Abstract
Purpose
The separate effects of whole grain (WG) and fruit and vegetable (F&V) diets on inflammatory biomarkers have not been assessed. Therefore, we evaluated these two high-fiber diets in relation to inflammation indices in obese and overweight women.
Study design
Parallel randomized clinical trial.
Methods
In the present study, 75 women were recruited and randomly assigned to three diet groups: a whole grain diet (WG-D) group, F&V group, and a combined whole grain and F&V diet group (WGFV-D) for 10 weeks. As a “feeding trial” all participants were asked to visit the clinic daily and eat prescribed foods in the presence of a nutritionist. Anthropometric indices and biochemical biomarkers were measured at baseline and after 10 weeks of the trial.
Results
Each of the three diet groups showed significant changes in serum biomarkers (CRP, TNF-α, IL-6, D-dimer, and serum fibrinogen) after following the diet for 10 weeks (P = 0.01). In adjusted models, significant changes were observed for CRP, TNF-α, IL-6, D-dimer, and serum fibrinogen (P = 0.01). In a model adjusted for malondialdehyde (MDA) level, a trend toward significance was observed (P = 0.05). Consumption of all three different diets for 10 weeks showed statistically significant change for all biomarkers (P < 0.05) the most notable changes in inflammatory indices were observed among participants following the WG diet.
Conclusions
Study results indicate that consumption of high-fiber diets, especially the WG diet, can help lower inflammatory levels and prevent subsequent adverse health consequences.
Level of Evidence
Level I, randomized controlled trial.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since 1975, the rising prevalence of obesity has become a major public health problem [1]. In 2016, more than 1.9 billion (39%) adults were overweight and 650 million (13%) were obese [1]. The prevalence of obesity in Iran was 57% for women and 42.8% for men [2]. Moreover, abdominal obesity in Iran was estimated to be 54% among women and 12.9% among men, which suggests that Iranian women are at higher risk for obesity-related comorbidities [2,3,4]. Obesity is characterized by imbalanced energy and expansion of adipose tissue mass and associated with low levels of physical activity, an unhealthy lifestyle, insufficient sleep and mental disorders such as stress and depression [5, 6]. Adipose tissue is now well recognized as an active metabolic and endocrine organ [5], containing a high percentage of adipocytes and macrophages [7]. These are important sources of inflammatory cytokine production, generating CRP, IL-6, and TNF-α, the cytokines most consistently observed to display elevated levels in obese persons [7]. High concentrations of TNF-a in adipose tissue can lead to serious consequences including impaired insulin action [8, 9]. Elevated IL-6 levels can induce liver production of CRP, causing a low-grade inflammatory state in obese persons [5, 10,11,12]. Obesity-induced inflammation is strongly associated with an increased risk of insulin resistance, type 2 diabetes and cardiovascular disease [13].
At present, dietary interventions are considered one of the primary therapeutic methods to treat obesity [14,15,16]. Use of a high fiber diet, as a feasible and effective way to control body weight, has led to a proliferation of research on the effects of higher consumption of dietary fiber, e.g. in the form of whole grain (WG) or fruits and vegetables (F&V) [17,18,19,20,21,22,23]. Higher intake of WG products has been associated with decreased levels of CRP and TNF-α [17, 22, 24]. Fewer studies examining high fiber consumption—in the form of fruits and vegetables—have also shown significant changes in inflammatory biomarkers [19, 20, 23]. Additionally, a recent meta-analysis investigating the association between dietary fiber intake and mortality found differences based on dietary sources. Risk of mortality was lower in participants with higher compared to lower intakes of cereal and vegetables, while no association was found between participants with high and low fruit fiber intakes [25]. While various trials have evaluated the effects of dietary fiber on inflammatory biomarkers, and a comprehensive systematic review and meta-analysis reported differences in the effects of high fiber diets on the risk of mortality, a comparison between sources of dietary fiber is lacking [19,20,21,22,23,24,25]. To our knowledge, our study is the first to specifically investigate the different effects of high dietary fiber from different sources on inflammatory markers in obese subjects. In particular, we aimed to compare the effects of high fiber from whole grains versus fruits and vegetables on inflammatory markers in obese individuals.
Methods
Participants
This parallel feeding trial was carried out in Isfahan, Iran between January and July 2016. We explained the study protocol to overweight and obese women admitted to the nutrition clinic after which they provided written consent. Inclusion criteria were (1) absence of noncommunicable diseases such as diabetes, hyperlipidemia, and hypertension (2) no history of liver and renal disease, hormonal disorders, food allergy, and other related diseases, (3) no use of medications such as weight-loss drugs, anti-inflammatory, or hormonal medications e.g. oral contraceptives and dietary supplements. Women were excluded if (1) they did not follow the prescribed diets or return for follow-up visits or (2) if they were taking medications or suffered from diseases which would interfere with the current study. The sample size was calculated based on the following formula [26]: N = 2 [(Z1−α/2 ± Z1−β)2 – S2]/d2; α (type 1 error) = 0.05 and β (type 2 error) = 0.20, d = 25 and SD = 2.6 indicated the required number of subjects was 17. Based on the calculation, we determined that 17 subjects were needed in each group to achieve adequate power (after taking into consideration probable dropout). Accordingly, we assigned 25 subjects to each feeding group. Participants were randomly assigned to three high-fiber diet groups for 10 weeks: (1) a whole grain high-fiber diet (WG-D), (2) a fruit and vegetable high-fiber diet (FV-D), or (3) a diet containing both whole grain and fruits and vegetables (WGFV-D). Computer-generated random numbers were used to randomize participants. The study was approved by the Research Council and Ethical Committee of the School of Nutrition and Food Science, Isfahan University of Medical Sciences, Isfahan, Iran (grant number: 194172). All participants gave written consent before study enrollment. This trial was registered at Clinical Trials.gov with code NCT03340298.
Study procedure
Prior to the start of the trial, all baseline levels of FBS (mg/dl), plasma insulin, TG (mg/dl), total cholesterol (mg/dl), LDL-C (mg/dl), HDL-c (mg/dl) and CRP (mg/l) were measured and anthropometric assessments including height, weight, waist circumference, systolic and diastolic blood pressure were performed.
After randomization, subjects were assigned to one of three feeding groups (stratified by age, sex and BMI), including WG, FV and both WG and F&V. All three groups consumed high-fiber foods containing 35 gs of fiber per day. The WG-D intervention group received 25 g from WG products and 10 g of F&V. In contrast, the FV-D group was provided 25-g of fiber from F&V (as the main source) and 10 g from WG sources. The third group received equal proportions from both sources (17.5 g from WG and 17.5 g from F&V). Required energy intake was calculated based on the Harris-Benedict formula and whole calorie intake was decreased to reduce weight by 0.5 kg every week. A dietitian calculated the energy requirement for each participant separately, based on 55% of energy intake from carbohydrates, 15% from protein and 30% from fats. The number of servings to be obtained from each food group was determined and given to each individual.
For each intervention group, participants were given a list of allowable foods and asked to visit the nutrition clinic daily to consume prescribed foods. To assure a diverse diet for all three groups in addition to an exchange list, a list of items in each food group and sub-food groups was provided. Every 2 weeks all participants were assessed for food intake and anthropometric measurements. Participants were requested to maintain a physical activity record between in the intervals between visits. They were also asked to record 24-h dietary intake for 3 days per week during weeks 2, 5, and 10 of the trial. Assessment of anthropometric and biochemical indices was performed both before and at the end of the trial.
Assessment of anthropometric measurements and blood pressure
Weight was measured to the nearest 100 g with a digital scale (Seca, Hamburg, Germany) while participants were wearing light clothes. Height was measured in a standing position after removal of shoes with a tape measure while shoulders were in a relaxed position. The narrowest part of the waist (between the last rib and the iliac crest) was used to measure waist circumference to the nearest 0.1 cm over light clothing. BMI was calculated as body weight (kg)/height 2 (m). We used a standard mercury sphygmomanometer to measure participant blood pressure after resting for 15 min. The measurement was performed three times in the sitting position at 1-min intervals. The average of three measurements was used as the final blood pressure. Systolic blood pressure was defined as the appearance of the first sound and diastolic blood pressure was defined as the disappearance of the sound (Korotkoff phase 5). All participants were asked to avoid coffee, tea and any caffeine-containing foods/beverages before the blood pressure measurement.
Assessment of biochemical measurements
Twelve-hour fasting blood samples (10 ml) were taken at baseline and after 10 weeks of the intervention to measure serum insulin level, blood glucose level and serum lipids (including total cholesterol, triglycerides, LDL- C, HDL-C) and inflammatory biomarkers (CRP, TNF-α).
Fasting blood glucose was measured by an enzymatic colorimetric method using glucose oxidase on the day of blood collection. Blood was centrifuged at 3000 rpm for 10 min to separate the plasma and samples were stored at − 70 °C until they were analyzed. Total cholesterol level and serum TG analysis were done using a Selectra 2 auto-analyzer (Vital Scientific, Spankeren, The Netherlands909) using an enzymatic colorimetric method with cholesterol esterase and cholesterol oxidase and glycerol phosphate oxidase (Pars Azmoun, Tehran, Iran). The determination of HDL-C was done after precipitation of apolipoprotein B containing lipoproteins with phosphotungstic acid. LDL-C was calculated by Friedewald’s formula when the TAG level was lower than 400 mg/dl; otherwise, LDL-C was determined by an enzymatic method. Malondialdehyde (MDA) measurements were determined by HPLC according to the method of Wong et al. [27]., high sensitivity C-reactive protein (hs-CRP) were measured using ultrasensitive latex-enhanced immunoturbidimetric assay (Randox laboratory Ltd, Belfast, United Kingdom). and TNF-α and Il-6 were determined with the use of ELISA by means of commercially available kits (Bender MedSystems and Biosource International, Vienna, Austria).
Statistical analysis
The Kolmogorov–Smirnov test was used to assess the normal distribution of the variables. Analysis of variance (ANOVA) and Chi square tests were used for quantitative and categorical variables, respectively. ANOVA was used to assess energy intake in the three different diet groups. We assessed dietary intake in the three groups with analysis of covariance (ANCOVA) after adjustment for energy intake. We used the ANOVA test to compare the baseline and final values among the three groups. Statistical significance was defined as P < 0.05.
Results
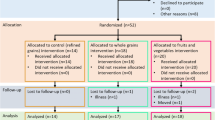
In this study, participants in the WG-D and FV-D groups completed the entire trial. However, in the WGFV-D group only one participant continued the trial until the fifth week, while others discontinued due to flatulence and stomach discomfort. Given these circumstances, we used intention-to-treat analyses. Further, laboratory data were entered at the end. The participant flow diagram is shown in Fig. 1. This diagram displays participant selection, allocation and follow-up in the intervention. Among the participants, no one was post-menopausal and all participants were non-smokers. Socio-demographic characteristics of participants in each of the three groups are shown in Table 1. All three diets (which contain high amounts of fiber) were well-tolerated among the women. No significant differences were observed by age, educational level, occupation, or history of diseases. No statistically significant differences in physical activity were observed across the intervention groups at baseline (P = 0.21). Weight in all groups changed significantly during the study: WG-D; 80.5 ± 1.3 kg at end of trial vs. 86.5 ± 1.6 kg at baseline, FV-D; 77.3 ± 1.6 kg vs. 81.6 ± 1.9 kg WGFV-D; and 78.3 ± 1.0 kg vs. 83.3 ± 1.5 kg. At the end of trial, serum levels of TG and HDL were lower in all groups as well as the LDL level in the WGFC-D group, however, these differences were not statistically significant. LDL was significantly lower in the WG-D (110 ± 7 vs. 99 ± 6; P = 0.01) and FV-D (114 ± 8 vs. 94 ± 5; P = 0.03) groups at the end of trial. Table 2 diplays dietary intakes of all participants in all three groups. A marginally significant difference was observed for vitamin C intake, in contrast to other intakes (e.g. calcium, fiber, etc.) which showed no significant changes. Baseline and endpoints of biochemical indices in all three trial groups are presented in Table 3. Baseline comparisons of biochemical indices showed marginally significant differences in fibrinogen and CRP levels, but no significant effect was observed for the other indices. In a comparison of inflammatory markers at the endpoint, significant changes in fibrinogen and CRP levels were observed and we also found suggestive differences in MDA and D-dimer levels. However, TNF-α and IL-6 levels did not change significantly. Between-group comparisons of variables showed that in the WF-D group, there were significant changes in CRP, D-dimer, fibrinogen, and MDA. In the WG-D and FV-D groups, we observed a significant change in fibrinogen level and marginally significant effect on CRP. On the other hand, in the FV-D group, none of the variables changed significantly except the D-dimer level, which showed a marginally significant effect.
We also checked the percent changes in biochemical indices among three different trial groups while adjusting for changes in weight. All inflammatory indicators were significantly changed after consumption of any of the three different diets for 10 weeks. Furthermore, the change for these three groups was statistically significant for all variables (P ≤ 0.05) and the most marked change in inflammatory indices was observed among participants following the WG-D.
Discussion
Following a 10-week feeding trial in obese and overweight women, inflammatory indicators (including CRP, serum fibrinogen, MDA, and D-dimer) decreased in the whole grain diet group as a result of the intervention. Similar reductions in fibrinogen were observed in the fruit and vegetable group during the intervention. The greatest reduction in these indicators was observed in the whole grain diet group and the smallest decline in the fruit and vegetable group (findings that remained even after adjusting for change in weight). Despite the significant reduction in CRP and fibrinogen in all groups, only the whole-grain group showed a major decline in the other biomarkers.
Some studies also have shown associations between fiber intake and inflammatory markers [17, 18, 22, 28,29,30,31,32]. As discussed, obesity results in an expansion of adipose tissue that induces a chronic state of inflammation. Adiet rich in fiber can decrease levels of systemic inflammation through a weight-reducing mechanism that limits the production of inflammatory cytokines by adipocytes [33, 34] and through the effect of fiber (unrelated to body weight) which is likely mediated through the intestinal microbiome [34].
Various possible mechanisms are thought to contribute to the anti-inflammatory activity of fiber, but two are considered the most probable. One is a mechanism suggesting indirect competition between commensal and pathogenic bacteria in the intestine. Short-chain fatty acids production due to fiber consumption, may result in preventing the colonization of pathogenic microorganism, as well as promote the growth of commensal. The anti-inflammatory effect of fiber may be related to elevated suppression of this pathogenic bacteria [34]. Metabolites of the intestinal microbiome, which appear in the intestine, plasma and urine are thought to be the other probable anti-inflammatory effect of fiber. Thus, fiber intake can cause anti-inflammatory effect through increasing the intestinal microbiome and substituting plasma metabolites profile. It is also possible that a cluster of microbial metabolites affects the inflammatory response [34]. The anti-inflammatory effect of short-chain fatty acids, especially butyrate is supported by various investigations [34,35,36].
Several studies support the relationship between dietary fiber intake from different sources (WG or FV) and lower levels of inflammation [22, 24, 37]. Similar to our study, a recent clinical trial by Vitaglione et al. that investigated the association between consumption of whole-grain fiber and inflammation in 80 healthy overweight and obese subjects who followed an isocaloric diet for 8 weeks, who showed a remarkable decline in TNF-a and IL-6 [22]. That study demonstrated that WG polyphenol is released and absorbed in the gut and is likely metabolized by gut microbiota, and is the most abundant circulating metabolite in overweight/obese subjects compared to subjects in a control group. It also showed a relation between metabolite release, mild modification of gut microbiota and reduction of inflammatory markers [22].
Another trial, by Katcher et al., compared two hypocaloric diets using WG or refined grains and their effects on CVD risk factors in obese subjects with metabolic syndrome [24]. They showed a similar beneficial effect, reporting a 38% reduction in CRP levels in participants consuming whole grains after 12 weeks [24]. The mechanism of the positive impact of a diet rich in whole grain can be explained by the interplay between gut microbiota and whole grain limited polyphenols. Martines et al. investigated the effects of three diet groups that consumed 60 g of (1) whole grains (2) brown rice and (3) a mixture of WG and brown rice on the gut microbiome and serum markers of systemic inflammation, showing alterations in the diversity of the gut microbiome. The greatest reduction in plasma IL-6 concentrations was observed among participants with higher proportions of Dialister and lower concentrations of Coriobacteriaceae. Prior studies have shown that these bacterial groups are related to chronic inflammation [22,23,24,25,26, 28,29,30,31,32,33,34,35,36,37]. The correlations between Dialister and Coriobacteriaceae with a IL-6 response suggests that these taxa may change the individual immunological response to whole grains [22, 37]. On the other hand, a number of studies have not documented a significant effect of WG on inflammatory markers. The result of a study by Tighe et al. showed no significant change in CRP and IL-6 levels in healthy middle-aged volunteers with normal to high BMI (18–35 kg/m2) after 12 weeks of substituting three servings of whole grains (whether in form of wheat or mixture of wheat and oats) for refined foods [38]. Anderson et al. found similar results in a study in which overweight healthy subjects (men and women) were given whole grain or refined foods (without changing the whole diet) for 6 weeks [39]. Differences in trial duration, participant BMI, dosage and various types of WG products, different sample sizes, could explain the variation of in results across studies. Furthermore, the calculation of required energy for each individual could explain the differences observed between the results of other studies and the associations we found between WG group and inflammatory markers. Results of the FV-D have also been contradictory. In a 12-week community trial, a large number of subjects (N = 1000, aged 18–85) were recruited to examine the relationship between inflammatory markers and quantity of F&V intake. F&V intakes were categorized into three groups based on daily servings. Their results showed that higher consumption of F&V inversely correlated with IL-6, TNF-a and CRP levels [32]. Furthermore, a cross-sectional study among 486 women aged 40-60 showed that participants in the highest quantile of F&V consumption had lower serum CRP concentrations [40]. Another study of a dietary pattern rich in F&V assessed its association with inflammatory markers documented a beneficial effect of higher intake of F&V and lower levels of inflammatory markers [29]. Inversely, Nadeem et al. examined the effects of increased F&V intake in different groups of healthy adults with a wide range of BMIs (a group with mild hypertension aged 40–60 and group of older adults aged 65–85), different trial durations (8 and 16 weeks) and different servings (1, 3, 6 servings per day for three groups of 40–60 year old adult with mild hypertension and in elderly group randomized to receive one or two serving for one group and at least five servings per day for other group). They reported no significant changes in CRP, IL-6, and E- selectin concentrations resulting from consumption of higher quantities of F&V in either groups [41]. F&V rich diet are believed to have a protective effect against disease-related markers of inflammation and oxidative stress. Possible mechanisms include mediation through a variety of vitamins, antioxidants and other micronutrients, including flavonoids, carotenoids, vitamin C and folic acid, as well as dietary fiber. These substances suppress the action of carcinogens and protect against oxidative DNA damage [32, 42, 43].
Although there are several trials documenting the effects of F&V and WG separately [17, 18, 22, 28,29,30,31,32, 44, 45], to our knowledge there no prior study has compared all these groups together. A strength of this study is that it was a feeding trial. In our study, required energy intake for all subjects was calculated and foods were prepared during daily clinic visits, giving us confidence in the amount of fiber consumed by each participant. Information for physical activity records and the 24-h dietary recall were recorded by the research team, which led to higher accuracy and prevention of unintended bias. Our study findings could be reinforced if the mechanisms for each dietary source and their effects on the inflammatory system through alteration of gut microbiota or other possible ways were investigated. A positive effect on biomarkers was seen for each group, as both WG and FV showed an ablative effect on inflammation. We expected the most marked change would be in the group combining WG and F&V but rather the most significant change was seen in WG-D group, the reason for which is unknown. Some limitations of this study must be noted. As all three diet groups were high in fiber, adherence to these diets for individuals with GI complication is not possible. All participants who enrolled were obese and overweight, therefore, it is unknown if the same positive effects on inflammatory indices would occur in subjects with normal BMIs.
Conclusion
In this randomized clinical trial among overweight and obese women, weight-reducing diets with different sources of fiber showed beneficial effects on inflammatory biomarkers, with the WG-D showing the most significant effects in reduction of inflammatory markers compared to the F&V group and WG and FV groups. Further trials are needed in larger populations to confirm these results. We suggest future studies investigate the effects of these dietary fiber sources in different populations, especially in those with normal BMI.
References
WHO Fact Sheet (2018) Obesity and overweight. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Accessed 8 Feb 2019
Barak F et al (2015) Adherence to the Dietary Approaches to Stop Hypertension (DASH) diet in relation to obesity among Iranian female nurses. Public Health Nutr 18(4):705–712. https://doi.org/10.1017/S1368980014000822
Janghorbani M et al (2007) First nationwide survey of prevalence of overweight, underweight, and abdominal obesity in Iranian adults. Obesity (Silver Spring) 15(11):2797–2808. https://doi.org/10.1038/oby.2007.332
Seidell JC et al (1996) Overweight, underweight, and mortality. A prospective study of 48,287 men and women. Arch Intern Med 156(9):958–963. https://doi.org/10.1001/archinte.1996.00440090054006
Goossens GH (2008) The role of adipose tissue dysfunction in the pathogenesis of obesity-related insulin resistance. Physiol Behav 94(2):206–218. https://doi.org/10.1016/j.physbeh.2007.10.010
Hauner H (2005) Secretory factors from human adipose tissue and their functional role. Proc Nutr S oc 64(2):163–169. https://doi.org/10.1079/PNS2005428
Fain JN (2006) Release of interleukins and other inflammatory cytokines by human adipose tissue is enhanced in obesity and primarily due to the nonfat cells. Vitam Horm 74:443–477. https://doi.org/10.1016/S0083-6729(06)74018-3
Hotamisligil GS (2003) Inflammatory pathways and insulin action. Int J Obes Relat Metab Disord 27(Suppl 3):S53–S55. https://doi.org/10.1038/sj.ijo.0802502
de Alvaro C et al (2004) Tumor necrosis factor alpha produces insulin resistance in skeletal muscle by activation of inhibitor kappaB kinase in a p38 MAPK-dependent manner. J Biol Chem 279(17):17070–17078. https://doi.org/10.1074/jbc.M312021200
Yudkin JS (2003) Adipose tissue, insulin action and vascular disease: inflammatory signals. Int J Obes Relat Metab Disord 27(Suppl 3):S25–S28. https://doi.org/10.1038/sj.ijo.0802496
Yudkin JS et al (2000) Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis 148(2):209–214. https://doi.org/10.1016/S0021-9150(99)00463-3
Yudkin JS et al (1999) C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol 19(4):972–978
Dixon JB (2010) The effect of obesity on health outcomes. Mol Cell Endocrinol 316(2):104–108. https://doi.org/10.1016/j.mce.2009.07.008
Kaul L, Nidiry J (1993) High-fiber diet in the treatment of obesity and hypercholesterolemia. J Natl Med Assoc 85(3):231–232
Sackner-Bernstein J, Kanter D, Kaul S (2015) Dietary intervention for overweight and obese adults: comparison of low-carbohydrate and low-fat diets. A meta-analysis. PLoS One 10(10):e0139817. https://doi.org/10.1371/journal.pone.0139817
Sayon-Orea C et al (2015) Adherence to Mediterranean dietary pattern and menopausal symptoms in relation to overweight/obesity in Spanish perimenopausal and postmenopausal women. Menopause 22(7):750–757. https://doi.org/10.1097/GME.0000000000000378
Hajihashemi P et al (2014) Whole-grain intake favorably affects markers of systemic inflammation in obese children: a randomized controlled crossover clinical trial. Mol Nutr Food Res 58(6):1301–1308. https://doi.org/10.1002/mnfr.201300582
Holt EM et al (2009) Fruit and vegetable consumption and its relation to markers of inflammation and oxidative stress in adolescents. J Am Diet Assoc 109(3):414–421. https://doi.org/10.1016/j.jada.2008.11.036
Macready AL et al (2014) Flavonoid-rich fruit and vegetables improve microvascular reactivity and inflammatory status in men at risk of cardiovascular disease–FLAVURS: a randomized controlled trial. Am J Clin Nutr 99(3):479–489. https://doi.org/10.3945/ajcn.113.074237
Oliveira A, Rodriguez-Artalejo F, Lopes C (2009) The association of fruits, vegetables, antioxidant vitamins and fibre intake with high-sensitivity C-reactive protein: sex and body mass index interactions. Eur J Clin Nutr 63(11):1345–1352. https://doi.org/10.1038/ejcn.2009.61
Sahyoun NR et al (2006) Whole-grain intake is inversely associated with the metabolic syndrome and mortality in older adults. Am J Clin Nutr 83(1):124–131. https://doi.org/10.1093/ajcn/83.1.124
Vitaglione P et al (2015) Whole-grain wheat consumption reduces inflammation in a randomized controlled trial on overweight and obese subjects with unhealthy dietary and lifestyle behaviors: role of polyphenols bound to cereal dietary fiber. Am J Clin Nutr 101(2):251–261. https://doi.org/10.3945/ajcn.114.088120
Yeon JY, Kim HS, Sung MK (2012) Diets rich in fruits and vegetables suppress blood biomarkers of metabolic stress in overweight women. Prev Med 54(Suppl):S109–S115. https://doi.org/10.1016/j.ypmed.2011.12.026
Katcher HI et al (2008) The effects of a whole grain-enriched hypocaloric diet on cardiovascular disease risk factors in men and women with metabolic syndrome. Am J Clin Nutr 87(1):79–90. https://doi.org/10.1093/ajcn/87.1.79
Kim Y, Je Y (2014) Dietary fiber intake and total mortality: a meta-analysis of prospective cohort studies. Am J Epidemiol 180(6):565–573. https://doi.org/10.1093/aje/kwu174
Esmaillzadeh A, Azadbakht L (2012) Legume consumption is inversely associated with serum concentrations of adhesion molecules and inflammatory biomarkers among Iranian women. J Nutr 142(2):334–339. https://doi.org/10.3945/jn.111.146167
Wong SH et al (1987) Lipoperoxides in plasma as measured by liquid-chromatographic separation of malondialdehyde-thiobarbituric acid adduct. Clin Chem 33(2 Pt 1):214–220
de Punder K, Pruimboom L (2013) The dietary intake of wheat and other cereal grains and their role in inflammation. Nutrients 5(3):771–787. https://doi.org/10.3390/nu5030771
Esposito K et al (2004) Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA 292(12):1440–1446. https://doi.org/10.1001/jama.292.12.1440
Lutsey PL et al (2007) Whole grain intake and its cross-sectional association with obesity, insulin resistance, inflammation, diabetes and subclinical CVD: the MESA Study. Br J Nutr 98(2):397–405. https://doi.org/10.1017/S0007114507700715
Qi L et al (2006) Whole-grain, bran, and cereal fiber intakes and markers of systemic inflammation in diabetic women. Diabetes Care 29(2):207–211. https://doi.org/10.2337/diacare.29.02.06.dc05-1903
Root MM et al (2012) Combined fruit and vegetable intake is correlated with improved inflammatory and oxidant status from a cross-sectional study in a community setting. Nutrients 4(1):29–41. https://doi.org/10.3390/nu4010029
Ghalandari H et al (2018) Comparison of two calorie-reduced diets of different carbohydrate and fiber contents and a simple dietary advice aimed to modify carbohydrate intake on glycemic control and inflammatory markers in type 2 diabetes: a randomized trial. Int J Endocrinol Metab 16(1):e12089. https://doi.org/10.5812/ijem.12089
Kuo SM (2013) The interplay between fiber and the intestinal microbiome in the inflammatory response. Adv Nutr 4(1):16–28. https://doi.org/10.3945/an.112.003046
Le Leu RK et al (2010) Synbiotic intervention of Bifidobacterium lactis and resistant starch protects against colorectal cancer development in rats. Carcinogenesis 31(2):246–251. https://doi.org/10.1093/carcin/bgp197
Samuel BS et al (2008) Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci USA 105(43):16767–16772. https://doi.org/10.1073/pnas.0808567105
Martinez I et al (2013) Gut microbiome composition is linked to whole grain-induced immunological improvements. ISME J 7(2):269–280. https://doi.org/10.1038/ismej.2012.104
Tighe P et al (2010) Effect of increased consumption of whole-grain foods on blood pressure and other cardiovascular risk markers in healthy middle-aged persons: a randomized controlled trial. Am J Clin Nutr 92(4):733–740. https://doi.org/10.3945/ajcn.2010.29417
Andersson A et al (2007) Whole-grain foods do not affect insulin sensitivity or markers of lipid peroxidation and inflammation in healthy, moderately overweight subjects. J Nutr 137(6):1401–1407. https://doi.org/10.1093/jn/137.6.1401
Esmaillzadeh A et al (2006) Fruit and vegetable intakes, C-reactive protein, and the metabolic syndrome. Am J Clin Nutr 84(6):1489–1497. https://doi.org/10.1093/ajcn/84.6.1489
Nadeem N et al (2014) Serum amyloid A-related inflammation is lowered by increased fruit and vegetable intake, while high-sensitive C-reactive protein, IL-6 and E-selectin remain unresponsive. Br J Nutr 112(7):1129–1136. https://doi.org/10.1017/S0007114514001639
Lock K et al (2005) The global burden of disease attributable to low consumption of fruit and vegetables: implications for the global strategy on diet. Bull World Health Organ 83(2):100–108
Organization, W.H. (2002) The world health report 2002: reducing risks, promoting healthy life. World Health Organization, Geneva
Gaskins AJ et al (2010) Whole grains are associated with serum concentrations of high sensitivity C-reactive protein among premenopausal women. J Nutr 140(9):1669–1676. https://doi.org/10.3945/jn.110.124164
Masters RC et al (2010) Whole and refined grain intakes are related to inflammatory protein concentrations in human plasma. J Nutr 140(3):587–594. https://doi.org/10.3945/jn.109.116640
Acknowledgements
The authors are grateful to all individuals who participated in this study.
Funding
This study was supported by Isfahan University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
NA and SF prepared the manuscript. ED and PM conducted the study. ED and LA checked and edited the article. LA supervised all aspects of the study.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of Isfahan University of Medical Sciences.
Informed consent
Informed consent was obtained from all study participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arabzadegan, N., Daneshzad, E., Fatahi, S. et al. Effects of dietary whole grain, fruit, and vegetables on weight and inflammatory biomarkers in overweight and obese women. Eat Weight Disord 25, 1243–1251 (2020). https://doi.org/10.1007/s40519-019-00757-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40519-019-00757-x