were measured in duplicate by the glucose oxidase method using a commercially available kit (RongSheng Biotech Co., Cat. No. 361500, Shanghai, China). The linear range of the kit is 0–28 mmol/L. The specification shows that when the reagent tests the glucose sample of 5.55 mmol/L, the difference of absorbance change should be (ΔA) ≥ 0.15A, and the absorbance difference detected by us is 0.216 following the requirements.
Statistical analysis
IBM® SPSS® software (Version 20) was used for data analysis of this study. Between-groups comparisons of demographic data were performed using the student’s t test. Kolmogorov–Smirnov one-sample test showed that none of the continuous variables followed a normal distribution pattern except the plasma glucose levels. Therefore, glucose data were analyzed with Independent-Sample T-Test. Then differences in asprosin levels, the subscales of the EDI-2, the total score of BDI and the total score of BAI between groups were compared by Mann–Whitney U tests. Correlations between variables were examined by Spearman’s rank-order correlation. Multivariate linear regression analysis used EDI-2 bulimia subscales as the dependent variable and clinical data as independent variables. The final model was determined by the stepwise method, and there was no collinearity among the independent variables. The validity of the model is determined by testing the normality of the residual. The significance levels for all tests were set at p < 0.05 (two-tailed).
Results
Demographic data and clinical assessments
Table 1 shows the comparison of demographic and clinical data between the AN and the HC group. The two groups were comparable in terms of age, sex, and level of education. The mean BMI of patients with AN was lower than that of the HC group (p < 0.001). The average age of onset in 46 patients with AN was 15.6 years, and the average duration of illness was 14.9 months.
As the subscales of the EDI-2, the total score of BDI and the total score of BAI were non-normally distributed, Mann–Whitney U tests were used to explore the difference between groups. The results of clinical and psychological assessments are presented in Table 1. Patients diagnosed with AN were higher than HC in each factor score of EDI-2 but significant for all scales except interpersonal distrust, which is consistent with the results of previous studies [45,46,47]. Compared to the HCs, AN patients also reported higher levels of depression and anxiety in BDI and BAI, which may be related to higher depression and anxiety traits in AN patients [48, 49].
Asprosin and glucose levels
On average, participants with AN had higher asprosin levels (2514.8 ± 1957.2 pg/mL vs. 1947.0 ± 2143.8 pg/mL, Z = − 2.136, df = 91, p = 0.033) and lower glucose levels (3.2 ± 0.6 mmol/L vs. 3.3 ± 0.4 mmol/L, F = 2.647, df = 91, p = 0.178) than those without AN (Table 1). Then, we compared the difference of plasma asprosin and plasma glucose levels between the two subtypes in patients with AN (see in Supplementary materials). The results showed that there was no significant difference in asprosin (2669.9 ± 1934.0 pg/mL vs. 2273.7 ± 2024.5 pg/mL, p = 0.380) and glucose (3.1 ± 0.4 mmol/L vs. 3.2 ± 0.7 mmol/L, p = 0.661) between AN-restricting-type and AN-binge-eating/purging-type groups.
Relationships between asprosin and clinical variables in AN patients
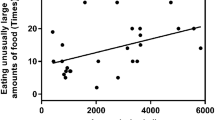
The correlation analysis between asprosin and 8 subscales of EDI-2, respectively, showed that it was positively correlated with the interoceptive awareness subscale scores (r = 0.320, p = 0.030) (Fig. 1). Correlation analysis showed that plasma asprosin levels were associated negatively with the duration of illness (r = − 0.310, p = 0.036) in AN patients (Fig. 2).
To examine the hypothesis that asprosin may contribute to the development of bulimia in AN, we performed a multivariate linear regression with the score of EDI-2 bulimia subscale as a dependent variable and plasma asprosin levels, glucose levels, and BMI as independent variables (F = 7.172, p = 0.001, r2 = 0.35) for bulimia (Table 2). Results show that increases in asprosin (p = 0.029), glucose (p = 0.024) and BMI (p = 0.003) were associated with an increase of the score of bulimia subscale.
No statistically significant correlations were found between plasma asprosin and glucose levels (r = − 0.150, p = 0.319). We did not find a correlation between plasma glucose levels and any of the EDI-2 subscales.
Discussion
In this study, we found an elevated plasma asprosin concentration in patients with AN. The secretion of asprosin is induced by fasting [28]. Considering its function as a fasting-induced orexigenic hormone, the increase of circulating asprosin in AN patients is likely to be an effect in response to starvation, which may be the compensation of the body for energy balance. On the other hand, asprosin can activate AgRP+ neurons in the arcuate nucleus of the hypothalamus through the blood–brain barrier and indirectly inhibit downstream anorexigenic POMC+ neurons, producing an appetite-promoting effect [30]. Our finding that AN patients have elevated levels of asprosin helps to explain the increase in AgRP+ [50, 51] and the decrease in POMC+ in these patients [52]. Although Duerrschmid et al. found that low BMI in neonatal progeroid syndrome individuals is caused by a lack of asprosin, it differs from AN in that neonatal progeroid syndrome is a congenital disease [53]. It is well known that AN is a disease caused by multiple factors in the biological psychosocial society. Just as the increased orexigenic hormone ghrelin in patients with AN [11], the increase of asprosin is more like the self-protection of the body in AN patients. Furthermore, some studies have shown that asprosin can also lead to insulin resistance and hyperinsulinemia [28]. Noteworthy, high levels of insulin suppress appetite and feeding behaviors [54, 55]. The asprosin–insulin–feeding inhibition pathway may be an underlying mechanism for the excessive harshness of diet in AN patients.
Our results indicate that the level of asprosin was negatively correlated with the duration of illness. Among AN patients, plasma asprosin levels decrease with longer duration of illness. Elevated levels of asprosin may be a compensatory response to food restriction in AN, but long-term insufficient energy intake, lack of nutrition and reduction of fat in patients with AN resulting in the decrease of asprosin synthesis.
Data showed a positive correlation between higher plasma asprosin levels and more severe deficits of interoceptive awareness. Interoceptive awareness not only refers to the perception of the physiological state of the body, such as warmth, pain, hunger, and vasomotor activity but also includes the observation and attention to the inherent drives and phenomena [56]. Since the 1960s, scholars have argued that a disturbance in the perception of one’s own body is at the core of AN psychopathology [57]. Numerous studies have shown that AN patients have more severe deficits in interoceptive awareness [46, 58]; some have suggested that such deficits may persist even after patients recover [59]. Studies have linked the lack of interoceptive awareness to dysfunctions in certain brain regions, especially the insula [60], which plays an important role in some of the symptoms of AN [61,62,63]. Interestingly, asprosin is a centrally acting hormone. Therefore, we hypothesized that the correlation observed between asprosin and interoceptive awareness scores in this study may be due to the abnormal interaction between asprosin and insula. Future work needs to further explore the interaction between asprosin and insula, and how they are associated with interoceptive symptoms of AN.
Multivariable linear regression showed that higher plasma asprosin levels, plasma glucose levels, and BMI could explain more severe bulimia-related psychopathology. Asprosin as an orexigenic hormone and BMI as an indicator of obesity, it is not difficult to understand that their elevation contributes to the development of bulimia symptoms. Elevated glucose increases satiety and reduces eating behavior, but why elevated glucose is associated with bulimia needs to be further studied.
The glucose levels in the AN group are lower than in the HC group, but there is no statistically significant difference between the two groups in line with some [14, 38, 64], but not all [65, 66]. Considering that lowering glucose can increase feeding behavior, the lower plasma glucose in AN patients may be the protection signal of the body. Although Asprosin promotes hepatic glucose release leading to raising blood glucose, AN patients have excessive restrictions on diet and insufficient energy intake, resulting in the depletion of glucogenic substrates, which makes blood glucose levels relatively low. In addition, there was no correlation between asprosin and fasting plasma glucose in the AN study [33, 34]. This may be related to the precise regulation of glucose by a series of hormones, and asprosin is only one of them. Although some previous studies have found a positive correlation between glucose and asprosin, participants in these studies were from diabetes [67, 68], glucose dysregulation [69], and polycystic ovary syndrome [68, 70]. These diseases were closely associated with abnormal glucose and insulin resistance, but our participants ruled out disorders of glucose metabolism.
As a preliminary exploration of the role of asprosin in AN, our study has a few limitations. First, the current study did not include any male subjects, as AN is an eating disorder that mostly affects women. Because of the limitations of detection techniques and ethics, we used the plasma of patients with AN to detect the concentration of asprosin. Although asprosin can have an appetite-promoting effect by crossing the blood–brain barrier and acting at the arcuate nucleus [30], it is not clear whether the peripheral level of asprosin fully represents its concentration in the central nervous system. Future experiments may be able to investigate the effect of AN on asprosin levels by detecting the concentration of asprosin in cerebrospinal fluid. In addition, due to the lack of sample size, there was no in-depth comparison of the differences between the two subtypes of AN. Future studies can explore whether there is a difference in the role of asprosin in AN-restricting and AN-binge/purging subtypes, and the role of asprosin in bulimia nervosa.
What is already known on this subject?
So far, most of the studies on asprosin have focused on obesity and glucose metabolism, exploring the role of asprosin in glucose regulation. Although previous studies have also suggested that the appetite-promoting effect of asprosin is closely related to weight, there is no in-depth study, especially in AN.
What this study adds?
Considering the development spectrum of AN, the response of asprosin to insufficient energy in the body may be one of the reasons for the development of AN-restricting subtype into AN-binge/purging subtype. This study attempts to elucidate the appetite-promoting and glucose-regulating effects of asprosin in patients with AN and to help understand the pathophysiology of AN.
Conclusions
To our knowledge, the current study is the first to examine the relationship between asprosin and AN. The results showed that asprosin increased in AN patients was associated with an interoceptive deficit, and asprosin levels decreased as the delay of the duration of illness. In addition, asprosin, glucose and BMI can be used to assess the bulimia symptoms of AN patients.
References
Westmoreland P, Krantz MJ, Mehler PS (2016) Medical complications of anorexia nervosa and bulimia. Am J Med 129(1):30–37. https://doi.org/10.1016/j.amjmed.2015.06.031
Klastrup C, Frolich J, Winkler LA, Stoving RK (2019) Hunger and satiety perception in patients with severe anorexia nervosa. Eat Weight Disord: EWD. https://doi.org/10.1007/s40519-019-00769-7
Heruc GA, Little TJ, Kohn M, Madden S, Clarke S, Horowitz M, Feinle-Bisset C (2018) Appetite perceptions, gastrointestinal symptoms, ghrelin, peptide YY and state anxiety are disturbed in adolescent females with anorexia nervosa and only partially restored with short-term refeeding. Nutrients 11(1):59. https://doi.org/10.3390/nu11010059
Holsen LM, Goldstein JM (2015) Valuation and cognitive circuitry in anorexia nervosa: disentangling appetite from the effort to obtain a reward. Biol Psychiatry 77(7):604–606. https://doi.org/10.1016/j.biopsych.2015.02.011
Eddy KT, Lawson EA, Meade C, Meenaghan E, Horton SE, Misra M, Klibanski A, Miller KK (2015) Appetite regulatory hormones in women with anorexia nervosa: binge-eating/purging versus restricting type. J Clin Psychiatry 76(1):19–24. https://doi.org/10.4088/JCP.13m08753
Lawson EA, Holsen LM, Desanti R, Santin M, Meenaghan E, Herzog DB, Goldstein JM, Klibanski A (2013) Increased hypothalamic-pituitary-adrenal drive is associated with decreased appetite and hypoactivation of food-motivation neurocircuitry in anorexia nervosa. Eur J Endocrinol 169(5):639–647. https://doi.org/10.1530/eje-13-0433
Aulinas A, Plessow F, Pulumo RL, Asanza E, Mancuso CJ, Slattery M, Tolley C, Thomas JJ, Eddy KT, Miller KK, Klibanski A, Misra M, Lawson EA (2019) Disrupted oxytocin-appetite signaling in females with anorexia nervosa. J Clin Endocrinol Metab 104(10):4931–4940. https://doi.org/10.1210/jc.2019-00926
Brooks SJ, Barker GJ, O’Daly OG, Brammer M, Williams SC, Benedict C, Schioth HB, Treasure J, Campbell IC (2011) Restraint of appetite and reduced regional brain volumes in anorexia nervosa: a voxel-based morphometric study. BMC Psychiatry 11:179. https://doi.org/10.1186/1471-244x-11-179
Dwyer DS, Horton RY, Aamodt EJ (2010) Role of the evolutionarily conserved starvation response in anorexia nervosa. Mol Psychiatry 16:595. https://doi.org/10.1038/mp.2010.95
Nakahara T, Harada T, Yasuhara D, Shimada N, Amitani H, Sakoguchi T, Kamiji MM, Asakawa A, Inui A (2008) Plasma obestatin concentrations are negatively correlated with body mass index, insulin resistance index, and plasma leptin concentrations in obesity and anorexia nervosa. Biol Psychiatry 64(3):252–255. https://doi.org/10.1016/j.biopsych.2007.08.005
Otto B, Cuntz U, Fruehauf E, Wawarta R, Folwaczny C, Riepl RL, Heiman ML, Lehnert P, Fichter M, Tschop M (2001) Weight gain decreases elevated plasma ghrelin concentrations of patients with anorexia nervosa. Eur J Endocrinol 145(5):669–673
Utz AL, Lawson EA, Misra M, Mickley D, Gleysteen S, Herzog DB, Klibanski A, Miller KK (2008) Peptide YY (PYY) levels and bone mineral density (BMD) in women with anorexia nervosa. Bone 43(1):135–139. https://doi.org/10.1016/j.bone.2008.03.007
Stoving RK, Chen JW, Glintborg D, Brixen K, Flyvbjerg A, Horder K, Frystyk J (2007) Bioactive insulin-like growth factor (IGF) I and IGF-binding protein-1 in anorexia nervosa. J Clin Endocrinol Metab 92(6):2323–2329. https://doi.org/10.1210/jc.2006-1926
Heruc GA, Little TJ, Kohn MR, Madden S, Clarke SD, Horowitz M, Feinle-Bisset C (2018) Effects of starvation and short-term refeeding on gastric emptying and postprandial blood glucose regulation in adolescent girls with anorexia nervosa. Am J Physiol Endocrinol Metab 315(4):E565–e573. https://doi.org/10.1152/ajpendo.00149.2018
Nakahara T, Kojima S, Tanaka M, Yasuhara D, Harada T, Sagiyama K-i, Muranaga T, Nagai N, Nakazato M, Nozoe S-i, Naruo T, Inui A (2007) Incomplete restoration of the secretion of ghrelin and PYY compared to insulin after food ingestion following weight gain in anorexia nervosa. J Psychiatr Res 41(10):814–820. https://doi.org/10.1016/j.jpsychires.2006.07.021
Hebebrand J, Muller TD, Holtkamp K, Herpertz-Dahlmann B (2007) The role of leptin in anorexia nervosa: clinical implications. Mol Psychiatry 12(1):23–35. https://doi.org/10.1038/sj.mp.4001909
Li D, Chang X, Connolly JJ, Tian L, Liu Y, Bhoj EJ, Robinson N, Abrams D, Li YR, Bradfield JP, Kim CE, Li J, Wang F, Snyder J, Lemma M, Hou C, Wei Z, Guo Y, Qiu H, Mentch FD, Thomas KA, Chiavacci RM, Cone R, Li B, Sleiman PA, Hakonarson H (2017) A genome-wide association study of anorexia nervosa suggests a risk locus implicated in dysregulated leptin signaling. Sci Rep 7(1):3847. https://doi.org/10.1038/s41598-017-01674-8
Hotta M, Ohwada R, Akamizu T, Shibasaki T, Takano K, Kangawa K (2009) Ghrelin increases hunger and food intake in patients with restricting-type anorexia nervosa: a pilot study. Endocr J 56(9):1119–1128
Hotta M, Ohwada R, Akamizu T, Shibasaki T, Kangawa K (2012) Therapeutic potential of ghrelin in restricting-type anorexia nervosa. Methods Enzymol 514:381–398. https://doi.org/10.1016/b978-0-12-381272-8.00024-6
Fazeli PK, Lawson EA, Faje AT, Eddy KT, Lee H, Fiedorek FT, Breggia A, Gaal IM, DeSanti R, Klibanski A (2018) Treatment with a ghrelin agonist in outpatient women with anorexia nervosa: a randomized clinical trial. J Clin Psychiatry. https://doi.org/10.4088/jcp.17m11585
Germain N, Galusca B, Grouselle D, Frere D, Billard S, Epelbaum J, Estour B (2010) Ghrelin and obestatin circadian levels differentiate bingeing-purging from restrictive anorexia nervosa. J Clin Endocrinol Metab 95(6):3057–3062. https://doi.org/10.1210/jc.2009-2196
Monteleone P, Serritella C, Martiadis V, Scognamiglio P, Maj M (2008) Plasma obestatin, ghrelin, and ghrelin/obestatin ratio are increased in underweight patients with anorexia nervosa but not in symptomatic patients with bulimia nervosa. J Clin Endocrinol Metab 93(11):4418–4421. https://doi.org/10.1210/jc.2008-1138
Harada T, Nakahara T, Yasuhara D, Kojima S, Sagiyama K, Amitani H, Laviano A, Naruo T, Inui A (2008) Obestatin, acyl ghrelin, and des-acyl ghrelin responses to an oral glucose tolerance test in the restricting type of anorexia nervosa. Biol Psychiatry 63(2):245–247. https://doi.org/10.1016/j.biopsych.2007.04.005
Misra M, Miller KK, Tsai P, Gallagher K, Lin A, Lee N, Herzog DB, Klibanski A (2006) Elevated peptide YY levels in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab 91(3):1027–1033. https://doi.org/10.1210/jc.2005-1878
Polli N, Scacchi M, Pecori Giraldi F, Sormani M, Zappulli D, Cavagnini F (2008) Low insulin-like growth factor I and leukopenia in anorexia nervosa. Int J Eat Disord 41(4):355–359. https://doi.org/10.1002/eat.20506
Cominato L, da Silva MM, Steinmetz L, Pinzon V, Fleitlich-Bilyk B, Damiani D (2014) Menstrual cycle recovery in patients with anorexia nervosa: the importance of insulin-like growth factor 1. Horm Res Paediatr 82(5):319–323. https://doi.org/10.1159/000367895
Nakai Y, Koh T (2001) Perception of hunger to insulin-induced hypoglycemia in anorexia nervosa. Int J Eat Disord 29(3):354–357
Romere C, Duerrschmid C, Bournat J, Constable P, Jain M, Xia F, Saha PK, Del Solar M, Zhu B, York B, Sarkar P, Rendon DA, Gaber MW, LeMaire SA, Coselli JS, Milewicz DM, Sutton VR, Butte NF, Moore DD, Chopra AR (2016) Asprosin, a fasting-induced glucogenic protein hormone. Cell 165(3):566–579. https://doi.org/10.1016/j.cell.2016.02.063
Li E, Shan H, Chen L, Long A, Zhang Y, Liu Y, Jia L, Wei F, Han J, Li T, Liu X, Deng H, Wang Y (2019) OLFR734 mediates glucose metabolism as a receptor of asprosin. Cell Metab 30(2):319–328.e318. https://doi.org/10.1016/j.cmet.2019.05.022
Duerrschmid C, He Y, Wang C, Li C, Bournat JC, Romere C, Saha PK, Lee ME, Phillips KJ, Jain M, Jia P, Zhao Z, Farias M, Wu Q, Milewicz DM, Sutton VR, Moore DD, Butte NF, Krashes MJ, Xu Y, Chopra AR (2017) Asprosin is a centrally acting orexigenic hormone. Nat Med 23(12):1444–1453. https://doi.org/10.1038/nm.4432
Beutler LR, Knight ZA (2018) A spotlight on appetite. Neuron 97(4):739–741. https://doi.org/10.1016/j.neuron.2018.01.050
Ugur K, Aydin S (2019) Saliva and blood asprosin hormone concentration associated with obesity. Int J Endocrinol 2019:2521096. https://doi.org/10.1155/2019/2521096
Wang CY, Lin TA, Liu KH, Liao CH, Liu YY, Wu VC, Wen MS, Yeh TS (2019) Serum asprosin levels and bariatric surgery outcomes in obese adults. Int J Obes (Lond) 43(5):1019–1025. https://doi.org/10.1038/s41366-018-0248-1
Long W, Xie X, Du C, Zhao Y, Zhang C, Zhan D, Li Z, Ning Q, Luo X (2019) decreased circulating levels of asprosin in obese children. Horm Res Paediatr. https://doi.org/10.1159/000500523
Mayer J (1955) Regulation of energy intake and the body weight: the glucostatic theory and the lipostatic hypothesis. Ann N Y Acad Sci 63(1):15–43. https://doi.org/10.1111/j.1749-6632.1955.tb36543.x
Chaput JP, Tremblay A (2009) The glucostatic theory of appetite control and the risk of obesity and diabetes. Int J Obes 33(1):46–53. https://doi.org/10.1038/ijo.2008.221
Benini L, Todesco T, Grave RD, Deiorio F, Salandini L, Vantini I (2004) Gastric emptying in patients with restricting and binge/purging subtypes of anorexia nervosa. Am J Gastroenterol 99(8):1448–1454. https://doi.org/10.1111/j.1572-0241.2004.30246.x
Miljic D, Djurovic M, Pekic S, Doknic M, Stojanovic M, Milic N, Casanueva FF, Ghatei M, Popovic V (2007) Glucose metabolism during ghrelin infusion in patients with anorexia nervosa. J Endocrinol Invest 30(9):771–775. https://doi.org/10.1007/BF03350816
Lee S, Lee AM, Leung T (1998) Cross-cultural validity of the eating disorder inventory: a study of Chinese patients with eating disorders in Hong Kong. Int J Eat Disord 23(2):177–188
Leung F, Wang J, Tang CW (2004) Psychometric properties and normative data of the eating disorder inventory among 12 to 18 year old Chinese girls in Hong Kong. J Psychosom Res 57(1):59–66. https://doi.org/10.1016/s0022-3999(03)00506-3
Yeung A, Howarth S, Chan R, Sonawalla S, Nierenberg AA, Fava M (2002) Use of the Chinese version of the beck depression inventory for screening depression in primary care. J Nerv Ment Dis 190(2):94–99. https://doi.org/10.1097/00005053-200202000-00005
Sun XY, Li YX, Yu CQ, Li LM (2017) Reliability and validity of depression scales of Chinese version: a systematic review. Zhonghua liu xing bing xue za zhi = Zhonghua liuxingbingxue zazhi 38(1):110–116. https://doi.org/10.3760/cma.j.issn.0254-6450.2017.01.021
Lin XL, Lu DL, Gottschling J, Segal DL, Tang SY (2017) Validation of a Chinese version of the geriatric anxiety scale among community-dwelling older adults in mainland China. J Cross Cult Gerontol 32(1):57–70. https://doi.org/10.1007/s10823-016-9302-4
Leyfer OT, Ruberg JL, Woodruff-Borden J (2006) Examination of the utility of the beck anxiety inventory and its factors as a screener for anxiety disorders. J Anxiety Disord 20(4):444–458. https://doi.org/10.1016/j.janxdis.2005.05.004
Nevonen L, Clinton D, Norring C (2006) Validating the EDI-2 in three Swedish female samples: eating disorders patients, psychiatric outpatients and normal controls. Nord J Psychiatry 60(1):44–50. https://doi.org/10.1080/08039480500504537
Clausen L, Rokkedal K, Rosenvinge JH (2009) Validating the eating disorder inventory (EDI-2) in two Danish samples: a comparison between female eating disorder patients and females from the general population. Eur Eat Disord Rev: J Eat Disord Assoc 17(6):462–467. https://doi.org/10.1002/erv.945
Nevonen L, Broberg AG (2001) Validating the eating disorder inventory-2 (EDI-2) in Sweden. Eat Weight Disord: EWD 6(2):59–67
Guarda AS, Schreyer CC, Boersma GJ, Tamashiro KL, Moran TH (2015) Anorexia nervosa as a motivated behavior: relevance of anxiety, stress, fear and learning. Physiol Behav 152(Pt B):466–472. https://doi.org/10.1016/j.physbeh.2015.04.007
Godart NT, Perdereau F, Rein Z, Berthoz S, Wallier J, Jeammet P, Flament MF (2007) Comorbidity studies of eating disorders and mood disorders. Critical review of the literature. J Affect Disord 97(1–3):37–49. https://doi.org/10.1016/j.jad.2006.06.023
Merle JV, Haas V, Burghardt R, Dohler N, Schneider N, Lehmkuhl U, Ehrlich S (2011) Agouti-related protein in patients with acute and weight-restored anorexia nervosa. Psychol Med 41(10):2183–2192. https://doi.org/10.1017/s0033291711000365
Moriya J, Takimoto Y, Yoshiuchi K, Shimosawa T, Akabayashi A (2006) Plasma agouti-related protein levels in women with anorexia nervosa. Psychoneuroendocrinology 31(9):1057–1061. https://doi.org/10.1016/j.psyneuen.2006.06.006
Galusca B, Prévost G, Germain N, Dubuc I, Ling Y, Anouar Y, Estour B, Chartrel N (2015) Neuropeptide Y and α-MSH circadian levels in two populations with low body weight: anorexia nervosa and constitutional thinness. PLoS ONE 10(3):e0122040–e0122040. https://doi.org/10.1371/journal.pone.0122040
O’Neill B, Simha V, Kotha V, Garg A (2007) Body fat distribution and metabolic variables in patients with neonatal progeroid syndrome. Am J Med Genet A 143a(13):1421–1430. https://doi.org/10.1002/ajmg.a.31840
Baskin DG, Figlewicz Lattemann D, Seeley RJ, Woods SC, Porte D Jr, Schwartz MW (1999) Insulin and leptin: dual adiposity signals to the brain for the regulation of food intake and body weight. Brain Res 848(1–2):114–123. https://doi.org/10.1016/s0006-8993(99)01974-5
Labouèbe G, Liu S, Dias C, Zou H, Wong JCY, Karunakaran S, Clee SM, Phillips AG, Boutrel B, Borgland SL (2013) Insulin induces long-term depression of ventral tegmental area dopamine neurons via endocannabinoids. Nat Neurosci 16:300. https://doi.org/10.1038/nn.3321. https://www.nature.com/articles/nn.3321#supplementary-information
Quadt L, Critchley HD, Garfinkel SN (2018) The neurobiology of interoception in health and disease. Ann N Y Acad Sci 1428(1):112–128. https://doi.org/10.1111/nyas.13915
Bruch H (1962) Perceptual and conceptual disturbances in anorexia nervosa. Psychosom Med 24:187–194. https://doi.org/10.1097/00006842-196203000-00009
Nyman-Carlsson E, Engstrom I, Norring C, Nevonen L (2015) Eating disorder inventory-3, validation in Swedish patients with eating disorders, psychiatric outpatients and a normal control sample. Nord J Psychiatry 69(2):142–151. https://doi.org/10.3109/08039488.2014.949305
Jenkinson PM, Taylor L, Laws KR (2018) Self-reported interoceptive deficits in eating disorders: a meta-analysis of studies using the eating disorder inventory. J Psychosom Res 110:38–45. https://doi.org/10.1016/j.jpsychores.2018.04.005
Critchley HD (2004) The human cortex responds to an interoceptive challenge. Proc Natl Acad Sci U S A 101(17):6333–6334. https://doi.org/10.1073/pnas.0401510101
Kaye WH, Fudge JL, Paulus M (2009) New insights into symptoms and neurocircuit function of anorexia nervosa. Nat Rev Neurosci 10(8):573–584. https://doi.org/10.1038/nrn2682
Friederich HC, Brooks S, Uher R, Campbell IC, Giampietro V, Brammer M, Williams SC, Herzog W, Treasure J (2010) Neural correlates of body dissatisfaction in anorexia nervosa. Neuropsychologia 48(10):2878–2885. https://doi.org/10.1016/j.neuropsychologia.2010.04.036
McCormick LM, Keel PK, Brumm MC, Bowers W, Swayze V, Andersen A, Andreasen N (2008) Implications of starvation-induced change in right dorsal anterior cingulate volume in anorexia nervosa. Int J Eat Disord 41(7):602–610. https://doi.org/10.1002/eat.20549
Roczniak W, Mikolajczak-Bedkowska A, Swietochowska E, Ostrowska Z, Ziora K, Balcerowicz S, Gorska-Flak K, Milan M, Oswiecimska J (2019) Serum interleukin 15 in anorexia nervosa: comparison to normal weight and obese girls. World J Biol Psychiatry 1:9. https://doi.org/10.1080/15622975.2019.1583370
Kinzig KP, Coughlin JW, Redgrave GW, Moran TH, Guarda AS (2007) Insulin, glucose, and pancreatic polypeptide responses to a test meal in restricting type anorexia nervosa before and after weight restoration. Am J Physiol Endocrinol Metab 292(5):E1441–1446. https://doi.org/10.1152/ajpendo.00347.2006
Kim Y, Hildebrandt T, Mayer LES (2019) Differential glucose metabolism in weight restored women with anorexia nervosa. Psychoneuroendocrinology 110:104404. https://doi.org/10.1016/j.psyneuen.2019.104404
Zhang L, Chen C, Zhou N, Fu Y, Cheng X (2019) Circulating asprosin concentrations are increased in type 2 diabetes mellitus and independently associated with fasting glucose and triglyceride. Clin Chim Acta 489:183–188. https://doi.org/10.1016/j.cca.2017.10.034
Li X, Liao M, Shen R, Zhang L, Hu H, Wu J, Wang X, Qu H, Guo S, Long M, Zheng H (2018) Plasma asprosin levels are associated with glucose metabolism, lipid, and sex hormone profiles in females with metabolic-related diseases. Mediat Inflamm 2018:7375294. https://doi.org/10.1155/2018/7375294
Wang Y, Qu H, Xiong X, Qiu Y, Liao Y, Chen Y, Zheng Y, Zheng H (2018) Plasma asprosin concentrations are increased in individuals with glucose dysregulation and correlated with insulin resistance and first-phase insulin secretion. Mediat Inflamm 2018:9471583. https://doi.org/10.1155/2018/9471583
Alan M, Gurlek B, Yilmaz A, Aksit M, Aslanipour B, Gulhan I, Mehmet C, Taner CE (2019) Asprosin: a novel peptide hormone related to insulin resistance in women with polycystic ovary syndrome. Gynecol Endocrinol 35(3):220–223. https://doi.org/10.1080/09513590.2018.1512967
Acknowledgements
The authors are grateful to all those who participated in this study.
Funding
This study was supported by Grants from Major Social Development Special Foundation of Ningbo (2017C510010), Medical and Health Science and Technology Plan Project of Zhejiang Province (2019KY564), Ningbo Health Branding Subject Fund (PPXK2018-01), the Important Weak Subject Construction Project of Health and Wellness System of Shanghai (2019ZB0201), National Natural Science Foundation of China (81771461), Shanghai Mental Health Center Special Discipline of Psychosomatic Medicine (2017-TSXK-01), the Study on Early Recognition and Comprehensive Prevention Model of Anxiety and Depressive Disorder Based on Three-level Linkage (XHLHGG201808).
Author information
Authors and Affiliations
Contributions
YRH, JC and LMR conceived and designed this study, YXX and YCZ reviewed and edited the article, YRH, QK, ZZL did plasma asprosin and glucose measurement, JC, QL and HC recruited patients, YXX, YCZ, YRH, QK, LG and CC recruit healthy controls, YRH and YXJ performed the data analyses. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethics Committee of the Shanghai Mental Health Center (2018-28) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
All participants or their guardians signed the informed consent to the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hu, Y., Xu, Y., Zheng, Y. et al. Increased plasma asprosin levels in patients with drug-naive anorexia nervosa. Eat Weight Disord 26, 313–321 (2021). https://doi.org/10.1007/s40519-020-00845-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40519-020-00845-3