Abstract
Anorexia nervosa (AN) is an eating disorder that most frequently afflicts females in adolescence. In these subjects, cardiovascular complications are the main cause of morbidity and mortality. Aim of this review is to analyze the hemodynamic, pro-arrhythmic and structural changes occurring during all phases of this illness, including re-feeding. A systematic literature search was performed on studies in the MEDLINE database, from its inception until September 2017, with PUBMED interface focusing on AN and cardiovascular disease. This review demonstrated that the most common cardiac abnormalities in AN are bradycardia and QT interval prolongation, which may occasionally degenerate into ventricular arrhythmias such as Torsades des Pointes or ventricular fibrillation. As these arrhythmias may be the substrate of sudden cardiac death (SCD), they require cardiac monitoring in hospital. In addition, reduced cardiac mass, with smaller volumes and decreased cardiac output, may be found. Furthermore, mitral prolapse and a mild pericardial effusion may occur, the latter due to protein deficiency and low levels of thyroid hormone. In anorectic patients, some cases of hypercholesterolemia may be present; however, conclusive evidence that AN is an atherogenic condition is still lacking, although a few cases of myocardial infarction have been reported. Finally, refeeding syndrome (RFS), which occurs during the first days of refeeding, may engender a critically increased risk of acute, life-threatening cardiac complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anorexia nervosa (AN) is an eating disorder characterized by a distorted perception of body image and weight and an irrational fear of gaining weight, which prompt behaviors that cause body weight to remain below the limit for age, sex and physical health [1, 2]. It is subdivided into two forms:
-
restricting type, when food intake is kept low, often by adhering to strict rules (e.g., limited number of meals, caloric restriction, food selection).
-
binge-eating/purging type, when food intake may be unrestricted but is followed by purging behaviors, such as self-induced vomiting or abuse of laxatives or diuretics.
AN is a typical disorder of adolescence and mostly affects young women (10:1 female–male ratio); nevertheless, AN may be under-diagnosed in males, who seem to have poor outcomes. The incidence of AN has recently been reported to be 270 per 100,000 person-years in adolescents [3]. However, these data are not conclusive and may underestimate the burden of AN, as the binge-eating/purging type is less commonly recognized. Furthermore, the diagnostic criteria for this latter type of AN were changed in the fifth edition of the Diagnostic and Statistical Manual of mental disorders—DSM V, leading to a marked increase in the number of diagnoses since 2013.
The etiology and pathophysiology of AN are complex, involving genetic, neurobiological, psycho-developmental, social and cultural factors, and the person as a whole is affected, in both the psychic dimension and the majority of biological functions [4, 5]. Since the 1980s, it has been known that the cardiovascular system is altered in AN, and that cardiac complications worsen the prognosis of the disease [6,7,8]. In AN, the most feared cardiac abnormalities are ventricular arrhythmias (e.g. Torsade des Pointes), since they may be the substrate of sudden cardiac death (SCD). A fivefold higher mortality rate has been described in AN than in the general population [9, 10], and it is thought that starvation- and purging-related arrhythmias are a significant cause of death, especially when alcohol or other substance abuse is also present [9, 11, 12]. However, cardiac disease in AN is multifaceted.
In this paper, we review the cardiac complications of AN and give practical indications for their recognition and management.
Methods
A systematic search was conducted in the MEDLINE database with PUBMED interface from 1st April 2017 to 30th August 2017 by two independent authors, using the following keywords: “anorexia nervosa” AND one or more among: “sudden death”, “long QT”, “QT prolongation”, “prolonged QT”, “cardiovascular”, “heart”, “arrhythmia”, “mortality”.
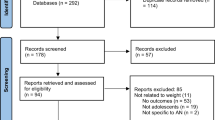
A total of 1987 records were found by means of these keywords. After the removal of duplicates, 292 records remained. We excluded 159 articles, in that they focused on eating disorders other than AN, were written in languages other than English or did not provide the full text.
Results
After collegial examination, 88 articles were considered suitable for this review. In anorectic patients, cardiovascular involvement was subdivided into conduction, structural and ischemic disease. RFS can display a broad spectrum of manifestations, including life-threatening cardiac events.
Conduction disease, ventricular repolarization and arrhythmias
Up to 87% of patients suffer from cardiovascular compromise early in AN [13]. Malnutrition causes cellular changes within cardiac muscle, leading to structural, functional or conduction complications [14]. Sinus bradycardia as an adaptation to the hypometabolic state is commonly observed [15]. Up to 85% may suffer from hypotension—less than 90/60 mmHg—usually secondary to chronic volume depletion and orthostatic changes, resulting in frequent episodes of dizziness and occasional frank syncope [16].
Patients have low cardiac output and increased peripheral vascular resistance, despite the presence of hypotension [6]. Starvation leads to low body weight, which may result in atrophic peripheral muscles, resulting in decreased venous return to the heart [17, 18]. Bradycardia during AN is reversible following nutritional treatment. It is important to point out that bradycardia may originate from conduction system disease; it may, therefore, require cardiac monitoring in hospital or, rarely, permanent pacemaker implantation [19,20,21]. In any case, according to international guidelines, permanent electrostimulation is indicated in class II in the case of heart rate < 30 bpm on waking or pause > 3 s during waking hours. However, these conditions are very rare in AN, and there is general consensus that, even if severe symptomatic bradycardia or high-grade atrioventricular block is observed, no temporary or permanent pacemaker implantation is indicated in anorexia patients [22].
On 12-lead ECG, prolongation of the QT interval, i.e. the time from the Q wave onset to the end of the T wave, indicates defects in ventricular repolarization. In the presence of a prolonged QT interval, early post-depolarization may determine ectopic ventricular beats that trigger ventricular arrhythmias, such as torsades des pointes (TdP) and ventricular fibrillation, potentially culminating in SCD. QT interval prolongation is a common finding in AN; thus, 12-lead ECG and QT interval analysis are recommended in these patients [23].
When measuring the QT interval, the heart rate must be taken into account, as it influences QT duration. This is even more important in AN, since vagal hyperactivity with sinus bradycardia often occurs in the early phase of the disease, while resting tachycardia is common in long-standing AN as a consequence of autonomic dysfunction or medical complications, such as infection or chronic hypovolemia [24, 25]. There are several formulas (Bazett, Framingham, Sagie, Fridericia and Hodges) for calculating the heart rate-corrected QT interval (QTc), but there is no general agreement on which is the most accurate in AN. Walter and colleagues suggested that Hodges’ formula was the best choice for patients with AN, because in healthy people it seems to be less correlated with heart rate [26]. The heterogeneity of ventricular depolarization can be also assessed by measuring the difference between the longest and the shortest QT interval on the same 12-lead ECG, so-called QT interval dispersion. Although this latter critically depends on heart rate, it has been associated with potentially fatal arrhythmias in AN, as in patients with congenital or acquired QT prolongation [27, 28].
A QTc > 500 ms and a QTc dispersion > 60 ms may be considered the thresholds above which the risk of ventricular arrhythmias is substantially increased, even though a strong correlation with arrhythmias has been reported only for QTc of 600 ms or more [12, 29]. Subjects with AN and a QTc > 500 ms should be admitted for biochemical assessment and continuous electrocardiographic recording by means of telemetry or serial ECGs. When the QTc is longer than > 470 ms, patients should be promptly evaluated [7]. Importantly, the QT may be normal or mildly prolonged at rest, but become severely long during exercise, as in long-QT syndrome [30, 31]. QT prolongation can be demonstrated by means of exercise stress testing, which must be performed by experienced personnel trained in the management of cardiac arrhythmias and arrest. QT prolongation constitutes a very dangerous problem, since anorectic patients often engage in intense physical activity. In this regard, Nagata et al., in an interesting cross-sectional study, showed that adolescents with AN and other eating disorders reported high levels of exercise, with females in particular reporting greater participation in team sports. Their study revealed that, in these patients, bradycardia was associated with greater exercise frequency and participation in team sports [32].
Heart rate variability, which defines the physiological oscillations of the intervals between successive heart beats over time, is usually higher in AN than in controls because of enhanced vagal stimulation and sympathetic deficiency, especially before the disease progresses to an advanced stage, and this pattern has been related to prolonged QTc [33]. Nevertheless, the actual involvement of increased heart rate variability in QT-dependent ventricular arrhythmias has been questioned [34] and, at present, assessment of heart rate variability should be limited to research purposes and not used to guide clinical decisions.
Hydro-electrolytic disturbances are the main cause of QT lengthening in patients with AN; when serum levels of ions are normal, only a mild prolongation of the QTc is observed in AN, which probably reflects autonomic nervous system dysfunction [35].
Hyponatremia in AN may be due to malnutrition, the chronic use of diuretics or high water intake, though water restriction is more common in anorectic subjects. Hypokalemia is the most common electrolytic disorder and is most often due to a purging behavior, with abuse of diuretics and laxatives and self-induced vomiting [36]. In a retrospective study, Seidler and colleagues reviewed potassium levels on admission after resuscitation in 283 patients and found lowers values in those with eating disorders [37]. Hypomagnesemia, when combined with hypokalemia, increases the probability of TdP, ventricular fibrillation and heart failure; it rarely causes arrhythmias alone [38]. Hypermagnesemia may be also a complication of purging behavior, due to the chronic assumption of laxatives; when severe (> 5 mg/dL), it may result in arrhythmias and cardiac arrest [39]. Hypocalcemia and hypophosphatemia are not rare in AN and may contribute to electrical instability of the heart [40, 41].
Clinicians should bear in mind that electrolytic depletion is associated with several other changes in the ECG, besides QTc prolongation, such as ST-segment depression, prominent U waves, ventricular ectopic beats, and tall P waves.
Renal impairment surely plays a role in determining all these hydro-electrolytic disturbances in AN patients. In a retrospective study evaluating a cohort of adolescent AN patients, Stheneur et al. [42] documented renal impairment, which occurs especially when BMI and heart rate were very low. In their study, a strong association emerged between body mass index (BMI) and glomerular filtration rate (GFR), low heart rates being significantly associated with reduced GFR (according to the Cockroft–Gault equation).
Anti-depressants and neuroleptic agents, the drugs most commonly prescribed in AN, may increase the risk of SCD through direct interaction with ion channels [43]. This risk of pro-arrhythmic activity is aggravated by concomitant electrolytic alterations, comorbidities such as hepatic dysfunction or hypothyroidism, or a favorable anatomical substrate [44]. Neuroleptics, including such new-generation molecules as olanzapine, risperidone, quetiapine and aripiprazole, and tricyclic antidepressants (imipramine, desipramine, amitriptyline, clomipramine) affect sodium, potassium and calcium channels, leading to QT prolongation and an increased risk of TdP. Pharmacovigilance studies have pointed to an effect on the QTc of high doses of the most recent selective serotonin reuptake inhibitors, e.g. citalopram and escitalopram, with cases of fatal arrhythmias [29].
Psychotropic drugs should be prescribed after considering their intrinsic pro-arrhythmic risk (Table 1) and after QT interval analysis, correction of any electrolyte abnormality and, if necessary, cardiologist consultation. After starting these medications, patients should undergo regular ECG monitoring, and the dosage and/or type should be re-discussed if the QTc and QTc dispersion are > 500 ms and > 60 ms, respectively. Therapy may also be continued unchanged in asymptomatic subjects, but evaluation by a cardiologist is mandatory [23].
Structural heart disease
Pathological changes in the myocardium similar to those observed in various cardiomyopathies have been reported in AN, such as mitochondrial swelling and loss, the accumulation of lipofuscin or myxoid material, interstitial edema, fibrosis, and myofibrillar atrophy [45,46,47]. When the left ventricular structure and function are evaluated by means of echocardiography, reduced cardiac mass with smaller cavity diameters and decreased cardiac output are found in AN subjects in comparison with controls. Moreover, in a recent paper, Kuwabara et al. reported that anorectic patients present a low ventricular mass index, which correlates well with low body mass index [48,49,50,51,52] (Table 2).
Diastolic dysfunction has been also described [53, 54]. Mitral valve prolapse is common, but its clinical significance is uncertain [55]. Doppler tissue evaluation can help to identify subclinical forms and to stratify patients, especially in combination with N-terminal pro-type B natriuretic peptide measurement [56, 57]. Risk stratification may also be improved by evaluating inferior vena cava diameters and inspiratory collapsibility [58, 59]. Finally, the most recent echocardiographic techniques, such as speckle tracking echocardiography-derived strain imaging, which enables cardiac dysfunction to be detected prior to the appearance of overt symptoms, may be clinically useful in identifying those AN patients who are at increased risk of developing cardiac involvement. Morris et al. recently demonstrated that a subgroup of AN children who enacted purging behavior displayed left ventricular remodeling. Furthermore, all AN patients in their study present impaired regional ventricular function at the apex, as measured by strain imaging [60].
The pathophysiology of these alterations is only partially known. In addition to the effects of hypovolemia and caloric restriction, a role may be played by a deficiency of thiamine and, possibly, other vitamins, hormonal imbalances [61, 62] and direct toxicity of substances abused in purging behavior, such as ipecac, an emetic syrup easily available over the counter [63].
The active form of thiamine (thiamine pyrophosphate) is an essential coenzyme for decarboxylation in carbohydrate metabolism and energy production in the form of adenosine triphosphate (ATP). In the event of deficiency of this essential vitamin, particularly of the active form (beri–beri disease), pyruvate and some aminoacids become unavailable in many metabolic pathways, through the inhibition of the citric acid cycle and hexose monophosphate shunt; this leads to the accumulation of pyruvate and lactate, which is followed by intense vasodilation and high-output cardiac failure, with increased risk of death [64,65,66,67]. Although previous studies yielded conflicting results, with a prevalence of vitamin B1 deficiency that varied between 0 and 13%, Winston et al. observed a 38% prevalence of vitamin B1 deficiency (19% being particularly severe [61]). In their experience, these authors did not find any correlations with the duration of eating restriction, frequency of vomiting, or alcohol consumption. In any case, high-dose oral or parenteral supplementation is indicated. Specifically, Hofer et al. suggest 200–300 mg IV or PO daily during re-feeding [68].
Uncommonly, AN patients may present with acute heart failure [45]. Acute left ventricular dysfunction may also be due to Takotsubo cardiomyopathy [69]. This latter is characterized by transient myocardial stunning, typically involving only a part of the left ventricle, triggered by physical or psychological stress. In AN, triggers may be starvation, malnutrition, hypoglycemia and re-feeding. As in subjects without AN [69, 70], in anorectic patients Takotsubo cardiomyopathy may present with acute heart failure, but also ischemic stroke, heart perforation or fatal arrhythmias with SCD [71].
About 35% of AN patients have pericardial effusion, which is, however, most often mild and without hemodynamic significance [7]. This complication has been correlated with low body weight, protein deficiency, and levels of thyroid hormone and insulin-like growth factor-1, and generally reverts with weight restoration [72]. Indeed, cases of cardiac tamponade or pericardial effusion requiring drainage are anecdotal [73,74,75].
Ischemic heart disease: atherosclerosis and coronary artery disease
Hypercholesterolemia is frequent in AN as a result of heightened lipolysis, reduced cholesterol removal, and greater activity of the cholesterol-ester-transfer protein. Concentrations of total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol and apolipoprotein (A1, B, C2, C3, E) are higher in patients with AN, especially the binging-purging subtype, than in controls. These perturbations in lipid profile are mainly ascribed to the alterations of many endocrine axes, and generally normalize with weight gain [76,77,78]. The long-term use of antipsychotic drugs, particularly phenothiazine neuroleptics, has also been associated with the development of dyslipidemia, as well as of diabetes mellitus [29]. Since it is not known whether high cholesterol levels in AN require specific treatment and, if so, which values should be targeted, measuring cholesterol as part of standard biochemical evaluation is not advisable.
Solmi et al. showed that circulating markers of oxidative stress are higher in subjects with AN than in healthy controls, and chronic oxidative stress may promote endothelial dysfunction and plaque instability or rupture [79].
However, conclusive evidence that AN is an atherogenic condition is still lacking. For instance, Birmingham and colleagues measured the intima-medial thickness of the carotid artery, which is a strong predictor of atherosclerotic coronary artery and cerebrovascular disease, and found no difference between AN patients and controls [80]. Furthermore, ischemic heart disease does not seem to be more common in AN than in the general population, even though a few cases of acute myocardial infarction have been reported [81, 82].
Re-feeding syndrome
Re-feeding syndrome (RFS) defines a series of hemodynamic and electrolytic alterations occurring in AN during the first days of re-feeding after sustained caloric restriction; it is related to the abrupt transition of metabolism from catabolic to anabolic [83]. RFS can display a broad spectrum of manifestations, including life-threatening cardiac events (Fig. 1). Thus, efforts should be made to avoid it; patients must be admitted to hospital for monitoring and caloric intake must be immediately reduced if RFS is suspected [84]. Early nutritional support is mandatory to reduce AN morbidity and mortality.
The re-feeding phase should be carefully implemented to reduce the risk of life-threatening complications during the first 10 days. Preventive support should be gradually administered by means of electrolytes, vitamins (particularly B1) and fluids.
Phosphate levels (< 1 mmol/l or < 3 mg/dl) play a critical role in RFS; a level < 0.30 mmol/l could be a particularly life-threatening factor, which must be tightly controlled, and phosphate supplementation in the first days of re-feeding is mandatory.
The most serious complications are likely to occur in cases of severe malnutrition (BMI < 10) and of overfeeding in the early stages without adequate supplementation of micro-nutrients [68, 85,86,87,88].
Conclusions
AN may be complicated by heart disease, which may be clinically severe. This supports the paradigm that patients with AN should be cared for by a multidisciplinary team, including a psychiatrist, nutritionist, endocrinologist, and—specifically for cardiac disorders—internist and cardiologist.
The QT interval should be routinely monitored by means of standard 12-lead ECG and its measurement properly corrected for heart rate, since significant prolongation may herald potentially fatal ventricular arrhythmias. Moreover, the contribution of psychotropic drugs and exercise to QTc prolongation must be always taken into account.
Although structural heart disease is common in AN, echocardiography is indicated for AN subjects only when prompted by the clinical presentation, since most of the abnormalities that can be revealed by echocardiography do not imply any intervention in the absence of clinical correlates. Similarly, routine assessment of circulating cholesterol is not recommended, as patient management does not differ according to cholesterol values.
RFS constitutes a situation of critically increased risk of acute, life-threatening cardiac complications and must be addressed by adopting tailored re-feeding programs, in a dedicated environment and with the availability of the skills and equipment necessary for the treatment of medical emergencies (Table 3).
References
Keski-Rahkonen A, Hoek HW, Susser ES, Linna MS, Sihvola E, Raevuori A, Bulik CM, Kaprio J, Rissanen A (2007) Epidemiology and course of anorexia nervosa in the community. Am J Psychiatry 164(8):1259–1265
Bulik CM, Reba L, Siega-Riz AM, Reichborn-Kjennerud T. Anorexia nervosa: definition, epidemiology, and cycle of risk. Int J Eat Disord. 2005;37 Suppl:S2–S9
Keski-Rahkonen A, Hoek HW, Susser ES, Linna MS, Sihvola E, Raevuori A, Bulik CM, Kaprio J, Rissanen A (2007) Epidemiology and course of anorexia nervosa in the community. Am J Psychiatry 164(8):1259–1265
Becker AE, Grinspoon SK, Klibanski A, Herzog DB (1999) Eating disorders. N Engl J Med 340:1092–1098
Fairburn CG, Harrison PJ (2003) Eating disorders. Lancet 361:407–416
Casiero D, Frishman WH (2006) Cardiovascular complications of eating disorders. Cardiol Rev 14(5):227–231
Sachs KV, Harnke B, Mehler PS, Krantz MJ (2016) Cardiovascular complications of anorexia nervosa: a systematic review. Int J Eat Disord 49(3):238–248. https://doi.org/10.1002/eat.22481 (Epub 29 Dec 2015)
Keshaviah A, Edkins K, Hastings ER, Krishna M, Franko DL, Herzog DB, Thomas JJ, Murray HB, Eddy KT (2014) Re-examining premature mortality in anorexia nervosa: a meta-analysis redux. Compr Psychiatry 55(8):1773–1784
Neumarker KJ (1997) Mortality and sudden death in anorexia nervosa. Int J Eat Disord 21:205–212
Crisp AH, Callender JS, Halek C, Hsu LK (1992) Long-term mortality in anorexia nervosa. A 20-year follow-up of the St. George’s and Aberdeen cohorts. Br J Psychiatry 161:104–107
Miller RJH, Chew D (2016) Re-feeding syndrome and alcoholic cardiomyopathy: a case of interacting diagnoses. J Cardiol Cases 14(3):90–93
Jáuregui-Garrido B, Jauregui-Lobera I (2012) Sudden death in eating disorders. Vasc Health Risk Manag 8:91–98
Fohlin L (1977) Body composition, cardiovascular and renal function in adolescent patients with anorexia nervosa. Acta Paediatr Scand Suppl 268:1–20
Spaulding-Barclay MA, Stern J, Mehler PS (2016) Cardiac changes in anorexia nervosa. Cardiol Young 26(4):623–628
Yahalom M, Spitz m, Sandler L (2013) The significance of bradycardia in anorexia nervosa. Int J Angiol 22:83–94
Warren MP, Wande Viele RL (1973) Clinical and metabolic features of anorexia nervosa. Am J Obstet Gynecol 117:435–449
Sharp CW, Freeman CP (1993) The medical complications of anorexia nervosa. Br J Psychiatry 162:452–462
Debra K, Katzman DK (2005) Medical complications in adolescents with anorexia nervosa: a review of the literature. Int J Eat Disord 37(Suppl):S52–S59
López-Guzmán A, Taboada F, Alvarez Escolá C (2002) Sinus bradycardia in anorexia nervosa. Nutr Hosp 17(1):46–47
Golden NH, Katzman DK, Kreipe RE et al, Society for Adolescent Medicine (2003) Eating disorders in adolescents: position paper of the Society for Adolescent Medicine. J Adolesc Health 33(6):496–503
Kanbur N, Goldberg E, Pinhas L, Hamilton RM, Clegg R, Katzman DK (2009) Second-degree atrioventricular block (Mobitz type I) in an adolescent with anorexia nervosa: intrinsic or acquired conduction abnormality. Int J Eat Disord 42(6):575–578
Raghi G, Perucca A, Parravicini U et al (2006) Severe bradycardia in an asymptomatic young subject: is there an indication to permanent cardiac pacing? G Ital Cardiol (Rome) 7:299–302
American Psychiatric Association (2006) Treatment of patients with eating disorders, third edition. American Psychiatric Association. Am J Psychiatry 163(7 Suppl):4–54
Kollai M, Bonyhay I, Jokkel G, Szonyi L (1994) Cardiac vagal hyperactivity in adolescent anorexia nervosa. Eur Heart J 15(8):1113–1118
Tokumura M, Watanabe H, Esaki T (2012) Convalescent resting tachycardia predicts unfavorable outcome of anorexia nervosa. Pediatr Int 54(6):844–848
Walter C, Rottler E, von Wietersheim J, Cuntz U (2015) QT-correlation formulae and arrythmogenic risk in female patients with anorexia nervosa. Int J Cardiol 187:302–303. https://doi.org/10.2016/j.ijcard,2015.03.230
Day CP, McComb JM, Campbell RW (1990) QT dispersion: an indication of arrhythmia risk in patients with long QT intervals. Br Heart J 63(6):342–344
Swenne I, Larsson PT (1999) Heart risk associated with weight loss in anorexia nervosa and eating disorders: risk factors for QTc interval prolongation and dispersion. Acta Paediatr 88(3):304–309
Fanoe S, Kristensen D, Fink-Jensen A, Jensen HK, Toft E, Nielsen J, Videbech P, Pehrson S, Bundgaard H (2014) Risk of arrhythmia induced by psychotropic medications: a proposal for clinical management. Eur Heart J 35(20):1306–1315
Priori SG, Wilde AA, Horie M, Cho Y, Behr ER, Berul C, Blom N, Brugada J, Chiang CE, Huikuri H, Kannankeril P, Krahn A, Leenhardt A, Moss A, Schwartz PJ, Shimizu W, Tomaselli G, Tracy C (2013) Executive summary: HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Heart Rhythm 10(12):e85–e108
Padfield GJ, Escudero CA, DeSouza AM, Steinberg C, Gibbs K, Puyat JH, Lam PY, Sanatani S, Sherwin E, Potts JE, Sandor G, Krahn AD (2016) Characterization of myocardial repolarization reserve in adolescent females with anorexia nervosa. Circulation 133(6):557–565
Nagata JM, Carlson JL, Kao JM, Golden NH, Murray SB, Peebles R (2017) Characterization and correlates of exercise among adolescents with anorexia nervosa and bulimia nervosa. Int J Eat Disord 50(12):1394–1403
Murialdo G, Casu M, Falchero M, Brugnolo A, Patrone V, Cerro PF, Ameri P, Andraghetti G, Briatore L, Copello F, Cordera R, Rodriguez G, Ferro AM (2007) Alterations in the autonomic control of heart rate variability in patients with anorexia or bulimia nervosa: correlations between sympathovagal activity, clinical features, and leptin levels. J Endocrinol Invest 30:356–362
Facchini M, Sala L, Malfatto G, Bragato R, Redaelli G, Invitti C (2006) Low-K+ dependent QT prolongation and risk for ventricular arrhythmia in anorexia nervosa. Int J Cardiol 106:170–176
Guerrier K, Mitan L, Wang Y, Czosek RJ (2016) Risk for prolonged QT interval and associated outcomes in children with early restrictive eating patterns. Cardiol Young 26(4):644–649
Winston AP (2012) The clinical biochemistry of anorexia nervosa. Ann Clin Biochem 49(Pt 2):132–143
Seidler T, Jacobshagen C, Bauer M, Hasenfuss G, Waeschle RM (2011) Distribution of potassium levels on admission for CPR—severe hypokalaemia with dysmorphophobic eating disorders. Resuscitation 82(5):535–537
Fonseca V, Havard CW (1985) Electrolyte disturbances and cardiac failure with hypomagnesaemia in anorexia nervosa. Br Med J (Clin Res Ed) 291(6510):1680–1682
Kutsal E, Aydemir C, Eldes N, Demirel F, Polat R, Taspnar O, Kulah E (2007) Severe hypermagnesemia as a result of excessive cathartic ingestion in a child without renal failure. Pediatr Emerg Care 23(8):570–572
Palla B, Litt IF (1988) Medical complications of eating disorders in adolescents. Pediatrics 81(5):613–623
Abed J, Judeh H, Abed E, Kim M, Arabelo H, Gurunathan R (2014) “Fixing a heart”: the game of electrolytes in anorexia nervosa. Nutr J 13:90
Stheneur C, Bergeron SJ, Frappier JY, Jamoulle O, Taddeo D, Sznajder M, Lapeyraque AL (2017) Renal injury in pediatric anorexia nervosa: a retrospective study. Eat Weight Disord. https://doi.org/10.1007/s40519-017-0401-1
Krantz MJ, Sabel AL, Sagar U, Long CS, Barbey JT, White KV, Gaudiani JL, Mehler PS (2012) Factors influencing QT prolongation in patients hospitalized with severe anorexia nervosa. Gen Hosp Psychiatry 34(2):173–177
Timour Q, Frassati D, Descotes J, Chevalier P, Christé G, Chahine M (2012) Sudden death of cardiac origin and psychotropic drugs. Front Pharmacol 10:3:76
Birmingham CL, Gritzner S (2007) Heart failure in anorexia nervosa: case report and review of the literature. Eat Weight Disord 12:e7–e10
Lamzabi I, Syed S, Reddy VB, Jain R, Harbhajanka A, Arunkumar P (2015) Myocardial changes in a patient with anorexia nervosa: a case report and review of literature. Am J Clin Pathol 143(5):734–737. https://doi.org/10.1309/AJCP4PLFF1TTKENT
Sutton MSJ, Plappert T, Crosby L, Douglas P, Mullen J, Reichek N (1985) Effects of reduced left ventricular mass on chamber architecture, load, and function: a study of anorexia nervosa. Circulation 72(5):991–1000
Romano C, Chinali M, Pasanisi F, Greco R, Celentano A, Rocco A, Palmieri V, Signorini A, Contaldo F, de Simone G (2003) Reduced hemodynamic load and cardiac hypotrophy in patients with anorexia nervosa. Am J Clin Nutr 77(2):308–312
Kastner S, Salbach-Andrae H, Renneberg B, Pfeiffer E, Lehmkuhl U, Schmitz L (2012) Echocardiographic findings in adolescents with anorexia nervosa at beginning of treatment and after weight recovery. Eur Child Adolesc Psychiatry 21(1):15–21
Lelli L, Rotella F, Castellini G, Benni L, Lo Sauro C, Barletta G, Mannucci E, Castellani S, Di Tante V, Galanti G, Ricca V (2015) Echocardiographic findings in patients with eating disorders: a case-control study. Nutr Metab Cardiovasc Dis 25(7):694–696
Franzoni F, Galetta F, Cupisti A, Rolla M, Santoro G, Pentimone F (2003) Ultrasonic tissue characterization of the myocardium in anorexia nervosa. Acta Paediatr 92:297–300
Kuwabara M, Niwa K, Yamada U, Ohta D (2018) Low body mass index correlates with low left ventricular mass index in patients with severe anorexia nervosa. Heart Vessels 33(1):89–93
Galetta F, Franzoni F, Cupisti A, Morelli E, Santoro G, Pentimone F (2005) Early detection of cardiac dysfunction in patients with anorexia nervosa by tissue Doppler imaging. Int J Cardiol 101(1):33–37
Escudero CA, Potts JE, Lam PY, De Souza AM, Mugford GJ, Sandor GG (2016) An echocardiographic study of left ventricular size and cardiac function in adolescent females with anorexia nervosa. Eur Eat Disord Rev 24(1):26–33
Meyers DG, Starke H, Pearson PH, Wilken MK (1986) Mitral valve prolapse in anorexia nervosa. Ann Intern Med 105(3):384–386
Dini FL, Lattanzi F, Fontanive P, Rosa GM, De Tommasi SM (2009) Value of tissue Doppler imaging for risk stratification of patients with chronic systolic heart failure with or without restrictive mitral flow. Eur J Echocardiogr 10(4):562–566
Dini FL, Rosa GM, Fontanive P, Santonato V, Napoli AM, Ciuti M, Di Bello V (2010) Combining blood flow and tissue Doppler imaging with N-terminal pro-type B natriuretic peptide for risk stratification of clinically stable patients with systolic heart failure. Eur J Echocardiogr 11(4):333–340
Besli F, Kecebas M, Caliskan S, Dereli S, Baran I, Turker Y (2015) The utility of inferior vena cava diameter and the degree of inspiratory collapse in patients with systolic heart failure. Am J Emerg Med 33(5):653–657
Carbone F, Bovio M, Rosa GM, Ferrando F, Scarrone A, Murialdo G, Quercioli A, Vuilleumier N, Mach F, Viazzi F, Montecucco F (2014) Inferior vena cava parameters predict readmission in ischemic heart failure. Eur J Clin Invest 44(4):341–349
Morris R, Prasad A, Asaro J, Guzman M, Sanders L, Hauck A, Singh GK, Levy PT (2017) Markers of cardiovascular dysfunction in adolescents with anorexia nervosa. Glob Pediatr Health 4:2333794×17727423
Winston AP, Jamieson CP, Madira W, Gatward NM, Palmer RL (2000) Prevalence of thiamin deficiency in anorexia nervosa. Int J Eat Disord 28(4):451–454
Douyon L, Schteingart DE (2002) Effect of obesity and starvation on thyroid hormone, growth hormone, and cortisol secretion. Endocrinol Metab Clin N Am 31(1):173–189
Romig RA (1985) Anorexia nervosa, ipecac, and sudden death. Ann Intern Med 103(4):641
Cappelli V, Bottinelli R, Polla B, Reggiani C (1990) Altered contractile properties of rat cardiac muscle during experimental thiamine deficiency and food deprivation. J Mol Cell Cardiol 22:1095–1106
Astudillo L, Degano B, Madaule S et al (2003) Development of beriberi heart disease 20 years after gastrojejunostomy. Am J Med 115:157–158
Wooley JA (2008) Characteristics of thiamin and its relevance to the management of heart failure. Nutr Clin Pract 23:487–493
Sriram K, Manzanares W, Joseph K (2012) Thiamine in nutrition therapy. Nutr Clin Pract 27:41–50
Hofer M, Pozzi A, Joray M, Ott R, Hähni F, Leuenberger M, von Känel R, Stanga Z (2014) Safe refeeding management of anorexia nervosa inpatients: an evidence-based protocol. Nutrition 30(5):524–530
Grabowski A, Kilian J, Strank C, Cieslinski G, Meyding-Lamadé U (2007) Takotsubo cardiomyopathy—a rare cause of cardioembolic stroke. Cerebrovasc Dis 24(1):146–148
Valbusa A, Paganini M, Secchi G, Montecucco F, Rosa GM (2013) What happened to a thrombus during apical ballooning syndrome: a case report. Swiss Med Wkly 143:w13797
Volman MN, Ten Kate RW, Tukkie R (2011) Tako Tsubo cardiomyopathy, presenting with cardiogenic shock in a 24-year-old patient with anorexia nervosa. Neth J Med 69(3):129–131
Carlomagno G, Mercurio V, Ruvolo A, Senatore I, Halinskaya I, Fazio V, Affuso F, Fazio S (2011) Endocrine alterations are the main determinants of cardiac remodelling in restrictive anorexia nervosa. ISRN Endocrinol 2011:171460
Ramacciotti CE, Coli E, Biadi O, Dell’Osso L (2003) Silent pericardial effusion in a sample of anorexic patients. Eat Weight Disord 8(1):68–71
Docx MK, Gewillig M, Simons A, Vandenberghe P, Weyler J, Ramet J, Mertens L (2010) Pericardial effusions in adolescent girls with anorexia nervosa: clinical course and risk factors. Eat Disord 18(3):218–225
Kircher JN1, Park MH, Cheezum MK, Hulten EA, Kunz JS, Haigney M, Atwood JE (2012) Cardiac tamponade in association with anorexia nervosa: a case report and review of the literature. Cardiol J 19(6):635–638
Misra M, Freed N, Herzog DB, Goldstein M, Riggs S, Klibanski A (2006) Uncoupling of cardiovascular risk markers in adolescent girls with anorexia nervosa. J Pediatr 149(6):763–769
Ohwada R, Hotta M, Oikawa S, Takano K (2006) Etiology of hypercholesterolemia in patients with anorexia nervosa. Int J Eat Disord 39(7):598–601
Rigaud D, Tallonneau I, Vergès B (2009) Hypercholesterolemia in anorexia nervosa: frequency and changes during refeeding. Diabetes Metab 35(1):57–63
Solmi M, Veronese N, Manzato E, Sergi G, Favaro A, Santonastaso P, Correll CU (2015) Oxidative stress and antioxidant levels in patients with anorexia nervosa: a systematic review and exploratory meta-analysis. Int J Eat Disord 48(7):826–841
Birmingham CL, Lear SA, Kenyon J, Chan SY, Mancini GB, Frohlich J (2003) Coronary atherosclerosis in anorexia nervosa. Int J Eat Disord 34(3):375–377
García-Rubira JC, Hidalgo R, Gómez-Barrado JJ, Romero D, Cruz Fernández JM (1994) Anorexia nervosa and myocardial infarction. Int J Cardiol 15(2):138–140 45(
Abuzeid W, Glover C (2011) Acute myocardial infarction and anorexia nervosa. Int J Eat Disord 44(5):473–476
Crook MA (2014) Refeeding syndrome: problems with definition and management. Nutrition 30(11–12):1448–1455
Sachs K, Andersen D, Sommer J, Winkelman A, Mehler PS (2015) Avoiding medical complications during the refeeding of patients with anorexia nervosa. Eat Disord 23(5):411–421
Blank S, Zadik Z, Katz I, Mahazri Y, Toker I, Barak I (2002) The emergence and treatment of anorexia and bulimia nervosa. A comprehensive and practical model. Int J Adolesc Med Health 14:257–260
Ornstein RM, Golden NH, Jacobon MS, Shenker IR (2003) Hypophosphatemia during nutritional rehabilitation in anorexia nervosa: implications for refeeding and monitoring. J Adolesc Health 32:83–88
Mehanna HM, Moledina J, Travis J (2008) Refeeding syndrome: what it is, and how to prevent and treat it. BMJ 336:1495–1498
Rio A, Whelan K, Goff L, Reidlinger DP, Smeeton N (2013) Occurrence of refeeding syndrome in adults started on artificial nutrition support: prospective cohort study. BMJ Open 3(1): e002173
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Research involving human and/or participants
This article does not contain any studies performed by any of the authors involving human participants or animals.
Informed consent
For this type of study, formal consent is not required.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Giovinazzo, S., Sukkar, S.G., Rosa, G.M. et al. Anorexia nervosa and heart disease: a systematic review. Eat Weight Disord 24, 199–207 (2019). https://doi.org/10.1007/s40519-018-0567-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40519-018-0567-1