Abstract
Stress has been supposed to increase appetite. The biological basis of this phenomenon may be a stress-induced alteration of the secretion of GUT peptides such as ghrelin. Stress-induced changes in ghrelin secretion could be a biological basis of overeating and a factor contributing to the development of obesity. Aim of the study was to analyze the effect of acute psychosocial stress on ghrelin secretion in obese and normal weight women. We compared pre- and postprandial plasma ghrelin secretion of 42 obese and 43 normal weight women in a randomized crossover design. Ghrelin and cortisol concentrations were measured and ratings of stress were also recorded in response to a psychological stressor (Trier Social Stress Test, TSST). Ghrelin samples were collected in the fasting state one time before participating in the TSST and one time before a control session. After the TSST, respectively, control session participants had a standardized ad libitum meal. 30 and 60 min after the TSST, respectively, control session preprandial ghrelin was measured again. Obese women showed lower pre- and postprandial release of ghrelin than normal weight controls. Moreover, obese women showed inhibited postprandial decrease of ghrelin secretion. Stress did not affect postprandial ghrelin secretion, but inhibited food intake in all subjects. The present data provide further evidence of altered ghrelin release in obesity. Acute stress did not affect postprandial ghrelin secretion, but inhibited food intake in all subjects. Results are discussed with regard to biological and psychological regulation of hunger and satiety in obesity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Evidence linking stress as a risk factor to obesity is accumulating. Stress is widely thought to lead to overeating [1] and a factor contributing to the development of obesity. The underlying biological mechanisms of the relationship between stress and obesity are unclear. HPA axis dysregulation induced by acute or prolonged stress might play an important role resulting in increased visceral fat and obesity. Several studies suggest stress might alter hormones that control appetite. Herein the appetite hormone ghrelin might play a key role. Ghrelin is an orexigenic hormone which circulates in relation to food intake. It increases before meals and is rapidly suppressed by food intake [2, 3]. The clear preprandial rise and postprandial fall in plasma ghrelin levels support the hypothesis that ghrelin is an initiation signal for meal consumption [4]. Beyond its role in meal initiation, ghrelin also fulfills criteria for an adiposity-related hormone involved in long-term body-weight regulation [5]. Changes in ghrelin concentration associated with food intake are diminished in obese people [4]. According to Kristenssson et al. [9] ghrelin secretion is increased in response to stress. They measured stress-induced plasma ghrelin concentrations in rats. Rats were exposed to water avoidance stress for 60 min. They compared ghrelin levels before and after stress. Acute stress increased ghrelin production. Enhanced stress-induced ghrelin levels in rodents were also reported by Asakawa et al. [10]. In humans acute psychological stress also seems to raise ghrelin secretion. Monteleone et al. [11] measured the acute salivary ghrelin response to the Trier Social Stress Test (TSST) in ten women with acute bulimia nervosa (BN) and ten healthy controls. There were no group differences before stress, but the BN patients displayed enhanced ghrelin responses to TSST. In obese women stress-related elevation of ghrelin has been found too [12, 13]. On the contrary, Zimmermann et al. [14] found no effect of stress on ghrelin secretion. Induction of psychological stress through public speaking did not modify plasma total ghrelin levels. In another study of Rouach et al. [8] no alterations in plasma ghrelin levels through psychologically induced stress were found. They examined the effects of stress through the TSST on plasma ghrelin levels of 24 subjects. Their findings indicate that psychological stress did not affect plasma ghrelin. In consideration of the inconsistent results the present study investigated whether there is an altered release of ghrelin through an acute psychosocial stressor in obese and normal weight women. It was hypothesized that stress alters releasing of ghrelin and that stress-induced alterations are higher in obese than in normal weight women. In addition, differences between obese and normal weight people in food intake under stress were investigated.
Method
Eighty-five healthy women aged 18–30 years were recruited through advertisements in local newspapers in the area of Trier, Germany. 43 were obese (BMI 31.5 ± 1.8 kg/m2) and 42 were normal weight (BMI 21.7 ± 2.0 kg/m2). Exclusion criteria were smoking, regular alcohol consumption (more than seven drinks a week), regular medication (pharmacological or medical). To assess the presence of any of these exclusion criteria, participants were examined and interviewed by a physician. All women received € 80 for their participation. The study was approved by the ethics committee of the University of Trier in April 2012 and informed, written consent was obtained from all subjects.
Experimental design
The study was performed in a within-subject, randomized, crossover design. Each subject came to the laboratory on three occasions for: (1) measurement of anthropometry; (2) measurement of postprandial ghrelin in combination with a stress test; and (3) measurement of postprandial ghrelin in combination with a control condition. The sequence, whether stress or control condition was realized first, was varied by chance.
Aim of the study was to analyze the effect of acute psychosocial stress on postprandial ghrelin secretion in obese and normal weight women. As stress manipulation participants took part in a stress test for 15 min (stress condition) or read magazines for the same time period (control condition). To validate the effect of the stressor salivary cortisol measurements were obtained.
Measurements
Anthropometry Body weight was measured with subjects minimally clothed without shoes, using digital scales. Height was measured with subjects in a standing position without shoes, using a tape meter, with the shoulders in a normal state. BMI was calculated as weight in kilograms divided by height in squared meters.
Food intake To investigate food intake we used a Universal Eating Monitor (UEM), which is described in detail in Hubel et al. [22] and is of proven reliability.
Ghrelin Blood samples were collected using EDTA-treated tubes and immediately centrifuged to yield plasma for peptide determinations. For stabilization of ghrelin, AEBSF and HCl were added into blood collection tubes. Samples were frozen at −80 °C until laboratory analysis. Concentrations of ghrelin (catalogue no. EZGRT-89K) were determined by commercial ELISA based assays (Merck Millipore, Billerica, MA, USA).
Cortisol Saliva samples were collected using salivettes (Sarstedt, Nümbrecht, Germany), plastic vials with cotton dental rolls inside, and frozen at −20 °C until laboratory analysis. They were assayed using delayed Fluoreszenz-Immunoassays. Intraassay coefficients of variation were 4.0–6.7 %. The interassay coefficient of variation was 7.1–9.0 % [21].
VAS Aspects of psychological stress were assessed using 100-mm VAS with questions about feelings of being stressed. Opposing extremes of each feeling were described at either end of the 100-mm horizontal line, and subjects marked the line to indicate how they felt at that moment. Stress profiles were assessed before and after the stress test.
Stress test/control condition In the stress task participants had to take part in the Trier Social Stress Test (TSST) [15], a standardized laboratory stressor designed to elicit psychological stress and cortisol responses. The TSST consisted of a 3-min speech preparation period, a 5-min public speaking task in front of two evaluative, non-responsive audience members and a 5-min mental arithmetic task. The TSST is a validated tool to provoke psychobiological stress responses. In the control condition participants read magazines for the same duration as the TSST (15 min). The sequence whether the TSST was realized on the first or second occasion was randomized.
Procedure
Participants were exposed to 2 days of 2-h laboratory sessions, starting 2.00 pm each day. The maximum time period between the two laboratory sessions was 1 week. The process of every session was equal apart from the task (stress vs. control). To create standardized internal state of satiety, participants were asked to refrain from eating and drinking (excluding water) for 3 h before each occasion. On arrival at the laboratory they filled out a questionnaire, assessing the timing of eating and drinking before each session to confirm that they had followed the directions. Upon arrival, an intravenous catheter was inserted in the subject’s forearm vein and after a 40 min rest, a blood sample for hormonal and a salivary sample for cortisol determinations was drawn (baseline). At this point, subjects answered a short questionnaire regarding their subjective feelings of stress, using VAS to rate their answers. Hereafter participants took part in the TSST or read magazines as control condition. The stress period lasted about 15 min after which a second salivary sample was drawn. Subjects were taken then to another room, where they answered their feelings of being stressed on VAS again before having a standard test meal (500 g of chocolate or vanilla pudding; Brand: Dr. Oetker, Germany), whereby they could eat as much as they wanted. (Nutritional content per 100 g was for chocolate: 158 kcal, 3.1 g protein, 15.8 g carbohydrate, 9.1 g fat. For vanilla: 152 kcal, 2.9 g protein, 15.2 g carbohydrate, 9.1 g fat.) Afterwards blood and salivary was sampled again 30 and 60 min after the beginning of the stress test, respectively, control task. Time points of blood sampling were chosen according to former studies [23]. Macronutrients and energy of the test meal were used with respect to previous studies that have shown that such a meal is able to provoke maximum response of GUT peptides [24, 25].
Statistical analysis
For statistical analysis of ghrelin, two-way repeated-measures analysis of variance (ANOVA) was used to determine the effects of different factors: group (obese vs. normal weight), condition (stress vs. neutral) and measurement points (baseline, +30, +60 min).
To analyze food intake a two-way analysis of variance (ANOVA) was used.
Results
Manipulation check of stress
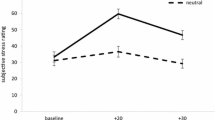
Success of stress induction was tested by subjective stress level and cortisol increase. The rise in subjective stress after the TSST was significant for both comparison groups (F (2, 148) = 24.29, p < 0.001). There was also a significant stress-induced increase in salivary cortisol over all subjects. A MANOVA for repeated measurement showed a significant effect of stress on secretion of salivary cortisol for obese as well as normal weight women (F (1, 80) = 9.9, p < 0.01). Rise and fall of cortisol is depicted in Fig. 1. In the control session there was no difference between measurement points.
In the stress condition cortisol changes were not significantly correlated to ghrelin changes. After the neutral stimulus there was a positive correlation of 0.23 (p < 0.05).
Ghrelin
ANOVA showed a main effect of groups on ghrelin secretion (F 1, 83 = 13.36; p < 0.001, η 2 = 0.14) and a main effect of measurement points (F 2, 166 = 137.993, p < 0.001, η 2 = 0.62). There was also a significant interaction between groups and measurement points (F 2, 166 = 3.40, p = 0.044, η 2 = 0.04). Post hoc tests showed ghrelin values decline after eating the test meal in both groups. Both pre- and postprandial concentrations were lower in obese women than in normal weight women (preprandial, 510.82 ± 226.28 vs. 726.13 ± 363.10 pg/ml and postprandial 526.97 ± 237.30 vs. 737.07 ± 341.58 pg/ml). There was also a difference in dynamics between both groups. The decrease in ghrelin secretion from baseline to measurement point + 60 min was lower in obese than in normal weight women (obese: 510.82 ± 226.28–420.06 ± 186.55 pg/ml; normal weight: 526.97 ± 237.30–605.24 ± 281.97 pg/ml). Stress did not affect ghrelin secretion (F < 1, n.s.). The plasma concentrations of ghrelin in pre- and postprandial state after stress and in the neutral condition are shown in Figs. 2 and 3.
Food intake
Food intake under stress for the obese was 224.2 ± 107.0 and 256.3 ± 131.4 g for the normal weight. ANOVA showed a significant effect of stress in all subjects (F 1, 66 = 11.47, p < 0.001; η 2 = 0.15). Food intake was significantly lower in the stress condition compared to the control condition.
Discussion
This study revealed weight-dependent, but no stress-dependent differences in ghrelin secretion in laboratory. Food intake under stress was inhibited for all subjects.
Ghrelin values decline precipitously to trough values within 60 min after meal ingestion [2]. Therefore, we chose measurement points in a time period (30 and 60 min after stress induction) where postprandial differences can be observed. Expectedly plasma ghrelin levels declined following food intake in both groups. This result has been previously demonstrated and supports the role for ghrelin in regulating food intake [2]. Moreover, we investigated weight-associated differences in ghrelin secretion. Pre- and postprandial obese women had lower ghrelin values than normal weight women. This result is consistent with other studies [6–8, 16]. English et al. [17] found no postprandial change in circulating ghrelin in obese subjects compared to normal weight controls. Our results do not confirm this because we found postprandial decrease in both groups. But obese women showed lower postprandial decline in ghrelin secretion than normal weight controls. This finding replicates results of Le Roux et al. [18] and Kojima and Kangawa [4] who also reported lower postprandial decrease of ghrelin concentrations in obese people. Therefore, the present data provide further evidence of altered ghrelin release in obesity. The reasons for the reduced plasma ghrelin levels are still unclear [18]. Lower ghrelin values in obese people may be caused biologically. Probably, decreased plasma ghrelin concentrations in obese people represent a compensatory reaction of the organism to the positive energy balance associated with obesity [6, 19]. That means, obese people have biologically lower hunger signals than normal weight subjects. A consequence of low ghrelin levels might be a disturbed ability to regulate the hunger and satiation process in so far as the biological signal does not represent their subjective state. We did not find an effect of acute psychosocial stress on postprandial ghrelin secretion either in obese or in normal weight women. This result is also reported by Zimmermann et al. [14] finding no effect of stress on ghrelin secretion. Rouach et al. [8] also found no differences in plasma ghrelin levels throughout the TSST between obese and normal weight subjects. In contrast, Monteleone et al. [11] measured enhanced salivary ghrelin responses after TSST in bulimia nervosa (BN) patients but not in healthy controls. Because of measuring salivary ghrelin and BN patients this result can hardly be compared to our results. Moreover, the extent to which salivary ghrelin mirrors circulating concentrations of the hormone is still a matter of debate. Although at present there is no certainty that salivary ghrelin reflects circulating ghrelin [11]. Gluck et al. [12] and Geliebter et al. [20] also reported higher stress-induced ghrelin release in obese women. Both studies report elevated plasma ghrelin secretion in response to an acute laboratory stressor in obese women. However, there are some fundamental differences between their and our study designs. Contrary to our study, they used a physiological stressor (cold pressor test, CPT) and measured only preprandial ghrelin. In addition they investigated only obese subjects without a control session. Low baseline ghrelin levels were associated with inhibited food intake under stress in the obese. This is consistent with previous studies proposing reduced intake after stress not only in eating disorder patients such as anorexia nervosa but also in obesity [3]. This study has several limitations that should be taken into account when interpreting the results. We did not standardize the nutritional content of our subjects’ last meal. We cannot certainly say that all subjects exactly keep the fasting time of 3 h. While we asked them how many hours ago they had their last meal before the study began, we cannot exclude that some subjects did not follow the instructions. Both factors might have had an influence on ghrelin secretion. Only women were included in the sample, because they are at a higher risk to eat in response to stress than males. Therefore the findings are restricted to females in a limited age range. According to Torres and Nowson [3], people eat less as the severity of a stressor increases. Severity of induced stress through TSST is moderate to high but hardly comparable to a naturalistic one. In general laboratory stressors are weaker and shorter than the most naturalistic stressors [1]. Therefore, it is difficult to generalize the results on stress-induced inhibition in food intake to life stressors. A further limitation has also to be considered. Implanting with the catheter could have been in itself stressful and lead to relatively high baseline measures that would preclude the observation of additional stress effects. However, in the present study the TSST seemed to be a more powerful stressor than simply implanting the catheter. The reported total ghrelin after stress is somewhat higher than in Mittelman et al. [26], but they measured only basal concentrations and investigated obese adolescents. In our study, we only measured total ghrelin and therefore could not examine the stress effect on the active ghrelin. This is a methodological disadvantage with regard to the elegant studies of Raspopow et al. [27, 28], who did measure active concentrations and found results different from our data. The present study enabled further insight into the weight dependency of ghrelin secretion, which seemed to be not affected by an acute stressor. In future studies it would be important to investigate the stress-induced interplay of this peptide with other GUT peptides such as CCK and PYY. Also, intervention studies are needed for documenting ghrelin changes during weight reducing diets.
References
Greeno CG, Wing RR (1994) Stress-induced eating. Psychol Bull 115:444–464. doi:10.1037/0033-2909.115.3.444
Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP et al (2002) Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med 346(21):1623–1630. doi:10.1056/NEJMoa012908
Torres SJ, Nowson CA (2007) Relationship between stress, eating behavior, and obesity. Nutrition 23(11):887–894. doi:10.1016/j.nut.2007.08.008
Kojima M, Kangawa K (2005) Ghrelin: structure and function. Physiol Rev 85(2):495–522. doi:10.1152/physrev.00012.2004
Cummings DE (2006) Ghrelin and the short-and long-term regulation of appetite and body weight. Physiol Behav 89(1):71–84. doi:10.1016/j.physbeh.2006.05.022
Tschöp M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML (2001) Circulating ghrelin levels are decreased in human obesity. Diabetes 50(4):707–709. doi:10.2337/diabetes.50.4.707
Ingelsson E, Larson MG, Yin X, Wang TJ, Meigs JB, Lipinska I et al (2008) Circulating ghrelin, leptin, and soluble leptin receptor concentrations and cardiometabolic risk factors in a community-based sample. J Clin Endocrinol Metabol 93(8):3149–3157. doi:10.1210/jc.2008-0207
Rouach V, Bloch M, Rosenberg N, Gilad S, Limor R, Stern N et al (2007) The acute ghrelin response to a psychological stress challenge does not predict the post-stress urge to eat. Psychoneuroendocrinology 32(6):693–702. doi:10.1016/j.psyneuen.2007.04.010
Kristenssson E, Sundqvist M, Astin M, Kjerling M, Mattsson H, Dornonville de la Cour C et al (2006) Acute psychological stress raises plasma ghrelin in the rat. Regul Pept 134(2):114–117. doi:10.1016/j.regpep.2006.02.003
Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Fujimiya M et al (2001) A role of ghrelin in neuroendocrine and behavioral responses to stress in mice. Neuroendocrinology 74(3):143–147. doi:10.1159/000054680
Monteleone P, Tortorella A, Scognamiglio P, Serino I, Monteleone AM, Maj M (2012) The acute salivary ghrelin response to a psychosocial stress is enhanced in symptomatic patients with bulimia nervosa: a pilot study. Neuropsychobiology 66(4):230–236. doi:10.1159/000341877
Gluck ME, Yahav E, Hashim SA, Geliebter A (2014) Ghrelin levels after a cold pressor stress test in obese women with binge eating disorder. Psychosom Med 76(1):74–79. doi:10.1097/PSY.0000000000000018
Sarker MR, Franks S, Caffrey J (2013) Direction of post-prandial ghrelin response associated with cortisol response, perceived stress and anxiety, and self-reported coping and hunger in obese women. Behav Brain Res 257:197–200. doi:10.1016/j.bbr.2013.09.046
Zimmerman US, Buchmann A, Steffin B, Dieterle C, Uhr M (2006) Alcohol administration acutely inhibits ghrelin secretion in an experiment involving psychological stress. Addict Biol 12:17–21. doi:10.1111/j.1369-1600.2006.00026.x
Kirschbaum C, Pirke KM, Hellhammer DH (1993) The ‘Trier Social Stress Test’—a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 28:76–81. doi:10.1159/000119004
Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, Frost GS et al (2003) Inhibition of food intake in obese subjects by peptide YY3–36. N Engl J Med 349(10):941–948. doi:10.1056/NEJMoa030204
English PJ, Ghatei MA, Malik IA, Bloom SR, Wilding JPH (2002) Food fails to suppress ghrelin levels in obese humans. J Clin Endocrinol Metabol 87(6):2984. doi:10.1210/jc.87.6.2984
Le Roux CW, Patterson M, Vincent RP, Hunt C, Ghatei MA, Bloom SR (2005) Postprandial plasma ghrelin is suppressed proportional to meal calorie content in normal-weight but not obese subjects. J Clin Endocrinol Metabol 90(2):1068–1071. doi:10.1210/jc.2004-1216
Cummings DE, Shannon MH (2003) Roles for ghrelin in the regulation of appetite and body weight. Arch Surg 138:389–396. doi:10.1001/archsurg.138.4.389
Geliebter A, Carnell S, Gluck ME (2013) Cortisol and ghrelin concentrations following a cold pressor stress test in overweight individuals with and without night eating. Int J Obes 37:1104–1108. doi:10.1038/ijo.2012.166
Dressendörfer RA, Kirschbaum C, Rohde W, Stahl F, Strasburger CJ (1992) Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. J Steroid Biochem Mol Biol 43:683–692. doi:10.1016/0960-0760(92)90294-S
Hubel R, Läßle R, Lehrke S et al (2006) Laboratory measurement of cumulative food intake in humans: results on reliability. Appetite 46:57–62. doi:10.1016/j.appet.2005.10.006
Di Francesco V, Barazzoni R, Bissoli L, Fantin F, Rizzotti P, Residori L et al (2010) The quantity of meal fat influences the profile of postprandial hormones as well as hunger sensation in healthy elderly people. J Am Med Direct Assoc 11:188–193. doi:10.1016/j.jamda.2009.08.004
Lorig F, Kießl G, Laessle R (2015) Stress-related cortisol response and laboratory eating behavior in obese women. Eat Weight Disord. doi:10.1007/s40519-015-0190-3
Kießl G, Laessle R (2016) Stress inhibits PYY secretion in obese and normal weight women. Eat Weight Disord 21(2):245–249. doi:10.1007/s40519-015-0231-y
Mittelman SD, Klier K, Braun S, Azen C, Geffner ME, Buchanan TA (2010) Obese adolescents show impaired meal responses of the appetite-regulating hormones ghrelin and PYY. Obesity (Silver Spring) 18(5):918–925. doi:10.1038/oby.2009.499
Raspopow K, Abizaid A, Matheson K, Anisman H (2010) Psychosocial stressor effects on cortisol and ghrelin in emotional and non-emotional eaters: influence of anger and shame. Horm Behav 58(4):677–684. doi:10.1016/j.yhbeh.2010.06.003
Raspopow K, Abizaid A, Matheson K, Anisman H (2014) Anticipation of a psychosocial stressor differentially influences ghrelin, cortisol and food intake among emotional and non-emotional eaters. Appetite 74:35–43. doi:10.1016/j.appet.2013.11.018
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
The study was approved by the ethics committee of the University of Trier in April 2012.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Kiessl, G.R.R., Laessle, R.G. Stress does not affect ghrelin secretion in obese and normal weight women. Eat Weight Disord 22, 79–84 (2017). https://doi.org/10.1007/s40519-016-0316-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40519-016-0316-2