Abstract
Purpose
Seasonal variation exists in the psychopathology of eating disorders. However, it is still unknown whether there is seasonal variation in eating disorder symptom severity. This study investigated seasonal trends in hospital admissions and birth dates among patients with eating disorders in Taiwan (25°N). Subgroup analyses by gender and comorbid affective disorders were also of interest.
Methods
Data on all hospital admissions between 2000 and 2013 were collected from the Taiwan National Health Insurance Research Database, and 1954 patients with eating disorders were identified. Hospital admissions and birth dates were recorded by day. The four seasons and cross-seasons were defined by solstices and equinoxes. The expected distribution of births was determined using data from all patients hospitalized from 2000 to 2013 (n = 13,139,306).
Results
Hospital admissions among patients with eating disorders exceeded the rate of expected hospital admissions in the summer season (p < 0.001) and the autumn cross-season (p < 0.001). However, the seasonal (p = 0.421) and cross-seasonal (p = 0.24) distributions of birth dates among these patients did not differ from the expected distributions. Interestingly, hospital admissions among patients with comorbid affective disorders exceeded the rates of hospital admissions among non-affective patients during the spring (p = 0.004). Moreover, the number of non-affective patients born during autumn exceeded the birth rates of affective patients during this season (p = 0.001). Gender and comorbid affective disorders were not associated with cross-seasonal differences in either hospitalizations or dates of birth.
Conclusions
Affective psychopathology in inpatients with eating disorders may substantially contribute to symptom severity that waxes and wanes with the seasons. Moreover, the seasonal distribution of birth dates was significantly different in patients without comorbid affective disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
All organisms on earth are subjected to a diurnal cycle of daylight and darkness. Living organisms have evolved built-in biological rhythms that allow them to anticipate and prepare for precise and regular environmental changes [1]. From an evolutionary perspective, an organism’s internal biological rhythms result in improved survival and competitive advantage. The biological clock in mammals, located in the hypothalamic suprachiasmatic nucleus, contributes to synchrony between the environmental light–dark cycle and internal biochemical processes [2]. It has been suggested that perturbation of the biological clock increases risks for several disorders, including obesity, diabetes mellitus, cardiovascular disease, thrombosis, and even inflammation [3]. A rigorous animal study clearly showed that disrupted biological rhythms could hamper metabolism, cognition, emotionality, and behavior [2]. This implies that biological rhythms play a central role in both physical and mental health. Indeed, desynchronized biological rhythms are frequently observed in patients with psychiatric disorders [4].
Hippocrates, the father of modern medicine, observed that some diseases were more apt to occur and to be exacerbated during certain seasons [5]. In recent decades, accumulating scientific evidence supports the view that certain diseases and physiological processes in humans exhibit seasonal variation [5, 6]. For example, seasonal variation is essential to sustaining ongoing cognitive processes [7]. Furthermore, bipolar disorders display psychopathological seasonality, with manic symptoms flaring up in fall and depressive symptoms peaking in winter [8]. However, mental illnesses may be more tightly linked with seasonal patterns than previously thought. A recent study found that Google searches for information about all major mental illnesses followed seasonal patterns [9]. With the aim of optimizing care, psychiatrists should better understand seasonal variations in mental illnesses.
Eating patterns also have been associated with biological rhythms. A literature review revealed specific seasonal variations in the psychopathological symptoms of eating disorders (ED) [10–15]. However, most previous studies have focused primarily on outpatients with ED. It is still unknown whether patients with ED who require hospitalization have a symptom severity that varies seasonally. Notably, seasonal variations in eating habits also occur in a subset of the general population [16–18]. In this regard, seasonal variation in the symptoms of ED may not reflect a waxing and waning in symptom severity with the seasons. It is important to delve into seasonal variation in hospital admissions for ED and specific trends in the demographics of these patients.
The principal aim of this study was to investigate seasonal variations in patients with ED who required hospitalization. Moreover, we explored whether gender and comorbid affective disorders were associated with seasonality in these patients. Interestingly, preliminary evidence has linked ED to the season in which a person was born [19–21], showing that autumn babies were at a greater risk of developing ED later in life. Therefore, this study also examined the seasonal distribution of births among ED patients in Taiwan. We believe that to gain a better understanding of ED seasonality, it is important to bridge the gap in our knowledge of ED patients whose acute symptoms exacerbation require hospitalization.
Materials and methods
To assess monthly trends in hospital admissions for ED, the present study utilized data collected from the National Health Insurance Research Database (NHIRD), which are derived from the claims data of the National Health Insurance (NHI) program of Taiwan. The NHI program was established in 1995 and delivers universal coverage provided by a government-run, single-payer compulsory insurance plan to centralize the disbursement of healthcare funding. By 2010, it covered 99.5 % of the Taiwanese population [22]. As the NHI program covers approximately 23 million residents in Taiwan, it is one of the largest and most comprehensive nationwide population databases in the world.
The NHIRD contains standard computerized claims submitted by medical institutions under contract with the NHI Program for reimbursement of medical expenses. The claims data include outpatient, emergency, and inpatient care records that are scrutinized by medical reimbursement specialists. Licensed medical records technicians review and verify the diagnostic coding before reimbursement claims are submitted. The Bureau of National Health Insurance (BNHI) performs quarterly expert reviews of a random sample of every 50–100 ambulatory and every 20 inpatient claims in each hospital and clinic and interviews patients to verify the accuracy of diagnoses. False reports of diagnoses result in severe penalties from the BNHI. Therefore, information obtained from the NHIRD is considered complete and accurate.
The NHIRD provides encrypted data including patient identification numbers, gender, birthdays, dates of admission and discharge, medical institutions providing the services, ICD-9-CM (International Classification of Diseases, 9th Revision, Clinical Modification) diagnostic and procedure codes (up to five each), outcome at hospital discharge (recovered, died or transferred out), order codes and fees charged for medical services. The data are updated biannually. Since 2003, the use of electronic medical records has been mandated and standardized across 321 hospitals, providing the foundation for digital health and data exchange. Importantly, researchers who seek to use the NHIRD and its data subsets are required to sign a written agreement declaring that they do not intend to obtain information that could potentially violate the privacy of patients or care providers. Undoubtedly, the NHIRD can provide a valuable window into seasonal variation in symptom severity among patients requiring hospitalization for an ED.
This was a retrospective study based on the NHIRD, with a study period spanning from January 1, 2000, through December 31, 2013. Data from the “detailed documents of hospitalization medical expenses” and “registry for contracted medical facilities” were extracted from the NIHRD. Figure 1 illustrates the flowchart for study design and patient selection. Among the 13,139,306 hospitalized patients, we identified 1954 patients with a diagnosis of ED according to ICD-9-CM codes. Among these ED patients, 979 patients had anorexia nervosa (AN) (307.1); 233 patients had bulimia nervosa (BN) (307.51); and 742 patients had an eating disorder, not otherwise specified (EDNOS) (307.5, 307.59). Patients who had a primary diagnosis of schizophrenia (295) or other psychotic disorders (297) were excluded (n = 125), as their ED could be drug-induced, transient, or atypical in nature [23]. Records with unknown gender were also excluded (n = 7). Diagnoses of affective disorders included major depressive disorder (296.2, 296.3); bipolar disorders (296.0, 296.1, 296.4, 296.5, 296.6, 296.7, 296.8); dysthymic disorder (300.4); and depressive disorder, not otherwise specified (311). The number of admissions for ED was recorded by day.
Seasonality was analyzed in two ways [24]. The first method compartmentalized the data into four seasons as defined by solstices and equinoxes. The four seasons were spring (March 21–June 20), summer (June 21–September 20), autumn (September 21–December 20), and winter (December 21–March 20). The second method divided the data into four cross-seasons defined by the 45-day periods before and after each solstice and equinox. This allowed for examination of the four quarters of the year with (1) the most amount of light (summer cross-season, May 6–August 5), (2) the least amount of light (winter cross-season, November 6–February 5), (3) the median amount of light with an increasing photoperiod (spring cross-season, February 6–May 5), and (4) the median amount of light with a decreasing photoperiod (autumn cross-season, August 6–November 5). The distribution of birth dates among all hospitalized patients was considered the expected probability distribution. Therefore, the percentages of expected births were 22.5 % in spring, 24.4 % in summer, 27.2 % in autumn, 25.9 % in winter, 23.7 % in the spring cross-season, 22.5 % in the summer cross-season, 26.7 % in the autumn cross-season, and 27.1 % in the winter cross-season.
The Charlson comorbidity index (CCI) was used to determine comorbidities [25], including acute myocardial infarction (410), ischemic cerebrovascular accident (430–432), hemorrhagic cerebrovascular accident (433–434), transient ischemic attack (435), unspecified stroke (436), diabetes mellitus (250), acute renal failure (584), chronic kidney disease (585–586), hypertension (401–405), dyslipidemia (272), gout (274), atrial fibrillation (427.31), liver disease (456.0–456.21, 571.2, 571.4–571.6, 572.2–572.8), chronic obstructive pulmonary disease (490–492, 494, 496), and heart failure (428).
The distribution of hospital admissions and births among ED patients over the four seasons and four cross-seasons were tested using the Chi-square goodness-of-fit test. Subgroup analyses by gender and comorbid affective disorders were performed using the Chi-square test of independence. All tests were two-sided, and p < 0.05 was considered significant. All statistical analyses were performed using IBM SPSS Statistics for Windows, Version 22.0 (IBM Corp., Armonk, NY) and GraphPad Prism version 6 for Windows (GraphPad Software, La Jolla, CA, USA).
Results
Table 1 summarizes the demographic characteristics within the AN, BN, and EDNOS subgroups of ED patients. The mean age of the 1954 inpatients with ED was 36 ± 22.5 years, which was higher than the peak age of onset of either AN (15–19 years) or BN (20–24 years) identified in previous epidemiological studies [26]. Unsurprisingly, a female predominance (75 %) existed in this population. The average length of hospitalization for ED was 12.6 ± 13.2 days. The proportion of ED patients with comorbid affective disorders was 32.2 %. As expected, patients with BN most frequently had comorbid affective disorders (63.5 %). The total costs of hospitalization and psychotherapy were 35.6 ± 41.1 thousand NT dollars and 7.2 ± 14.1 thousand NT dollars, respectively. Among the 1954 inpatients with ED, only 101 patients recovered. A total of 1638 patients were referred to outpatient departments for follow-up treatment. Importantly, 151 patients left the hospital against medical advice.
Table 2 shows the seasonal variations in hospital admissions and birth dates. Hospitalizations among patients with ED exceeded the expected rate of hospital admissions in summer season (χ 2 = 29.1, p < 0.001). However, the distribution of hospital admissions among male patients with ED did not differ from the distribution of hospital admissions among female patients with ED (χ 2 = 0.84, p = 0.841). Interestingly, ED patients with comorbid affective disorders had a different seasonal hospital admission distribution than patients without comorbid affective disorders (χ 2 = 8.55, p = 0.036). That is, affective cases had higher rates of hospital admissions in spring (χ 2 = 13.52, p = 0.004), while non-affective cases had similar seasonal trends to those of inpatients with ED overall. On the other hand, birth dates in inpatients with ED overall did not follow a seasonal pattern. Considering the subgroup analysis by gender, birth dates among male patients with ED did not have a different distribution than the birth dates of female patients with ED (χ 2 = 2.34, p = 0.506). Interestingly, seasonal birth trends differed between affective and non-affective patients with ED (χ 2 = 7.85, p = 0.049). Non-affective patients with ED had a birth rate that exceeded that of affective patients in autumn (χ 2 = 17.7, p = 0.001).
Table 3 shows cross-seasonal variations in hospital admissions and birth dates. Patients with ED had higher rates of hospital admissions in the autumn cross-season than would be expected (χ 2 = 18.86, p < 0.001). However, subgroup analyses did not reveal any associations between gender and affective disorder status and seasonality. That is, male and female and affective and non-affective patients had similar cross-seasonal distributions in hospital admissions. The distributions of birth did not follow cross-seasonal variations in patients with ED or their subgroups.
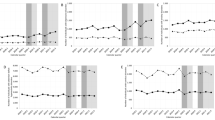
Figure 2 depicts the seasonal trends in hospital admissions and births for patients with ED. Apparently, affective patients with ED had a distribution of hospital admissions that differed from inpatients with ED overall and non-affective cases (χ 2 = 13.52, p = 0.004). They had higher rates hospital admissions in spring. In contrast, non-affective patients with ED had a distribution of births that differed from inpatients with ED overall and affective cases (χ 2 = 17.7, p = 0.001). Births among non-affective ED patients occurred more frequently in autumn.
Seasonal variation in hospital admissions and births. Among patients with eating disorders, seasonal variations existed in hospital admissions (χ 2 = 29.1, p < 0.001) but not in births (χ 2 = 2.82, p = 0.421). Interestingly, affective patients with eating disorders had a different hospital admission distribution compared with patients with eating disorders overall and non-affective cases (χ 2 = 13.52, p = 0.004). They had higher rates of hospital admissions in spring. In contrast, non-affective patients with eating disorders had a different birth date distribution than inpatients with ED overall and affective cases (χ 2 = 17.7, p = 0.001). The birth rate was of these patients was higher in autumn
Discussion
In clinical practice, ED are underdiagnosed conditions, as patients with ED usually conceal their illness and avoid seeking professional care [26]. Gaining a better understanding of patients with ED who require hospitalization could help describe symptom severity and its potential seasonal variation within these patients. Here, we found that patients with ED had a periodicity in hospital admissions, implying a seasonal variation in disease severity. However, ED inpatients with comorbid affective disorders showed a different seasonality, with a rate of hospital admissions in spring exceeding that of non-affective patients. Interestingly, non-affective patients with ED had a rate of autumn births that differed from the expected distribution of births among all hospitalized patients. Considering gender differences, male inpatients with ED exhibited similar seasonal distributions in hospital admissions and births as female inpatients with ED. In brief, affective psychopathology in inpatients with ED appeared to substantially contribute to the observed seasonality in symptom severity. Moreover, the seasonal distribution of births among these patients may be associated with comorbid affective disorders.
In the present study, patients with both ED and affective disorders had a number of hospital admissions in the spring that exceeded that of non-affective ED patients. This spring predominance is not in accordance with findings of previous studies. A growing body of evidence has substantiated the existence of seasonality in affective disorders [27]. That is, depressive symptoms in patients with bipolar disorders and seasonal affective disorder (SAD) may flare up in winter [8, 27]. Indeed, ED and SAD may have similar etiopathogenesis [11], and previous studies reported approximately 13–27 % of outpatients with ED also satisfied the criteria for winter SAD [11, 28]. In this regard, patients with both ED and SAD may experience exacerbated symptoms in winter and require hospitalization. However, we observed a different seasonality among inpatients with ED. Rather than symptoms flaring up in winter, inpatients with ED developed exacerbated symptoms in spring, implying a different effect of seasonality in these two populations. However, another possible explanation is that the seasonality in symptom expression might not precisely mirror symptom severity. Further research on ED could explore the differences between inpatients and outpatients in symptom severity and seasonality of symptom expression.
Another striking finding was that inpatients with ED who did not have comorbid affective disorders had a rate of births in autumn that exceeded that of affective ED patients, implying that autumn babies are at a greater risk of developing non-affective ED later in life. The etiology of mental illnesses is largely multi-factorial and involves a mix of genetic factors and environmental exposures interacting with each other across the entire lifespan [29]. It has been made increasingly apparent that seasonal changes markedly modulate human biology [30] and influence susceptibility to certain diseases. A seminal animal study reported that perinatal photoperiod could have persistent effects on biological rhythm function [31]. Another human study found seasonal transcriptional signatures within immune systems [6]. Seasonal imprinting of the biological clock and gene expression might explain the seasonality observed in the births of patients with a number of psychiatric disorders including ED. Indeed, evidence has linked dysregulated biological rhythms to psychiatric disorders [4]. However, research surrounding the seasonality in ED patients’ births continues to show mixed results [19–21, 32], including an excess in births during spring [20, 32] or autumn [19, 32] births or insignificant findings [21, 32]. In fact, being born in spring has been found to increase the risk of SAD [33], and ED usually co-occurs with SAD [11, 28]. In this scenario, the excess in spring births among ED patients might have been confounded by ED cases with co-occurring SAD. Further research investigating this issue should be conducted to tease out the impact of comorbid affective disorders.
The relative age effect could be translated into a relative weight effect, accounting for the greater risk of developing non-affective ED in autumn babies. A previous study examined seasonal birth patterns in women with BN and in those who ever endorsed bingeing or purging [19]. The authors found the greatest number of births in autumn for the three categories and suggested that a relative age effect might play a role. In Taiwan, the entry age for kindergarten and elementary school is determined by the date of birth occurring on or before September 1. Therefore, despite the same grade, children born in the autumn would be 3–9 months older and weigh more than children born in the other seasons. In this regard, cultural pressures for thinness may be particularly strong for these autumn-born, earlier-maturing children, thereby predisposing them to ED.
Intrinsic features among inpatients with ED may be different from those among outpatients with ED. For example, in our study, the mean age of inpatients with ED was 34.7 ± 17.6 years, which is much older than the mean age of onset for AN (15–19 years) and BN (20–24 years) [26]. Moreover, patients with ED who require hospitalization represent a more severe form of ED. The heterogeneity between inpatients and outpatients might contribute to differences in seasonality. However, seasonal periodicity can change with several environmental and social variables. These changes are repeated annually, creating a natural experiment for exploring the links between seasonal exposures and disease. Importantly, seasonality is still a fairly distal risk factor that may be linked etiologically with ED through several mediating factors. Factors that vary with time of year may counterbalance each other and be undetectable in an analysis of seasonal exposures. Therefore, the precise causes of seasonal variations in ED remain poorly understood. However, several potential mechanisms have been mentioned in previous studies [19–21, 32].
At present, little is known regarding the impact of seasonality on inpatients with ED. To address this gap, the present study used a large national health insurance database. However, the limitations inherent to working with this type of data need to be acknowledged. First, independent verification of diagnoses was not possible, as diagnosis of ED was based on diagnostic codes registered by the treating physicians. Second, the data are descriptive and cannot capture the reasons underlying clinical hospitalization decisions. Therefore, hospital admissions for ED may not truly reflect symptom severity, as patients with ED may be hospitalized for other reasons such as suicidal or parasuicidal behavior. It is also possible that the number of excess hospital admissions in spring may be due to the timing of hospitalization decisions. ED inpatients with comorbid affective disorders might experience exacerbations of their symptoms in winter and then be hospitalized the next spring. However, the NIHRD did not provide the time lag between the symptom exacerbation and admission to the hospital. Third, inpatients with ED may be heterogeneous in nature. The mean length of hospital stay was 12.6 ± 13.2 days, a duration longer than would be needed for crisis intervention but too short to safely achieve complete weight restoration. Moreover, a standard deviation for hospital stays of 13.2 days implies remarkable individual differences in inpatients with ED. We also noted that 151 patients left the hospital against medical advice, which supports the existence of heterogeneity within this population. Fourth, there was no specific ICD-9-CM code for SAD; therefore, we could not specifically tease out the impact of SAD on seasonality. Fifth, a large of proportion of patients had a diagnosis of EDNOS, which is a heterogeneous diagnosis that limits generalizability. Finally, we excluded duplicate subject records, preventing the examination of recurrent admissions within a season.
Observation is a fundamental step to test a hypothesis, understand a process and create a conclusion. Researchers have observed that climate conditions have differential effects on disease expression, and the interplay between these factors have been found to generate seasonal variation in disease incidence and symptom expression. Although time-dependent factors responsible for the seasonality of ED remain an enigma, the present study adds to the evidence suggesting that seasonality in inpatients with ED might be different from that in ED outpatients. Inpatients with ED and comorbid affective disorders revealed symptoms that flared up in spring, and non-affective inpatients with ED had a rate of autumn births that exceeded that of affective patients. As ED are phenotypically heterogeneous entities with multifactorial etiologies [32], further research should be conducted to disentangle the differences between inpatients and outpatients in seasonality and clinical characteristics.
References
Hut RA, Beersma DG (2011) Evolution of time-keeping mechanisms: early emergence and adaptation to photoperiod. Philos Trans R Soc Lond B Biol Sci 366(1574):2141–2154. doi:10.1098/rstb.2010.0409
Karatsoreos IN, Bhagat S, Bloss EB, Morrison JH, McEwen BS (2011) Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc Natl Acad Sci USA 108(4):1657–1662. doi:10.1073/pnas.1018375108
Maury E, Ramsey KM, Bass J (2010) Circadian rhythms and metabolic syndrome: from experimental genetics to human disease. Circ Res 106(3):447–462. doi:10.1161/CIRCRESAHA.109.208355
Wulff K, Gatti S, Wettstein JG, Foster RG (2010) Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat Rev Neurosci 11(8):589–599. doi:10.1038/nrn2868
Naumova EN (2006) Mystery of seasonality: getting the rhythm of nature. J Public Health Policy 27(1):2–12. doi:10.1057/palgrave.jphp.3200061
Dopico XC, Evangelou M, Ferreira RC, Guo H, Pekalski ML, Smyth DJ, Cooper N, Burren OS, Fulford AJ, Hennig BJ, Prentice AM, Ziegler AG, Bonifacio E, Wallace C, Todd JA (2015) Widespread seasonal gene expression reveals annual differences in human immunity and physiology. Nat Commun 6:7000. doi:10.1038/ncomms8000
Meyer C, Muto V, Jaspar M, Kussé C, Lambot E, Chellappa SL, Degueldre C, Balteau E, Luxen A, Middleton B, Archer SN, Collette F, Dijk D-J, Phillips C, Maquet P, Vandewalle G (2016) Seasonality in human cognitive brain responses. Proc Natl Acad Sci USA 113(11):3066–3071. doi:10.1073/pnas.1518129113
Akhter A, Fiedorowicz JG, Zhang T, Potash JB, Cavanaugh J, Solomon DA, Coryell WH (2013) Seasonal variation of manic and depressive symptoms in bipolar disorder. Bipolar Disord 15(4):377–384. doi:10.1111/bdi.12072
Ayers JW, Althouse BM, Allem JP, Rosenquist JN, Ford DE (2013) Seasonality in seeking mental health information on Google. Am J Prev Med 44(5):520–525. doi:10.1016/j.amepre.2013.01.012
Hardin TA, Wehr TA, Brewerton T, Kasper S, Berrettini W, Rabkin J, Rosenthal NE (1991) Evaluation of seasonality in six clinical populations and two normal populations. J Psychiatr Res 25(3):75–87. doi:10.1016/0022-3956(91)90001-Q
Brewerton TD, Krahn DD, Hardin TA, Wehr TA, Rosenthal NE (1994) Findings from the Seasonal Pattern Assessment Questionnaire in patients with eating disorders and control subjects: effects of diagnosis and location. Psychiatry Res 52(1):71–84. doi:10.1016/0165-1781(94)90121-X
Fornari VM, Braun DL, Sunday SR, Sandberg DE, Matthews M, Chen IL, Mandel FS, Halmi KA, Katz JL (1994) Seasonal patterns in eating disorder subgroups. Compr Psychiatry 35(6):450–456. doi:10.1016/0010-440X(94)90228-3
Lam RW, Goldner EM, Grewal A (1996) Seasonality of symptoms in anorexia and bulimia nervosa. Int J Eat Disord 19(1):35–44. doi:10.1002/(SICI)1098-108X(199601)19:1<35:AID-EAT5>3.0.CO;2-X
Yamatsuji M, Yamashita T, Arii I, Taga C, Tatara N, Fukui K (2003) Seasonal variations in eating disorder subtypes in Japan. Int J Eat Disord 33(1):71–77. doi:10.1002/eat.10107
Shuman NK, Krug I, Maxwell M, Pinheiro AP, Brewerton T, Thornton LM, Berrettini WH, Brandt H, Crawford S, Crow S, Fichter MM, Halmi KA, Johnson C, Kaplan AS, Keel P, Lavia M, Mitchell J, Rotondo A, Strober M, Woodside DB, Kaye WH, Bulik CM (2010) Is season of birth related to disordered eating and personality in women with eating disorders? Eat Weight Disord 15(3):e186–e189. doi:10.1007/BF03325297
Perry JA, Silvera DH, Rosenvinge JH, Neilands T, Holte A (2001) Seasonal eating patterns in Norway: a non-clinical population study. Scand J Psychol 42(4):307–312. doi:10.1016/j.amepre.2013.01.012
Chotai J, Smedh K, Johansson C, Nilsson LG, Adolfsson R (2004) An epidemiological study on gender differences in self-reported seasonal changes in mood and behaviour in a general population of northern Sweden. Nord J Psychiatry 58(6):429–437. doi:10.1080/08039480410006052
Winthorst WH, Roest AM, Bos EH, Meesters Y, Penninx BW, Nolen WA, de Jonge P (2014) Self-attributed seasonality of mood and behavior: a report from the Netherlands study of depression and anxiety. Depress Anxiety 31(6):517–523. doi:10.1002/da.22130
Brewerton TD, Dansky BS, O’Neil PM, Kilpatrick DG (2012) Seasonal patterns of birth for subjects with bulimia nervosa, binge eating, and purging: results from the National Women’s Study. Int J Eat Disord 45(1):131–134. doi:10.1002/eat.20898
Disanto G, Handel AE, Para AE, Ramagopalan SV, Handunnetthi L (2011) Season of birth and anorexia nervosa. Br J Psychiatry 198(5):404–405. doi:10.1192/bjp.bp.110.085944
Winje E, Torgalsboen AK, Brunborg C, Lask B (2013) Season of birth bias and anorexia nervosa: results from an international collaboration. Int J Eat Disord 46(4):340–345. doi:10.1002/eat.22060
Hsing AW, Ioannidis JP (2015) Nationwide population science: lessons from the Taiwan National health insurance research database. JAMA Intern Med 175(9):1527–1529. doi:10.1001/jamainternmed.2015.3540
Bou Khalil R, Hachem D, Richa S (2011) Eating disorders and schizophrenia in male patients: a review. Eat Weight Disord 16(3):e150–e156. doi:10.1007/BF03325126
Brewerton TD, Berrettini WH, Nurnberger JI Jr, Linnoila M (1988) Analysis of seasonal fluctuations of CSF monoamine metabolites and neuropeptides in normal controls: findings with 5HIAA and HVA. Psychiatry Res 23(3):257–265. doi:10.1016/0165-1781(88)90016-9
Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA (2005) Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 43(11):1130–1139. doi:10.1097/01.mlr.0000182534.19832.83
Hoek HW, van Hoeken D (2003) Review of the prevalence and incidence of eating disorders. Int J Eat Disord 34(4):383–396. doi:10.1002/eat.10222
Magnusson A (2000) An overview of epidemiological studies on seasonal affective disorder. Acta Psychiatr Scand 101(3):176–184. doi:10.1034/j.1600-0447.2000.101003176.x
Ghadirian AM, Marini N, Jabalpurwala S, Steiger H (1999) Seasonal mood patterns in eating disorders. Gen Hosp Psychiatry 21(5):354–359. doi:10.1016/S0163-8343(99)00028-6
Rutter M (2005) How the environment affects mental health. Br J Psychiatry 186:4–6. doi:10.1192/bjp.186.1.4
Foster RG, Roenneberg T (2008) Human responses to the geophysical daily, annual and lunar cycles. Curr Biol 18(17):R784–R794. doi:10.1016/j.cub.2008.07.003
Ciarleglio CM, Axley JC, Strauss BR, Gamble KL, McMahon DG (2011) Perinatal photoperiod imprints the circadian clock. Nat Neurosci 14(1):25–27. doi:10.1038/nn.2699
Winje E, Willoughby K, Lask B (2008) Season of birth bias in eating disorders–fact or fiction? Int J Eat Disord 41(6):479–490. doi:10.1002/eat.20540
Pjrek E, Winkler D, Heiden A, Praschak-Rieder N, Willeit M, Konstantinidis A, Stastny J, Kasper S (2004) Seasonality of birth in seasonal affective disorder. J Clin Psychiatry 65(10):1389–1393. doi:10.4088/JCP.v65n1014
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
None.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The Institutional Review Board for the Protection of Human Subjects at the Tri-Service General Hospital, a medical teaching hospital within the National Defense Medical Center in Taiwan, approved the protocol.
Informed consent
Informed consent was originally obtained by the National Health Research Institutes in Taiwan. The privacy of each individual’s information was protected using encrypted personal identification to avoid the potential for ethical violations related to the data.
Rights and permissions
About this article
Cite this article
Liang, CS., Chung, CH., Tsai, CK. et al. Seasonality of hospital admissions and birth dates among inpatients with eating disorders: a nationwide population-based retrospective study. Eat Weight Disord 23, 233–240 (2018). https://doi.org/10.1007/s40519-016-0326-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40519-016-0326-0