Abstract
Background and purpose
The association between weight status with simple cognitive tasks such as reaction time (RT) may not be observed in young people as cognitive functioning development has reached its peak. In the present study, we aimed to examine the association between overall and central adiposity with overall and central processing of RT in a sample of young adult men with different weight status from Ardabil, Iran.
Methods
Eighty-six young males between June-July 2018 completed RT tests as well as premotor time (PMT) using surface electromyography changes in isometric contraction response to an audio stimulus.
Results
No significant associations were observed between RT and PMT and different body mass index categories (underweight, normal weight, overweight and obese), as well as fat mass and fat to skeletal muscle mass ratio quartiles (Q). However, participants with greater waist to height ratio (WHtR) had longer PMT (but not RT) than their peers with lower WHtR (Q3 than Q2 and Q1 groups; p < 0.05, d = 1.23). Participants in the skeletal muscle mass quartile Q2 tended to have longer RT than participants in Q3 in an adjusted comparison model (p = 0.05, d = 0.72).
Conclusions
Although the association between weight status and RT might be elusive in young adults, our results show that higher central adiposity is negatively associated with PMT in young adults. Longitudinal studies are needed to explore the changes in obesity indexes and process speed in longer terms.
Level of evidence
Level I, experimental study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ability to respond quickly to an external stimulus is important for athletic performance, but also for dealing with sudden incidents during daily life. For example, individuals having a fast reaction time (RT) can better prevent a fall [1] or bypass a car when exposed to a sudden external stimulus [2]. RT shortens from childhood to adulthood reaching its peak at young adulthood (18–35), and then again increases towards old ages [3, 4].
RT tests are among the most basic measures of information processing speed [4]. The speed of information processing is an important aspect of cognitive development since it depicts an individuals ability to process complex information quickly [5, 6]. Improvements in information processing speed during the developmental period of life leads to improved working memory and in this way benefits intelligence [5, 6]. Moreover, changes in information processing speed can mediate the impact of ageing on inhibition, working memory, and arithmetical skills [7, 8]. Therefore, knowing factors that are associated with fast processing speed at younghood may help in designing interventions that can provide lifelong benefits.
Several factors influence RT, but their contribution may change from childhood to old ages. Socioeconomic status (SES) is associated with RT, such that both children and adults with a lower SES perform worse in RT tasks as compared to those with a higher SES [9, 10]. More physically active individuals are able to respond faster to a presented stimulus and process information more quickly, as compared to their less active peers [11]. Both higher blood pressure and higher depressive symptoms are negatively associated with cognitive function, but positively with overweight and obesity [12,13,14].
Furthermore, it has been shown that obesity is detrimentally associated with various complex neurocognitive domains such as executive function, complex attention, decision making, motor skill, verbal and visual memory in children, adolescents and mid adults [12, 13]. However, lower skeletal muscle mass (SMM) can negatively influence cognitive function in older people [15, 16]. In children, adolescents and young adults [17,18,19], various obesity indices (i.e., BMI, %fat, waist circumference, waist to hip ratio and waist to height ratio) have been explored for a possible association with simple or choice RT, but there is limited information on whether body composition (i.e. SMM) has an effect on this association.
Several recent studies in young people were inconsistent concerning the association between weight status and RT. For example, young normal weight participants have performed better in RT tasks as compared to their overweight or underweight peers in some studies [20,21,22], whereas other most recent studies have found no differences [17,18,19]. Some methodological factors, like low statistical power [20,21,22], or lack of adjustment for important covariates like age, physical activity (PA), and SES [20,21,22] may explain the inconsistent results between RT and obesity indices in youth.
On the other hand, another reason for the inconsistent results underlying the association between weight status and RT observed in the literature may be due to the reason that the brain reaches the peak development in young adults. Therefore, the association between weight status and RT may be elusive, and in this regard, sensitive methodologies are needed. In this way, another important consideration is the method applied for RT measurement. RT can be measured as a time interval between the application of a stimulus and the appearance of a motor response. RT tasks can be divided into simple RT and multiple choice RT tests [4]. Independent of the task, RT can be divided into two fractionated parts including central visuomotor (premotor or cognition time) and peripheral motor processes (motor time). The central processing time or premotor time (PMT) can be measured by recording surface electromyography (EMG) signal during performing isometric contraction in response to a stimulus [23]. The time between the onset or offset of the response signal in relation to the initial or terminal EMG activity can determine the PMT or duration of a cognitive process [23]. The PMT consists of two stages, including: (a) whether the stimulus has been presented (to identify it) and (b) response selection stage. Since obesity impacts central nervous system as well as autonomic and somatic components of the peripheral nervous system and as a consequence causes impaired cognitive function [24] it is speculated that PMT is a more sensitive factor than overall RT to observe whether RT is affected by weight status in young adults and to the best of our knowledge, no study has explored the PMT difference between various weight status groups.
The purpose of this study is to explore whether weight status is associated with RT in young adult males while controlling for covariates (i.e. SES, physical activity, depression and blood pressure). In the present study, we included various obesity indices (central and overall), important body composition variables such as SMM, as well as overall computerized RT and central component of RT. We hypothesize that higher adiposity would have a negative impact on PMT rather than overall RT, which could explain the previous inconsistent results in young adults [17,18,19,20,21,22, 25].
Methods
Participants and procedures
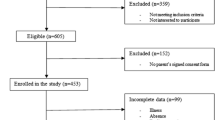
Eighty-six young males (age 16–24 years) participated in the present study. Participants with various weight statuses were recruited using research invitation advertisement from general participant pools (i.e., public libraries, public high schools, and University of Mohaghegh Ardabili). Three subjects did not meet the inclusion criteria (e.g., presenting musculoskeletal problems or chronic diseases, using medications or not interested in participating) and EMG RT data of three other participants were excluded from the data analysis due to inappropriate EMG signal, misses, or anticipations. The final sample included 80 young males. All participants gave written informed consent, and the study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Mohaghegh Ardabili University.
Data were collected in the course of 6 weeks between June-July 2018 in a physiological test lab at the University of Mohaghegh Ardabili. Each participant visited the lab twice. During the first visit (9-a.m to 14.30-p.m) participants were familiarized with the procedures of the study and participants’ demographic information and body composition data were recorded. The RT tests were introduced to participants and they were instructed to shave their skin on the positions were the EMG electrodes would be placed. Furthermore, they were requested to avoid consumption of food and caffeine-containing beverages 3–4 h before the RT measurements, and to avoid vigorous PA at least 24 h before the RT measurements. On the second visit (24 h later from the first visit and from 9-a.m to 14.30-p.m) computerized RT tests and surface electromyographic analysis of RT (EMG RT) data collection were performed.
Anthropometry
Body mass, fat mass, and SMM were measured by using an X-Contact 356 body composition analyzer (Jawon Medical, South Korea). The instrument with handlebar and footpad electrodes utilizes bio-electrical impedance analysis (BIA) technology. Height was measured barefoot in the Frankfurt horizontal plane with a telescopic height measuring instrument (Type SECA 225) to the nearest 1 mm. Both height and weight were measured twice at the first visit. If there were differences (≥ ± 0.1 kg) between measurements, a third measurement was performed and the average was calculated and reported. BMI was calculated as body weight in kilograms divided by the square of height in meters (kg/m2). Waist circumference was measured with an inelastic measuring tape between the superior border of the iliac crest and the lowest rib at the end of normal expiration to the nearest 0.1 mm. Waist circumference was divided by the height for waist to height ratio (WHtR) determination. Both BMI and fat mass were used as the overall obesity indices and WHtR was used as the central obesity index. WHtR was chosen as the central obesity index because it has been suggested a better index than waist circumference or waist to hip ratio in predicting visceral adipose tissue, body %fat, metabolic syndrome and early health risk [26,27,28].
Assessment of computerized RT tests
Computerized RT tests were assessed using the Cambridge Neuropsychological Test Battery (CANTAB), which is a validated computerized program for measuring various cognitive tasks [29, 30]. The CANTAB program was installed in a touch screen PC (ASUS, ET2012EUTS - 20.1 inches) to measure the following: a) Motor Screening Task (MST), b) Simple and five choices RT (performed using the touch screen), and c) two choices RT (performed using keyboard buttons). All the tasks including a 2 min break between the tests lasted approximately 15 min.
-
(a)
Motor screening test: The motor screening test is an introductory task to ensure that participants has no visual or comprehension problems. In the MST a pink cross appears on a black touch screen and participants are requested to touch the center of the cross with the dominant hand.
-
(b)
Simple and five choice RT: The participant must hold his finger in a rectangle in the touch screen until the yellow circle appears on the screen above the rectangle. The stimulus (yellow circle) appears in either a single (SRT) or in one of five possible locations (5-CRT). Then, the subject must release his finger upon detecting the yellow circle presentation and immediately afterwards touch the screen where the yellow circle appeared. Single stimulus (SRT) condition is identified first, and five stimuli (5-CRT) is identified in the second condition.
-
(c)
Two-choice RT (2-CRT): The test has two possible stimuli (left and right) and the participant needs to respond with two possible options (left and right buttons). The participant must press the right button (i.e., F8) on the keyboard when the stimuli (an arrow-shape) is displayed on the right-hand side of the screen; and press the left button (i.e., F7) on the keyboard when the stimuli are displayed on the left-hand side of the screen.
Electromyographic analysis of RT (EMG RT) for measuring premotor time (PMT)
Extensor carpi radialis electromyographic activity was used for measuring RT in initiation and termination during isometric contraction. For this mean, EMG (portable wireless surface EMG sensor, Biometrics Ltd., LE230, UK) was used applying the integral dry reusable surface electrodes (inter-electrode distance of 20 mm) for signal recording. For recording EMG activity, the electrode was placed over the belly of the extensor carpi radialis to record isometric wrist extension. For this mean, the participant’s dominant forearm and hand were placed on an armchair (special 60 × 12 cm wooden arms) and fixed with belts (to stabilize wrist). All participants performed wrist extension with a total time of 1 min. Before starting, procedures were explained to all participants with the tests and requested to do wrist extension as quickly and forcefully as possible against the confinement of the devices in response to an audible beep and to relax their muscle as fast as possible as soon as the beep sound finished. The auditory beep signal comprised a total of six audio signals consisting of three trials of 3-s contractions and three trials of 6-s contractions. To minimize participants’ anticipation, the auditory beep signals were presented as follows: (a) the sounds were presented in a balanced random order; (b) the time between sounds (beep) was also randomized to be either 3, 5 or 6 s. The audio beep signals were presented with the same sound volume for all participants to remove the confounding effects of intensity [31]. The recorded EMG activities were saved in the personal computer for visual detection of the delay in initiation or termination of contraction. Visual detection has been shown to correlate very highly with computer-based techniques (r = 0.999), and with higher reliability [32]. In this method, the delay in initiation or termination of the EMG signal is defined as the time interval between onset or offset of the auditory beep signal and onset or offset of the EMG signal. The EMG signal was sampled at 1000-Hz, band-pass filtered between 10 and 490 HZ and amplified (common mode rejection ratio higher than 96 dB at 60 Hz, and total gain = 1000). Data was processed through Lab View software (2015v15.0). Measures included 12 onsets and offset RT which by averaging decreased to 4 onsets and offset RTs. The four electromyographic RT measures were as follows: (a) Mean RT in initiation of 3 s contractions (Onset3); (b) Mean RT in termination of 3 s contractions (Offset3); (c) Mean RT in initiation of 6 s contractions (Onset6); (d) Mean RT in termination of 6 s contractions (Offset6).
Covariates
On the first visit, the following data were measured. SES was computed from parents’ educational and occupational status as explained in a previous study [19]. Long form International Physical Activity Questionnaire (IPAQ) was used as a standardized measure for measuring total physical activity (total-PA) with acceptable validity and reliability in 18- to 65-year-old adults [33]. The Beck Depression Inventory-II (BDI-II) was used as a reliable and valid measure of depressive symptoms [34, 35]. Blood pressure was measured using standard mercury sphygmomanometers (Model 1002/Presameter, Riester, Germany) three times and averaged to provide measures of systolic and diastolic blood pressure.
Statistical analyses
Data were checked for normality and outliers by using the Kolmogorov–Smirnov test and graphical models (e.g. boxplot, histogram, etc.). All independent values except for total-PA showed normal distribution. Therefore, natural log transformation data of total-PA was applied. Furthermore, although, Offset3, SRT, and 5-CRT, showed non-normal distribution, none of them showed substantial skewness and kurtosis (i.e., >+ 2 or < − 2). Therefore, according to Tabachnick and Fidell, the variables were included in the factor analysis with the other variables [36].
Factor analysis was used to decrease the numbers of dependent variables. Computerized RT (SRT; 5-CRT; 2-CRT) and EMG RT (onset3; offset3; onset6; offset6) yielded a total of two factors including: a) PMT and b) RT with Varimax rotation and principal components analysis (Table 2).
Multivariate analysis of covariance (MANCOVA) by using unadjusted and adjusted (controlling for age, SES, blood pressure, total-PA, and depressive symptoms) was conducted for comparison of composite computerized RT scores and composite PMT scores between the weight status groups. BMI cut off points [37, 38] defining weight status were used for categorizing the participants in one of the four groups including underweight, normal weight, overweight and obese. Fat mass, WHtR, SMM and fat to SMM ratio were compared between quartiles (25, 50, 75 and 100 percentiles). Bonferroni post hoc test was used for multiple comparisons. Cohen’s d (i.e., negligible for |d| < 0.2; small for 0.2 ≤ |d| < 0.5; medium for 0.5 ≤ |d| < 0.8; and large for |d| ≥ 0.8) was calculated based on the partial η2 statistics to interpret the magnitude of the effect size [39]. Assuming a power of 0.80, and an alpha of 0.05, a sample of 80 is required for MANCOVA to explore the large effect size for analyses with four groups [40]. Results of Levene’s test of equality of error variance was found non-significant for all the dependent variables (p > 0.05).
All calculations were performed using SPSS v.21.0 software for Windows (IBM, Corp., Armonk, NY, USA). Statistical significance was set at p < 0.05.
Results
General characteristics
Mean of the demographic, physiological, and physical characteristics, as well as EMG RT and computerized RT of the participants, are shown in Table 1. The prevalence of underweight, normal weight, overweight and obesity was 18.8% (n = 15), 42.5% (n = 34), 21.2% (n = 17), and 17.5% (n = 14), respectively.
Factor analysis
The results of factor analysis (Table 2) indicated that the two factors accounted for 65.30% of the total variance. Kaiser–Meyer–Olkin (KMO) measure of sampling adequacy was found 0.66, which shows the sample size is adequate. Bartlett’s test of sphericity (Approx. χ2 = 218.40; p < 0.001) indicated that the variables are related to each other and therefore are suitable for structure detection.
Comparison approach
There were no significant differences in the composite computerized RT or PMT scores between the BMI categories as well as between the fat mass or fat to SMM quartiles in the unadjusted and adjusted models (Figs. 1, 2, 3).
Composite computerized RT scores and composite PMT scores between the BMI categories. PMT premotor time which was measured using RT on initiation and termination of upper extremities contraction against audio signal; RT total computerized reaction time. Natural log transformation data of total-PA was used as covariate. Composite of T score of systolic and diastolic blood pressure was used a covariate. #Values are reported after adjustment for age, SES, depressive symptoms, total PA, and blood pressure
Composite computerized RT scores and composite PMT scores between the fat mass quartiles. PMT premotor time which was measured using RT on initiation and termination of upper extremities contraction against audio signal; RT total computerized reaction time. Natural log transformation data of total-PA was used as covariate. Composite of T score of systolic and diastolic blood pressure was used a covariate. #Values are reported after adjustment for age, SES, depressive symptoms, total PA, and blood pressure
Composite computerized RT scores and composite PMT scores between the fat mass to SMM ratio quartiles. PMT premotor time which was measured using RT on initiation and termination of upper extremities contraction against audio signal; RT total computerized reaction time; SMM skeletal muscle mass. Natural log transformation data of total-PA was used as covariate. Composite of T score of systolic and diastolic blood pressure was used a covariate. #Values are reported after adjustment for age, SES, depressive symptoms, total PA, and blood pressure
Differences between WHtR quartiles in composite RT scores and composite PMT scores are shown in Fig. 4. The results of unadjusted analysis indicted a longer composite PMT scores for the Q3 group than the Q2 (Unadj. p = 0.02, Fig. 4a). Likewise, using adjusted analysis, a longer composite PMT scores was observed for the Q3 group compared to the Q1 (Unadj. p = 0.02, Fig. 4b) and Q2 group (Unadj. p = 0.007, Fig. 4b). This suggests that the individuals with a higher central obesity had a longer PMT than their peers with a lower central obesity. The observed effect size for the PMT differences between the WHtR quartiles and for the unadjusted (d = 1.05) and adjusted (d = 1.23) analysis indicates a large negative impact of central obesity on PMT in young adult males. No composite RT scores differences were observed between WHtR quartiles in either unadjusted or adjusted analysis (Fig. 4a, b).
Composite computerized RT scores and composite PMT scores between the WHtR quartiles. PMT premotor time which was measured using RT on initiation and termination of upper extremities contraction against audio signal; RT total computerized reaction time; WHtR waist to height ratio. Natural log transformation data of total-PA was used as covariate. Composite of T score of systolic and diastolic blood pressure was used a covariate. #Values are reported after adjustment for age, SES, depressive symptoms, total PA, and blood pressure
Using either unadjusted or adjusted comparisons no composite PMT scores differences were observed between the SMM quartiles (Fig. 5a, b). Finally, although not statistically significant a longer composite RT scores were observed in the Q2 group compared to the Q3 group in the adjusted analysis (p = 0.05, d = 0.72; Fig. 5b), such that individuals with a higher SMM tended to have a shorter RT.
Composite computerized RT scores and composite PMT scores between the SMM quartiles. PMT premotor time which was measured using RT on initiation and termination of upper extremities contraction against audio signal; RT total computerized reaction time. Natural log transformation data of total-PA was used as covariate. Composite of T score of systolic and diastolic blood pressure was used a covariate. #Values are reported after adjustment for age, SES, depressive symptoms, total PA, and blood pressure
Discussion
No statistically significant differences were observed for RT or PMT between overall obesity or body composition groups. However, when compared between central obesity groups higher central adiposity (WHtR) was associated with a longer PMT, such as that individuals with higher central adiposity had inferior PMT. This partially supports our hypothesis that PMT is a more sensitive variable than overall RT to detect possible associations with adiposity or body composition in young adults.
RT is an important marker for cognitive ability during youth ages and is associated with functionality at older ages. Therefore, it is important to understand factors that can assist in developing RT already at youth. Because the association between weight status and RT in young adults has been inconsistent [17,18,19,20,21,22, 25], we used novel methods to test if RT (indicating overall computerized RT) and PMT (indicating a central component of RT) differ by adiposity and body composition.
Adiposity is associated with neurological disorders and cognitive impairment, which may develop during adulthood and emerge at old age [24, 41]. A limitation of previous studies underlying the association between weight status and RT is the use of BMI as the only measure of obesity index [21, 22, 25]. However, other obesity indices such as central obesity, are more closely associated with health risk factors [42]. Furthermore, subcutaneous fat percentage has been suggested as a better overall body obesity predictor than BMI, due to the reason that BMI as a measure of overall obesity index does not take into account for varying proportions of fat, bone, and muscle mass [43].
Systemic inflammation, impaired insulin regulation, and elevated triglycerides have been suggested as some of the biological mechanisms in which greater adiposity can impair cognitive function [44]. However, these mechanisms have solely been examined in old populations or in animals, and further studies in a young population are needed [44] for a better understanding on the biological mechanisms of the association between central adiposity and PMT in the young adult males.
Even though overweight/obesity is associated with impaired cognitive function, recent review papers [12, 13] have discussed the importance of covariates (i.e. age, SES, blood pressure, depression, etc.) which might influence this association. For example, inconsistent results have been reported concerning the association between weight status and RT in the literature. Skurvydas et al. observed that normal weight males performed better in simple RT test than their overweight peers [21]. Similarly, Nene et al. found that underweight females achieved the worst scores while performing simple visual RT tasks compared to normal weight females. However, the performance was similar between the overweight and the normal weight or underweight for visual RT [22]. However, Deore et al. noted that normal weight females performed better in simple visual RT compared to underweight and overweight participants [20]. Finally, Esmaeilzadeh observed no association between weight status (using various obesity indices) and a simple clinical measure of RT (RTclin) in schoolboys after adjustment for age [17]. However, Moradi and Esmaeilzadeh, found an association between percentage fat and simple visual RT [19] in schoolboys and adjusting for age, SES and PA. Most recently, Esmaeilzadeh et al. found no association between weight status (i.e. BMI) and RT (simple and choice RT) when examined a sample of young adult men and adjusting for age, SES and PA [18]. As a summary, some studies have reported an association between simple RT and weight status (i.e., BMI) in young people [20,21,22], while others did not observe this association [17, 18, 25].
A possible reason for observing no difference in RT between the overall obesity indices may be due to the participants’ age, as the potential changes might only emerge at an older age [41]. It should be noted that the existing literature underlying the association between cognitive function and weight status has mainly focused on elderly people when there is a cognitive decline, or children when the brain is still developing. The present study included individuals at the age between 17 and 25 years, which is a distinct developmental period, lying between childhood and adulthood. Therefore, the association between weight status and simple cognitive tasks such as RT might not be evident at this age [17,18,19, 25] since cognitive functioning development has reached a peak, and therefore being elusive. However, a novel finding of this study is that higher central adiposity had a negative impact on cognitive processing in young adult males measured by PMT.
Another interesting finding of the present study was the investigation of computerized RT and PMT differences between SMM and fat to SMM ratio quartiles. We hypothesized that higher SMM would be positively associated with RT in young males. Although PMT was similar between the SMM quartiles, the computerized RT tended to be better in participants with higher SMM compared to participants with lower SMM. This indicates that in contrast to higher adiposity which might have a negative impact on central processing in the young adult males, higher SMM seems has a positive impact on RT. Recent evidence suggests an association between the decrease in SMM and impaired cognitive function independent of adjustment for covariates such as sex, SES, smoking, etc. in older people [15, 16, 45]. It has been suggested that a loss of SMM in older people is linked with pathophysiological pathways in the brain, leading to an early stage of cognitive impairment [46]. Therefore, a higher SMM is likely to be a protective factor for cognitive diminish in older people [16, 45, 46]. However, little is known about the association between SMM and cognitive function in young people. Furthermore, the results of the present study need to be interpreted with caution, due to medium effect size (d = 0.72) for the computerized RT difference and the difference which was observed only between Q2 and Q3 groups.
Strengths and limitations
The study has some strengths including the novel methodology used to measure PMT and RT, use of objective methods for measuring fat mass and SMM, including various obesity and body composition variables and various weight status groups (such as underweight or obesity). The study also used both linear and comparison approaches and controlled for known potential covariates such as age, SES, PA, blood pressure and depressive symptoms. However, the study has some limitations including its cross-sectional nature, small sample size and inclusion of male participants only. Therefore, longitudinal studies in both males and females are required to confirm these findings.
Conclusion
Since the brain and cognitive function reach a peak during young adulthood, sensitive methodologies are needed to explore the association between weight status and processing speed in this period of life. We used novel methods to test if computerized RT (indicating the overall RT) and PMT (indicating a central component of RT) differ by adiposity and body composition. We found that central adiposity was associated with a longer PMT, whereas SMM was associated with a shorter overall computerized RT in the young males. These findings provide the basis for further studies exploring the longitudinal association between central obesity and muscle mass and processing speed impairment at a youth age. This is important as higher adiposity or lower SMM might increase at an older age and affect processing speed [41, 45, 46], therefore optimizing brain function at youth may benefit lifelong brain remodelling [47].
Abbreviations
- BDI-II:
-
BECK depression inventory-II
- BIA:
-
Bio-electrical impedance analysis
- BMI:
-
Body mass index
- CANTAB:
-
Cambridge Neuropsychological Test Battery
- EMG:
-
Electromyography
- EMG RT:
-
Electromyographic analysis of RT
- 5-CRT:
-
Five-choice RT
- KMO:
-
Kaiser–Meyer–Olkin
- IPAQ:
-
Long form international physical activity questionnaire
- Onset3:
-
Mean RT in initiation of 3 s contractions
- Offset3:
-
Mean RT in termination of 3 s contractions
- Onset6:
-
Mean RT in initiation of 6 s contractions
- Offset6:
-
Mean RT in termination of 6 s contractions
- MT:
-
Motor time
- MANCOVA:
-
Multivariate analysis of covariance
- PMT:
-
Premotor time
- RT:
-
Reaction time
- SRT:
-
Simple RT
- SMM:
-
Skeletal muscle mass
- SES:
-
Socioeconomic status
- 2-CRT:
-
Two-choice RT
- WHtR:
-
Waist to height ratio
References
Wang D, Zhang J, Sun Y et al (2016) Evaluating the fall risk among elderly population by choice step reaction test. Clin Interv Aging 11:1075–1082. https://doi.org/10.2147/CIA.S106606
Salvia E, Petit C, Champely S, Chomette R, Di Rienzo F, Collet C (2016) Effects of age and task load on drivers’ response accuracy and reaction time when responding to Traffic lights. Front Aging Neurosci 8:169. https://doi.org/10.3389/fnagi.2016.00169
Der G, Deary IJ (2006) Age and sex differences in reaction time in adulthood: results from the United Kingdom health and lifestyle survey. Psychol Aging 21:62–73. https://doi.org/10.1037/0882-7974.21.1.62
Sheppard LD, Vernon PA (2008) Intelligence and speed of information-processing: a review of 50 years of research. Pers Individ Dif 44(3):535–551. https://doi.org/10.1016/j.paid.2007.09.015
Fry AF, Hale S (1996) Processing speed, working memory and fluid intelligence: evidence for a developmental cascade. Psychol Sci 7:237–241. https://doi.org/10.1111/j.1467-9280.1996.tb00366.x
Kail R (2000) Speed of information processing: developmental change and links. J Sch Psychol 38:51–61. https://doi.org/10.1016/S0022-4405(99)00036-9
McAuley T, White DA (2011) A latent variables examination of processing speed, response inhibition, and working memory during typical development. J Exp Child Psychol 108(3):1–16. https://doi.org/10.1016/j.jecp.2010.08.009
Nettelbeck T, Burns NR (2010) Processing speed, working memory and reasoning ability from childhood to old age. Pers Individ Dif 48:379–384. https://doi.org/10.1016/j.paid.2009
Henneberg M, Brush G, Harrison GA (2001) Growth of specific muscle strength between 6 and 18 years in contrasting socioeconomic conditions. Am J Phys Anthropol 115:62–70. https://doi.org/10.1002/ajpa.1057
Noble KG, Norman MF, Farah MJ (2005) Neurocognitive correlates of socioeconomic status in kindergarten children. Dev Sci 8:74–87. https://doi.org/10.1111/j.1467-7687.2005.00394.x
Gomez-Pinilla F, Hillman C (2013) The inluence of exercise on cognitive abilities. Compr Physiol 3:403–428. https://doi.org/10.1002/cphy.c110063
Liang J, Matheson BE, Kaye WH, Boutelle KN (2014) Neurocognitive correlates of obesity and obesity-related behaviors in children and adolescents. Int J Obes Lond 38(4):494–506. https://doi.org/10.1038/ijo.2013.142
Pricketta C, Brennanc L, Stolwyk R (2015) Examining the relationship between obesity and cognitive function: a systematic literature review. Obes Res Clin Pract 93:113. https://doi.org/10.1016/j.orcp.2014.05.001
Eleuteri S, Norton MC, Livi F et al (2018) Sleep quality as predictor of BMI in non-depressed caregivers of people with dementia. Eat Weight Disord 23(5):553–560. https://doi.org/10.1007/s40519-018-0560-8
Noh HM, Oh S, Song HJ et al (2017) Relationships between cognitive function and body composition among community-dwelling older adults: a cross-sectionalstudy. BMC Geriatrics 17:259. https://doi.org/10.1186/s12877-017-0651-9
Nourhashémi F, Andrieu S, Gillette-Guyonnet S et al (2002) Is there a relationship between fat-free soft tissue mass and low cognitive function? results from a study of 7105 women. J Am Geriatr Soc 50:1796–1801. https://doi.org/10.1046/j.1532-5415.2002.50507.x
Esmaeilzadeh S (2014) Reaction time: does it relate to weight status in children? HOMO J Comp Hum Biol 65:171–178. https://doi.org/10.1016/j.jchb.2013.09.007
Esmaeilzadeh S, Farzizadeh R, Kalantari HA et al (2018) Is obesity associated with impaired reaction time in youth? Eat Weight Disord 15:20. https://doi.org/10.1007/s40519-018-0614-y
Moradi A, Esmaeilzadeh S (2017) Simple reaction time and obesity in children: whether there is a relationship? Environ Health Prev Med 22:2. https://doi.org/10.1186/s12199-017-0612-0
Deore DN, Surwase SP, Masroor S, Khan ST, Kathore V (2012) A cross sectional study on the relationship between the body mass index (BMI) and the audiovisual reaction time (ART). J Clin Diagn Res 6:1466–1468. https://doi.org/10.7860/JCDR/2012/4440.2534
Skurvydas A, Gutnik B, Zuoza AK et al (2009) Relationship between simple reaction time and body mass index. HOMO J Comp Hum Biol 60:77–85. https://doi.org/10.1016/j.jchb.2008.06.006
Nene AS, Pazare PA, Sharma KD (2011) A study of relation between body mass index and simple reaction time in healthy young females. Indian J Physiol Pharmacol 55:288–291
Botwinick J, Thompson LW (1966) Premotor and motor components of reaction time. J Exp Psychol 71(1):9–15. https://doi.org/10.1037/h0022634
O’Brien PD, Hinder LM, Callaghan BC, Feldman EL (2017) Neurological consequences of obesity. Lancet Neurol 16(6):465–477. https://doi.org/10.1016/S1474-4422(17)30084-4
Gunstad J, Spitznagel MB, Paul RH et al (2008) Body mass index and neuropsychological function in healthy children and adolescents. Appetite 50:246–251. https://doi.org/10.1016/j.appet.2007.07.008
Rodea-Montero ER, Evia-Viscarra ML, Apolinar-Jiménez E (2014) Waist-to-height ratio is a betteranthropometric index than waist circumference and BMI in predicting metabolic syndrome among obese mexican adolescents. Int J Endocrinol 2014:195407. https://doi.org/10.1155/2014/195407
Swainson MG, Batterham AM, Tsakirides C, Rutherford ZH, Hind K (2017) Prediction of whole-body fat percentage and visceral adipose tissue mass from five anthropometric variables. PLoS ONE 12(5):e0177175. https://doi.org/10.1371/journal.pone.0177175
Ashwell M, Gibson S (2016) Waist-to-height ratio as an indicator of ‘early health risk’: simpler and more predictive than using a ‘matrix’ based on BMI and waist circumference. BMJ Open 6:e010159. https://doi.org/10.1136/bmjopen-2015-010159
Sahakian BJ, Owen AM (1992) Computerized assessment in neuropsychiatry using cantab—discussion paper. J R Soc Med 85:399–402 (PMID:1629849 PMCID:PMC1293547)
Smith PJ, Need AC, Cirulli ET, Chiba-Falek O, Attix DK (2013) A comparison of the Cambridge Automated Neuropsychological Test Battery (CANTAB) with “traditional” neuropsychological testing instruments. J Clin Exp Neuropsychol 35:319–328. https://doi.org/10.1080/13803395.2013.771618
Nissen MJ (1977) Stimulus intensity and information processing. Percept Psychophys 22:338–352. https://doi.org/10.3758/BF03199699
Hodges PW, Bui BH (1996) A comparison of computer-based methods for the determination of onset of muscle contraction using electromyography. Electroencephalogr Clin Neurophysiol 101:511–519. https://doi.org/10.1016/S0921-884X(96)95190-5
Craig CL, Marshall AL, Sjöström M et al (2003) International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 35(8):1381–1395. https://doi.org/10.1249/01.MSS.0000078924.61453.FB
Beck AT, Steer RA, Brown GK, Lindfors J (2006) BDI-II: beck depression inventory: manual, svensk version. Stockholm, Psykologifo ¨rlaget
Ghassemzadeh H, Mojtabai R, Karamghadiri N, Ebrahimkhani N (2005) Psychometric properties of a Persian-language version of the Beck depression inventory-second edition: bDI-II-PERSIAN. Depression Anxiety 21:185–192. https://doi.org/10.1002/da.20070
Tabachnick BG, Fidell LS (2014) Using Multivariate Statistics, 6th edn. Pearson new international edition, Harlow, United Kingdom
Cole TJ, Bellizzi MC, Flegal KM, Dietz WH (2000) Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 320:1240–1243
Cole TJ, Flegal KM, Nicholls D, Jackson AA (2005) Body mass index cut offs to define thinness in children and adolescents: international survey. BMJ 335:194–197
Cohen J (1988) Statistical power analysis for the behavioral sciences. Lawrence Erlbaum, Mahwah
Faul F, Erdfelder E, Lang AG, Buchner A (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39:175–191. https://doi.org/10.3758/BF03193146
Wang J, Freire D, Knable L et al (2015) Childhood and adolescent obesity and long-term cognitive consequences during aging. J Comp Neurol 523(5):757–768. https://doi.org/10.1002/cne.23708
Lindqvist P, Andersson K, Sundh V et al (2006) Concurrent and separate effects of body mass index and waist-to-hip ratio on 24-year mortality in the population Study of Women in Gothenburg: evidence of age-dependency. Eur J Epidemiol 21:789–794. https://doi.org/10.1007/s10654-006-9074-1
Nooyens AC, Koppes LL, Visscher TL et al (2007) Adolescent skinfold thickness is a better predictor of high body fatness in adults than is body mass index: the Amsterdam Growth and Health Longitudinal Study. Am J Clin Nutr 85:1533–1539. https://doi.org/10.1093/ajcn/85.6.1533
Smith E, Hay P, Campbell L, Trollor JN (2011) A review of the association between obesity and cognitive function across the lifespan: implications for novel approaches to prevention and treatment. Obes Rev 12:740–755. https://doi.org/10.1111/j.1467-789X.2011.00920.x
Bae S, Shimada H, Park H et al (2017) Association between body composition parameters and risk of mild cognitive impairment in older Japanese adults. Geriatr Gerontol Int 17(11):2053–2059. https://doi.org/10.1111/ggi.13018
Burns JM, Johnson DK, Watts A, Swerdlow RH, Brooks WM (2010) Reduced leanmass in early Alzheimer disease and its association with brain atrophy. Arch Neurol 67:428–433. https://doi.org/10.1001/archneurol.2010.38
Fuchs E, Flugge G (2014) Adult neuroplasticity: more than 40 years of research. Neural Plast 2014:e541870. https://doi.org/10.1155/2014/541870
Funding
No funding was received for performing the present study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the Human Ethics Committee of University of Mohaghegh Ardabili and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of topical collection on Males and eating and weight disorders.
Rights and permissions
About this article
Cite this article
Narimani, M., Esmaeilzadeh, S., Pesola, A.J. et al. Impact of obesity on central processing time rather than overall reaction time in young adult men. Eat Weight Disord 24, 1051–1061 (2019). https://doi.org/10.1007/s40519-019-00752-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40519-019-00752-2