Abstract
Vitamin D is a fat-soluble vitamin and a steroid hormone that plays a central role in maintaining calcium-phosphorus and bone homeostasis in close interaction with parathyroid hormone, acting on its classical target tissues, namely, bone, kidney, intestine, and parathyroid glands. However, vitamin D endocrine system regulates several genes (about 3 % of the human genome) involved in cell differentiation, cell-cycle control, and cell function and exerts noncalcemic/pleiotropic effects on extraskeletal target tissues, such as immune and cardiovascular system, pancreatic endocrine cells, muscle, and adipose tissue. Several studies have demonstrated the role of vitamin D supplementation in the prevention/treatment of various autoimmune diseases and improvement of glucose metabolism, muscle, and adipose tissue function. Hence, this review aims to elucidate the effects of vitamin D on extraskeletal target tissues and to investigate the potential therapeutic benefit of vitamin D supplementation among a broad group of pathological conditions, especially with regard to metabolic and autoimmune diseases. In addition, we focused on the best daily intakes and serum levels of vitamin D required for extraskeletal benefits which, even if still controversial, appear to be higher than those widely accepted for skeletal effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Physiology of vitamin D endocrine system: from synthesis to peripheral actions of vitamin D
Vitamin D is a fat-soluble vitamin and a steroid hormone that plays a central role in the regulation of calcium homeostasis and bone health. It can be either produced from human skin upon UVB irradiation or obtained from external sources, such as vegetable or animal food containing vitamin D2 (or ergocalciferol) and vitamin D3 (VD3 or cholecalciferol), respectively (Fig. 1). At temperate climates, 80 % of vitamin D requirements are guaranteed by sun exposure, with remaining 20 % being secured by nutrition [1]. The largest part of vitamin D is produced from 7-dehydrocholesterol (7-DHC) present in keratinocytes via a UVB light-mediated photochemical synthesis, which leads to the conversion of 7-DHC to VD3. The skin storage depots of 7-DHC decrease with age, along with the capacity of the human skin to synthesize cholecalciferol [2]. Vitamin D hereby produced by the skin probably binds directly to an alpha-globulin known as vitamin D binding protein (DBP) and is then transported to the liver, where it undergoes 25-hydroxylation and is released as 25-hydroxyvitamin D3 [25(OH)vitamin D3 or 25(OH)D3]. The unbound (free) form of vitamin D represents the metabolically active one.
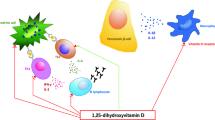
Classical vitamin D metabolic pathways and extraskeletal target tissues. Under solar UVB radiation, 7-dehydrocholesterol is converted to vitamin D3 (or cholecalciferol) in the skin. Vitamin D3 is then transported to the liver and converted to 25(OH)D3, the major circulating metabolite of vitamin D, by the action of vitamin D 25-hydroxylase. Thereafter, 25(OH)D3 is converted to 1,25(OH)2D3, the biologically active form of VD3, by renal 25(OH)D 3 1-alpha-hydroxylase. The latter enzyme (the rate-limiting enzyme of vitamin D metabolism) is stimulated by PTH, whereas it is inhibited by 1,25(OH)2D3 and other factors, such as phosphorus, calcium, and FGF-23, through a negative feedback mechanism. Finally, both 25(OH)D3 and 1,25(OH)2D3 undergo a further hydroxylation on carbon 24 by the enzyme 1,25(OH) 2 D 3 24-hydroxylase, which leads to their catabolism and prevents potential vitamin D intoxication. 1,25(OH)2D3 targets bone, kidney, intestine, and parathyroid glands to regulate calcium/phosphate homeostasis and inhibit parathyroid hormone production and 25(OH)D 3 1-alpha-hydroxylase activity, thus modulating its own synthesis. Apart from these classical target tissues, vitamin D acts on various extraskeletal tissues, exerting other noncalcemic/pleiotropic functions (red box in the figure). Green boxes show the 25(OH)D3 desirable (evidence based) serum levels to be reached for each non-classical target tissue which are above those established to be necessary for full effects on calcium–phosphorus homeostasis; the recommended vitamin D supplementation should be revised and eventually increased accordingly
Two additional hydroxylations are necessary for the biological activity of vitamin D, one at C25 and one at C1α, in the liver and in the kidney, respectively. The hydroxylation on C25 is catalysed by several enzymes, among which the most important is CYP2R1, also known as vitamin D 25-hydroxylase [3]; interestingly, this enzyme has been shown to be present in the human testis, where it is produced by Leydig cells under the influence of luteininzing hormone (LH) and contributes to the circulating levels of 25(OH)D3 in males [4]. However, a crucial step for the synthesis of metabolically active 1,25(OH)2D3—the one mainly involved in feedback mechanisms—is the 1α-hydroxylation occurring in the kidney by means of CYP27B1, also known as 25-hydroxyvitamin D 3 1α-hydroxylase. The proximal renal tubule represents the main site of action for this process, which is subject to both negative (by 1,25-dihydroxyvitamin D3, phosphate, calcium, and FGF-23) and positive feedback mechanisms (PTH, calcitonin, and insulin-like growth factor 1) [5–7] through complex chromatin and DNA promoter regulations [8, 9]. High levels of 1α-hydroxylase mRNA and enzyme activity have also been detected in human keratinocytes [10], in macrophages [11], and several other tissues [12]. Finally, an alternative hydroxylation of 1, 25(OH)2D occurs on carbon 24 (C24) through CYP24A1 activity (also known as 1,25-dihydroxyvitamin D 3 24-hydroxylase), a mitochondrial enzyme detected in virtually all nucleated cells and involved in the degradation of 1α25(OH)2D3 by hydroxylation of the side chain to form calcitroic acid.
1,25(OH)2D3 mainly exerts its effects through the activation of VDR nuclear receptor (a member of the steroid receptor transcription factor family), of which VD3 represents a high-affinity ligand. VDRs are largely expressed in several tissues, such as endothelium [13, 14], vascular smooth muscle [15], and cardiomyocytes [16]. Classical target tissues for vitamin D peripheral actions are represented by bone, kidney, intestine, and parathyroid glands. Vitamin D in the bone interacts with 1,25(OH)2D3 receptors expressed by osteoblasts, showing dual effects on bone, both stimulating osteoclastogenesis and bone resorption and modifying osteoblast function and bone mineralization. Under adequate calcium intake, 1,25(OH)2D3 helps stimulating calcium absorption and allows mineral deposition, whereas in calcium deficiency conditions, it supports serum calcium homeostasis at the expense of the bone, increasing bone resorption and inhibiting mineral bone deposition. In the kidney, the proximal renal tubule is the site for 1α-hydroxylation of 25(OH)D3 and for the synthesis of the active form 1,25(OH)2D3. In the kidney, 1,25(OH)2D3 increases the distal tubular reabsorption of calcium, involving TRP channels (transient receptor potential cation channels), especially TRPV5, and other proteins. In the intestine, 1,25(OH)2D3 increases calcium uptake through the production and activity of several proteins in the small intestine, including TRPV5, TRPV6, and calbindin-D9 K, which facilitate the transport of the calcium ion by means of increased permeability of the intercellular “tight junctions”. In the parathyroid glands, 1,25(OH)2D3 exerts an inhibitory role on parathyroid hormone synthesis and controls critical parathyroid genes, such as the CaSR (Calcium-sensing receptor) and parathyroid hormone gene.
Pleiotropic and extraskeletal effects of vitamin D: non-classical target tissues
Although the role of vitamin D in calcium-phosphorus metabolism regulation and mineral skeletal homeostasis is the most acknowledged, its role in different extraskeletal actions is largely documented. It is known that VDR receptor expression is ubiquitous, there are different extra-renal forms of the 1α-hydroxylase enzyme, and, finally, approximately 3 % of mice or human genome is, directly and/or indirectly, regulated by the active form of vitamin D [12]. In addition, it has been observed that vitamin D plays an important regulatory role in the physiology of immune system, skeletal muscle and adipose tissue, glucose metabolism, skin, cardiovascular and reproductive system, and neurocognitive functions, along with the modulation of cell proliferation (Fig. 1) [17, 18].
Immune system: type 1 diabetes mellitus, multiple sclerosis, autoimmune thyroid disorders
The influence of vitamin D on immune function has been profoundly investigated; however, a causal link between poor vitamin D status and autoimmune disease is still unclear. To date, there is still no consensus about recommended targeted serum levels and the optimal vitamin D supplementation in autoimmune diseases.
All immune cells express a functional VDR at determined stages of their differentiation, and antigen presenting cells (APC) are able to produce 1,25(OH)2D3 through the same enzyme expressed at kidney level, but only upon immune stimuli, such as IFN-α; in pathological conditions, such as sarcoidosis, this can lead to systemic excess of 1,25(OH)2D3 and severe hypercalcemia [19].
Vitamin D exerts its action on innate immune system as well as on acquired immune system, even though with opposed effects. Regarding innate immunity, vitamin D and its metabolites stimulate macrophage differentiation and activation, together with local production of defensins, such as cathelicidin and β2-defensin [20]. Mice fed a VD3 deficient diet show impairment of IL-6, TNFα, and IL-1 production and antimicrobial activity [21]. In addition, the activation of Toll-like receptors (TLRs) in human macrophages following infectious stimuli up-regulates the expression of VDR and 1α-hydroxylase genes, leading to the induction of antimicrobial peptides. Interestingly, a study on Mycobacterium tuberculosis has shown that African-American individuals, known to have increased susceptibility to tuberculosis, presented low 25(OH)D3 levels, a result supporting the presence of a potential link between TLRs and vitamin D-mediated innate immunity. This suggests that different susceptibilities to microbial infections among various human populations could be based on interracial differences in ability to produce vitamin D [22]. Furthermore, it has also been shown that patients with chronic kidney disease display low levels of 25(OH)D3: in these subjects, the administration of paricalcitol, a vitamin D analog, leads to a downregulation of IL-6 and TNFα, supporting a role of 25(OH)D3 in the regulation of immune and inflammatory processes [23].
On the other hand, the overall effect of vitamin D on the acquired immune system mostly consists in inhibitory effects. Indeed, 1,25(OH)2D3 inhibits surface expression of MHC class II and co-signaling molecules on antigen presenting cells, decreases the activity of Th1 and Th17 cells, and up-regulates regulatory T cells (Tregs). Hence, vitamin D could cause a shift of T cells from an effector phenotype, involved in autoimmune diseases, to a regulatory or protective one [24]. It is interesting to note that VDR and mineralocorticoid receptor (MR), members of the same receptor’s family, have opposite effects on immune and inflammatory responses [25]. Physiological doses of 1,25(OH)2D3 inhibit cytokine production by Th1 and Th17 cells in a VDR-dependent manner [26], whereas aldosterone can induce the priming to Th17 cells that are involved in autoimmune reaction [27]. Moreover, MR drives macrophage polarization towards a pro-inflammatory phenotype, as demonstrated by the fact that macrophage-specific MR genetic deletion and pharmacological antagonism in vitro are both able to induce a switch from a pro-inflammatory (M1) into an anti-inflammatory (M2) phenotype [28].
These data could explain the beneficial effect of vitamin D supplementation on the onset and progression of autoimmune diseases in both deficient and normal vitamin D status. For example, vitamin D prevents both insulitis and type 1 diabetes mellitus (T1DM) in mouse models of T1DM, and retrospective studies have shown apparent beneficial effects of vitamin D supplementation in early life on the risk of developing T1DM [29, 30]. In this regard, a recent randomized clinical trial on young patients with T1DM has shown that cholecalciferol supplementation (70 IU/kg body weight/day) improves the suppressive ability of regulatory T cells that is well known to be impaired in T1DM patients [31]. Giulietti et al. have demonstrated that genetically predisposed non-obese diabetic mice showed higher IL-1 levels in pancreatic islets and an aberrant cytokine profile in peritoneal macrophages (low IL-1 and IL-6 and high IL-15) when they were exposed to vitamin D deficiency early in life [32]. This highlights the importance of preventing vitamin D deficiency in early childhood to reduce the incidence of T1DM in subjects at risk, as also pointed out in other studies [33]. In addition, it has been shown that CYP27B1 and other genetic polymorphisms involved in vitamin D metabolism are associated with increased susceptibility to the onset of T1DM [34]; however, a meta-analysis Guo et al. found no evidence of a link between VDR receptor polymorphisms and T1DM risk in either case–control studies or family-transmission studies [35]. Epidemiological studies in humans indicate that vitamin D supplementation early in life could decrease the risk of developing T1DM: risk reduction varied between 26 % with cod liver oil to 78 % with 2000 IU/day vitamin D during the first year of life, with an overall effect of 30 % reduction in five studies [36]. Moreover, a seasonal pattern of disease onset has also been described for T1DM, suggesting an inverse correlation between sunlight and the disease; notably, vitamin D has been claimed as a potential mediator of this sunlight-mediated effect [37]. Likewise, the addition of vitamin D to insulin therapy among patients with new-onset T1DM has been associated with a slower decline of residual β-cell function compared with insulin alone [38]. Interestingly, we recently reported the case of a 14-year-old patient with new onset of T1DM, treated with a combination of high-dose Omega-3 fatty acids and high-dose cholecalciferol oral therapy (25,000 IU/week), showing an increase in peak C-peptide of about 20 % from baseline after 12 months, suggesting a potential role of this therapeutic strategy in decreasing the pancreatic β-cell inflammatory response, along with a longer preservation of β-cell mass in patients with new-onset T1DM [39].
Following on autoimmune diseases, low vitamin D plasma levels have been associated with an increased risk of developing multiple sclerosis (MS) [40]. An important prospective, nested case–control study conducted by Munger et al, has shown that among whites, the risk of MS significantly decreased with increasing levels of 25(OH)D3, suggesting that high-circulating levels of vitamin D are associated with a lower risk of MS. Among blacks and Hispanics, who had lower 25(OH)D3 levels than whites, no significant associations between vitamin D levels and MS risk were found [41]. Moreover, cholecalciferol supplementation may influence both clinical activity and magnetic resonance imaging disease activity in patients with established MS: in fact, a study showed that the addition of VD3 to treatment with interferon-β-1b significantly reduced disease activity in MS compared to patients undergoing interferon-β-1b alone [42].
Vitamin D deficiency may also be involved in the pathogenesis of various autoimmune thyroid disorders, such as Graves’ disease, Hashimoto’s autoimmune thyroiditis (HT), and postpartum thyroiditis [43]. Indeed, genetic polymorphism of VDR, DBP, and 1α-hydroxylase enzyme seems to predispose to the development of HT and Graves’ disease [44]. Moreover, an inverse correlation between vitamin D levels and anti-thyroid antibody levels (anti-thyroid peroxidase, or anti-TPO, and anti-thyroglobulin, or anti-Tg) has been found [45], although the mechanism underlying this association is still unclear. Evliyaoğlu et al. have shown that in children and adolescents affected by HT, the prevalence of vitamin D deficiency is significantly higher than that in the control group [46]. Whether high-dose vitamin D therapy has preventive or therapeutic effect in autoimmune thyroid disorders is currently under investigation. A recent study conducted on 218 euthyroid patients with HT living on the island of Crete has shown that the majority (85.3 %) of the Greek Caucasian patients with HT had low 25(OH)D3 serum levels, which were inversely correlated with serum anti-TPO thyroid antibodies. Vitamin D deficient patients received VD3 orally, 1200-4000 IU every day for 4 months aiming to maintain serum 25(OH)D3 levels ≥40 ng/mL. After 4 months, these patients showed a significant decrease (20.3 %) of serum anti-TPO levels, suggesting that vitamin D deficiency may be related to the pathogenesis of HT and that its supplementation could contribute to an improvement of the disease [47]. A correlation between vitamin D deficiency/insufficiency and inflammatory bowel disease (IBD), such as Crohn’s disease and ulcerative colitis, in northern european countries compared with southern counterparts has also been described, along with a seasonal variation in onset and exacerbations of the disease [48].
These results altogether strengthen the need of intervention clinical trials to demonstrate the existence of a potential causal link between vitamin D deficiency/insufficiency and autoimmune diseases, such as type 1 diabetes mellitus, multiple sclerosis, autoimmune thyroid disorders, and inflammatory bowel disease.
Adipose tissue and glucose/lipid metabolism: obesity and type 2 diabetes mellitus
Vitamin D is a fat-soluble vitamin distributed through the lymphatic system almost exclusively to the adipose tissue, from which it is released in small amounts, depending on the stored quantity; as a consequence, a greater adipose mass dilutes available vitamin D, explaining why risks associated with its deficiency are higher in obese patients. Several researchers focused on the role of vitamin D in adipose tissue biology and most observational studies in humans link vitamin D deficiency with almost all the aspects of metabolic syndrome, such as obesity, insulin resistance, hypertension, dyslipidemia, impaired fasting glucose, and type 2 diabetes mellitus (T2DM) [36, 49].
Several studies demonstrated a negative correlation between vitamin D levels and leptin and resistin, and a positive association with adiponectin [50, 51]. Marcotorchino et al. studied the preventive effect of VD3 supplementation (15,000 IU/kg of food for 10 weeks) on the onset of obesity in a mouse model, concluding that VD3 supplementation counteracted weight gain induced by high-fat diet, in parallel with an improvement of glucose homeostasis, an effect probably due to the up-regulation of genes involved in fatty acid oxidation and mitochondrial metabolism, followed by a significant rise in energy expenditure. These results suggest that VD3 supplementation may represent part of the strategy to face the onset of obesity and associated metabolic disorders [52]. Moreover, a study on a diet-induced obesity mouse model proved that high intake of vitamin D, especially if associated with high doses of calcium, leads to the activation of calcium-mediated apoptotic pathway in adipose tissue, counteraction of body and fat weight gain, and improvement in adiposity markers (plasma concentrations of glucose, insulin, and adiponectin) [53].
A large genetic study showed that higher body mass index (BMI) levels and genetic predisposition for obesity were associated with a decrease in 25(OH)D3 concentrations, while the effects of lower 25(OH)D3 levels on BMI are likely to be small [54]. Equally, vitamin D supplementation did not decrease BMI in four interventional studies [55]. Thus, efforts in reducing BMI are expected to decrease the prevalence of vitamin D deficiency. A Chinese randomized placebo-controlled trial involving 126 subjects with metabolic syndrome and vitamin D deficiency demonstrated that a supplementation with 700 IU/day of cholecalciferol for 12 months did not improve metabolic syndrome risk factors, such as BMI, blood pressure, fasting glucose, fasting insulin, and lipid profile [56]. Similar negative findings on conventional metabolic syndrome risk factors were obtained in a study recruiting 305 healthy postmenopausal women receiving a daily oral dose of 400 or 1000 IU cholecalciferol for 1 year [57].
We recently demonstrated that VD3 inhibits adipogenesis in murine 3T3-L1 preadipocytes [58], one of the best characterized and widely used cellular model to study adipocyte differentiation [59]. Cells exposed to VD3 and/or alendronate (ALN), showed an increase in VDR mRNA expression and a significant reduction in levels of peroxisome proliferator-activated receptor gamma (PPARγ), the master gene of adipogenesis. We concluded that both VD3 and ALN have a marked anti-adipogenic effect and that the latter is exerted via the activation of VDR. Therefore, since in the elderly the shift in bone marrow mesenchymal stem cells differentiation towards adipogenesis instead of osteoblastogenic lineage is one of the causes of bone mass reduction, VD3 supplementation should always be considered in patients undergoing ALN therapy to achieve maximal anti-osteoporotic efficacy and to attenuate the increase of marrow adipose tissue in the aging bone (Fig. 2).
Vitamin D3 inhibitory effect on 3T3-L1 adipogenesis. (a) 3T3-L1 preadipocytes were induced to differentiate both in the presence or absence of increasing concentration of VD3 (10−9 to 10−7 M), then examined by microscopy after oil red O staining and tested for lipid accumulation, after 7 days. *p < 0.05; **p < 0.01; ***p < 0.001; VD3-treated versus untreated cells (UT). (b) Representative microphotographs of the corresponding histograms shown in a (scale bar 70 µm)
As regard glucose metabolism, pre-clinical studies have shown that adequate vitamin D concentrations and functioning VDR are needed for normal activity of pancreatic islets β-cells and that VD3 has modest stimulatory effects on insulin synthesis and secretion, probably due to the effects of calcium on β-cell function, since insulin secretion is a calcium-dependent process [60]. However, in addition to the regulation of extracellular calcium concentration and flux through the β-cell [61], vitamin D seems to display a direct stimulatory effect on insulin secretion via a VDR-mediated effect on pancreatic β-cells [62]. In fact, mice lacking a functional VDR show impaired insulin secretion following a glucose load due to decreased insulin synthesis by the β-cell [63]. Moreover, vitamin D affects insulin sensitivity through different mechanisms. First, vitamin D stimulates the expression of insulin receptors, activating the VDR-retinoic acid X-receptor (RXR) complex, which binds to a vitamin D response element in the promoter region of insulin receptor gene. This in turn leads to an enhanced transcriptional activation of the insulin receptor gene and a subsequent increase in the total number of insulin receptors [64]. Vitamin D also enhances insulin sensitivity by: (1) the activation of Peroxisome proliferator-activated receptor delta (PPAR-δ), a transcription factor that regulates the metabolism of fatty acids in skeletal muscle and adipose tissue [65] and (2) through its regulatory role in extracellular calcium concentration and flux through cell membranes, since calcium is essential for insulin-mediated intracellular processes in insulin-responsive tissues, such as muscle and fat [66]. Finally, vitamin D insufficiency is associated with increased fat infiltration in skeletal muscle contributing to a decreased peripheral insulin sensitivity [67] and increased serum levels of parathyroid hormone (PTH), which has been associated with insulin resistance [68].
In diabetic patients, there is a high prevalence of VD3 deficiency [69]. A meta-analysis has shown that vitamin D supplementation in diabetic patients has a small effect on fasting glucose and a small improvement in insulin resistance, with no effect on HbA1c, whereas no effects were shown in patients with normal fasting glucose [70]. Chandler et al. recently conducted a prospective, randomized, double-blind, placebo-controlled clinical trial of oral cholecalciferol supplementation. It was found that vitamin D3 supplementation at any dose significantly increased C-peptide by 0.82 ng/mL, suggesting that vitamin D supplementation could be considered for the prevention and management of diabetes, along with weight loss and increased physical activity [71].
Finally, a recent study demonstrated that vitamin D supplementation with 16,000 IU of calcifediol orally once a week for a minimum of 8 weeks decreased fasting glucose in T2DM patients with vitamin D deficiency [72]. The Medical Research Council Ely Prospective Study 1990–2000 showed that baseline serum 25(OH)D3 is predictive of future glycemic status and insulin resistance: in this study, baseline 25(OH)D3 serum levels were inversely associated with 10-year risk of hyperglycemia, insulin resistance, and metabolic syndrome, suggesting an inverse relationship between baseline 25(OH)D3, glycemia, and insulin resistance [73]. The exact mechanism by which vitamin D affects lipid profile is still unclear. Therefore, the effect of vitamin D on lipid profile in patients undergoing a high-dose supplementation is still controversial. A randomized, controlled, double-blind study recently demonstrated that subjects with metabolic syndrome and vitamin D deficiency/insufficiency randomized to receive 50,000 IU of cholecalciferol weekly for 16 weeks displayed a significant reduction in triglycerides serum levels after 4 months, with a parallel improvement in vitamin D status, whereas the cholesterol concentration and other cardiometabolic risk factors were not significantly altered [74]. A meta-analysis by Jafari et al. evaluated the effect of vitamin D on serum lipid profile in patients suffering from T2DM, showing that vitamin D significantly reduced serum total cholesterol, LDL, and triglycerides, with negligible effects on HDL levels. Specifically, this meta-analysis demonstrated that vitamin D supplementation at doses of ≤2000 IU/day in a duration of ≤12 weeks determines more beneficial effects on lipid profile compared with higher doses and longer treatments [75]. In summary, these findings suggest that vitamin D could be considered as an adjuvant therapy for dyslipidemia, which is frequently associated with T2DM and metabolic syndrome.
Skeletal muscle
Skeletal muscle is a special target of vitamin D. VD3 promotes muscular proteins synthesis and activates calcium uptake in sarcoplasmic reticulum, hence maintaining muscle trophism and contractile efficiency. Under deficient vitamin D status, proximal myopathy, sarcopenia, reduced muscle strength, balance disorders, and a significant increase in risk of falls have been described [76, 77]. In osteomalacia, histopathological changes in skeletal muscle were described, represented by scattered muscle fiber atrophy, necrosis, derangement of intermyofibrillar network, and replacement of muscle tissue with adipocytes and fibrous connective tissue [78]. Observational studies have shown a correlation between low vitamin 25(OH)D3 and muscle weakness in children and elderly subjects [79]. A significant decrease in risk of falls and, as a result, a reduced fracture risk has also been described after vitamin D supplementation, particularly in the elderly [80], where the bone detrimental effects of vitamin D deficiency can further complicate those due to the physiological age-related sarcopenia. Indeed, vitamin D supplementation can improve energy recovery after exercise, muscle function, and body sway [81]. Fornari et al. recently investigated the role of lean mass in obese subjects, undergoing body composition analysis by dual X-ray absorptiometry and screened for metabolic and hormone profile. Interestingly, authors found that higher amounts of lean mass are directly linked to a lower inflammatory profile and better insulin sensitivity, but also to the presence of higher levels of vitamin D and IGF-1, suggesting that higher levels of lean mass correlate with a better metabolic profile and a lower inflammatory status [82]. Similarly, Rondanelli et al. showed that a daily dietary supplementation with whey protein, essential amino acids and vitamin D, in association with regular and age-appropriate physical activity, promoted a greater increase in fat-free mass, relative skeletal muscle mass, and muscle strength in sarcopenic elderly people [83]. Nevertheless, a Cochrane meta-analysis and another systematic review [84] have shown that muscle strength is not significantly improved by vitamin D supplementation, except for the improvement of proximal muscle strength in a subgroup of subjects with very low vitamin D status at baseline. More importantly, the risk of falls in elderly subjects and especially in nursing home residents can be reduced by ~20 % with a vitamin D supplementation of 800 IU/day. Thus, vitamin D effects on muscle are currently disputed, because there is conflicting evidence about the presence of VDR protein in mature skeletal muscle cells. Wang et al. have shown that VDR is undetectable in skeletal, cardiac, and smooth muscle when highly specific VDR antibodies are used, suggesting that the function of vitamin D on muscle is either indirect or does not involve a known receptor [85]. However, it was observed that VDR-null mice develop a specific phenotype with smaller muscle fibers and prolonged expression of immature muscle-specific genes and that mice with cardiomyocyte-specific deletion of VDR develop cardiac hypertrophy [86]. Moreover, VDR-null mice exhibit the expression of embryonic markers even after weaning and regardless of a high calcium diet [87]. A large number of studies showed genomic and rapid signaling changes in response to 1,25(OH)2D3 in myoblast or myocyte cultures in vitro, associated with a negative regulation of myostatin, the major inhibitory myokine [88]. The latter findings could be explained by the fact that skeletal muscle has a very high turnover (1 % per day) and probably needs even greater repair mechanisms than bone microdamage repair, whereby the expression of VDR in muscle satellite cells and myoblasts is more important than its presence in mature muscle cells. Thus, both cardiac and skeletal muscle defects appear in global VDR-null mice [89].
Anorexia nervosa and eating disorders
Several organic complications can occur in anorexia nervosa (AN), among which alterations in bone metabolism (osteoporosis and osteopenia, frequently associated with low-circulating levels of vitamin D) are usually observed [90]. In addition, depressive mood and self injurious behaviors, which are also frequent in AN, have been inversely associated with the vitamin D status [91, 92]. Although various evidences indicate that vitamin D status is poor in AN, other studies reported normal levels of vitamin D in AN patients [93–95]. A recent meta-analysis of 15 cross-sectional studies focused on vitamin D parameters and dietary vitamin D intake in patients with AN and healthy controls showed that both serum 25(OH)D3 and 1,25(OH)2D3 levels were significantly lower in AN than healthy controls, despite AN patients reported similar intake of vitamin D compared to healthy controls [96]. This finding seems to be paradoxical, since AN patients usually show higher levels of physical activity compared to age-matched healthy controls [97] and vitamin D is fat soluble. Hence, AN patient should have higher circulating 25(OH)D3 levels because of their decreased stores of fat mass [98, 99]. Various explanations for this contrasting data could be considered. For example, AN patients are often prone to overestimate their dietary intake [100] and they also practice mainly indoor physical activities that are less effective in maintaining optimal 25(OH)D3 serum levels than outdoor activities [101]; furthermore, AN patients often wear covering clothes minimizing light exposure due to shame sensations and reduced thermogenesis [102]. Finally, even though low serum levels of 25(OH)D3 are typically observed in obese people, it has been shown that low serum 25(OH)D3 is also associated with undernutrition, AN, and neoplastic cachexia [98, 103]. Moreover, the same meta-analysis revealed that AN patients treated with cholecalciferol supplementation displayed significantly higher serum levels of 25(OH)D3 compared to healthy controls [98]. These results bring to the conclusion that in AN patients with low 25(OH)D3 serum levels, cholecalciferol supplementation could be useful to reverse the poor vitamin D status and to prevent/counteract bone loss and its complications. Gatti et al. found a strong relationship between vitamin D status and bone mineral density (BMD) in AN [104]; there was a trend of higher hip BMD values in AN patients with 25(OH)D3 levels >30 ng/mL as compared with patients with 25(OH)D3 values between 20 and 30 ng/mL, suggesting that vitamin D allowance in amenorrheic patients with AN should be higher enough to increase 25(OH)D3 levels above 30 ng/mL. However, VD3 deficiency in patients with eating disorders is not only correlated with the risk of osteoporosis and bone loss. In this regard, a recent study reports the case of a woman with long-term anorexia nervosa and of a woman with long-term bulimia nervosa both complicated by severe hypovitaminosis D3 [25(OH)D3 serum levels between 1 and 4 ng/mL], decrease of VDR in blood cells, leukopenia, and the S allele of the 5-hydroxytryptamine transporter polymorphism [105]. This finding leads to the hypothesis that severe VD3-hypovitaminosis might be responsible for lack of inflammatory response and also for reduction in mood and depressive symptoms among patients with long-term eating disorders. Since the antimicrobial, anti-inflammatory, and immunomodulatory functions of VD3 are well known [106], using VD3 as an anti-inflammatory molecule could be a useful therapeutic strategy in the treatment of eating disorders.
Vitamin D therapy
Optimal serum levels and recommended daily intakes
Serum concentration of vitamin 25(OH)D3 is considered the best biomarker to determine vitamin D status, due to its long circulating half-life (approximately 2–3 weeks). Nevertheless, there is no consensus on the 25(OH)D3 thresholds for vitamin D deficiency or insufficiency between the main guidelines by the Institute of Medicine (IOM), the Endocrine Society, and the SIOMMMS (Table 1) [107–109].
The differences are due to the fact that IOM focuses on the general healthy population and intervention studies, considering that serum levels of 25(OH)D3 >20 ng/mL are sufficient to gain favourable skeletal outcomes and that there are no evidences of benefits of higher levels for non-skeletal outcomes, such as diabetes. Moreover, IOM considers serum levels of 25(OH)D3 >50 ng/mL a reason for concern about potential adverse events and toxicity risk. On the other hand, the Endocrine Society guidelines are mainly focused on people at risk for vitamin D deficiency and on epidemiologic observational studies, considering that serum levels of 25(OH)D3 >30 ng/mL are optimal for skeletal outcomes, and do not identify any vitamin 25(OH)D3 threshold above which risks for safety arise.
For the same reasons, there are differences among the two main guidelines also in the recommended daily intakes to prevent bone complications of vitamin D deficiency/insufficiency (rickets, osteomalacia). Indeed, the IOM report on reference dietary intakes for calcium and vitamin D indicates 600 IU per day of vitamin D for individuals 9–70 years and 800 IU for those older than 70 years as the recommended dietary allowance (RDA), which is defined as the average daily level of intake sufficient to meet the nutrient requirements of nearly all (97.5 %) healthy population [110]. The IOM report also concludes that the tolerable upper intake level (UL), defined as the maximum daily intake above which the potential for adverse health effects may increase with chronic use, is 4000 IU per day. In contrast, the Endocrine Society guidelines affirm that daily vitamin D intakes of 1500–2000 IU are needed to raise the blood level of 25(OH)D3 constantly above 30 ng/mL (Table 2) [109]. Several intervention studies conclude that serum 25(OH)D3 levels increase by nearly 1 ng/mL for each additional 100 IU of vitamin D supplement per day, though vitamin D necessary amount for obtaining a minimum serum level of vitamin 25(OH)D3 above 30 ng/mL depends also on the baseline 25(OH)D3 level, UVB body exposition, and dietary vitamin D intake [111].
Regarding the extraskeletal effects of vitamin D, several data support a correlation between a poor vitamin D status and various non-skeletal diseases, such as diabetes mellitus and multiple sclerosis. However, the existence of a causal relationship between vitamin D status and these disorders still remains unclear, as well as vitamin D supplementation efficacy, the recommended daily intake and serum vitamin 25(OH)D3 thresholds [112]. High-dose vitamin D therapy, also known as Stoss therapy, originated in Germany in the late 1930s for the rapid repletion of vitamin D status in the prevention and/or treatment of rickets, currently represents a reference for the treatment and maintenance treatment regimens in pathologic conditions which can potentially be improved by vitamin D, such as infections and chronic kidney diseases [113, 114].
A prospective intervention study by Masood et al. evaluated the efficacy of a high-dose oral or intramuscular VD3 therapy in maintaining target serum levels of vitamin 25(OH)D3 over time in patients with vitamin D deficiency (i.e., <20 ng/mL), randomizing 100 individuals to a bolus dose of 200,000 or 600,000 IU orally or intramuscularly. The results obtained 2 months after vitamin D administration showed that 87.5 and 93.8 % of individuals treated with intramuscular VD3 bolus (200,000 and 600,000 IU, respectively) achieved serum levels of 25(OH)D3 > 20 ng/mL, whereas only 70.6 and 83.3 % of individuals who underwent oral VD3 bolus (200,000 and 600,000 IU, respectively) reached the same serum levels of vitamin 25(OH)D3. After 6 months, more than 80 % of subjects who underwent intramuscular VD3 bolus of 600,000 IU maintained serum levels of 25(OH)D3 above 20 ng/mL, while less than one-third of subjects who underwent the lower dose of intramuscular VD3 bolus (200,000 IU) and the oral VD3 bolus (both 200,000 and 600,000 IU) maintained the same 25(OH)D3 serum concentrations. No differences in adverse events were found after the high-dose vitamin D bolus, indicating that both oral and intramuscular administrations of high-dose VD3 are safe and efficacious in correcting vitamin D deficiency in most patients treated, and this occurs within the first 2 months [115]. Intramuscular administration could also be useful in the case of non-adherence to treatment or individuals at risk of vitamin D malabsorption, or in subjects allergic to oral supplementations. Nevertheless, it appears likely that for the optimal benefits of vitamin D supplementation, vitamin D supplements should be provided daily to ensure that stable vitamin D circulating concentrations are maintained over time.
A few cases of infectious diseases and anemia-related illnesses represent pathological conditions where high-dose VD3 therapy has shown effectiveness; in particular, vitamin D could improve anemia, causing a decrease of pro-inflammatory cytokines and reducing hepcidin levels. Hepcidin is a peptide found in high amounts under inflammatory conditions, responsible for decreased iron absorption and simultaneous iron deprivation in macrophages [116, 117]. Intervention trials are needed to assess the exact dose and frequency of high-dose VD3 in these conditions.
To improve skeletal muscle function and reduce risk of falls, vitamin D supplements of 700–1100 IU daily have been shown to be modestly efficacious [79]. In 2011, a meta-analysis of 26 studies confirmed that vitamin D supplementation reduced the risks of falling when associated with calcium intake [118]. In another meta-analysis of eight controlled and randomized studies, Bishoff-Ferrari et al. further showed that daily supplementation of 700-1000 IU VD3 or its active metabolites reduced the risk of falls in the elderly, respectively, of 19 and 22 %. The effect could not be reached if the VD3 supplementation was <700 IU/day, or in the presence of blood levels of 25(OH)D3 <24 ng/mL [119]. In 2014, the American Geriatrics Society Workgroup on vitamin D supplementation for older adults concluded that a serum 25(OH)D3 concentration of at least 30 ng/mL should be considered as the minimum goal to achieve in older adults, especially in frail adults at higher risk of falls and fractures, recommending an average intake of vitamin D from all sources of 4000 IU/day, without any risk of intoxication. The workgroup also considered 1000 IU as the minimum daily supplement effective in reducing the risk of falls, along with a calcium supplementation at doses ranging from 500 to 1200 mg daily [120]. In contrast, others found that a very high dose of vitamin D may actually increase fractures, even though the reason for this evidence is unclear [121]. In fact, Sanders in 2010 showed that the administration of 500,000 IU per year of vitamin D increased the risk of falling (15 %), fractures (26 %), even when levels of 25(OH)D3 were >30 ng/mL [122]. These data could be explained by the fact that a high-dose vitamin D supplementation, especially among the elderly, could lead to increased physical activity and, as a consequence, higher risk of falls. Nevertheless, a recent randomized clinical trial has focused on the effectiveness of high-dose vitamin D in improving lower extremity function and lowering the risk of functional decline. The study cohort included 200 participants undergoing high-dose vitamin D monthly treatment and divided into three groups: a low-dose control group receiving 24,000 IU of VD3, a group receiving 60,000 IU of VD3, and a group receiving 24,000 IU of VD3 plus 300 mcg calcifediol. After a 12-month follow-up, it was found that the 60,000 group and 24,000 plus calcifediol group were not more effective in improving lower extremity function and had a higher risk of falls compared with the low-dose group (24,000 group), although they reached 25(OH)D3 levels of >30 ng/mL more consistently than the low-dose group [123]. Finally, cross-sectional studies indicate that levels of 25(OH)D3 between 30 and 40 ng/mL are associated with the greatest risk reduction for autoimmune and metabolic diseases, probably due to an adequate local paracrine production of 1,25(OH)2D3 in extra-renal tissues [124]. These evidence altogether suggest that 25(OH)D3 levels between 30 e 40 ng/mL could give additional benefits outside of the bone; to reach these levels in more than 97 % of the population, a supplementation of at least 2000 IU/day would be required, regardless of increased exposure to UVB [125].
Regarding high-dose vitamin D therapy in autoimmune diseases, Sotirchos et al. studied 40 patients with relapsing-remitting multiple sclerosis, randomized to receive 10,400 IU or 800 IU cholecalciferol daily for 6 months [126]. After 6 months, mean increase of 25(OH)D3 levels was higher in the high-dose group than in the low-dose group (34.9 ng/mL and 6.9 ng/ml, respectively) and only the high-dose group achieved 25(OH)D3 levels between 40 and 60 ng/mL, which was considered the optimal target for patients with MS [127]. In addition, only in the high-dose group, there was a reduction in the proportion of IL17+CD4+ T cells, considered a major contributor to the immunopathogenesis of MS, with a concomitant increase in the proportion of central memory CD4+ T cells and naïve CD4+ T cells, concluding that high-dose cholecalciferol therapy exhibits in vivo pleiotropic immunomodulatory effects in MS. Adverse events were minor and did not differ between the two groups, indicating that 10,400 IU cholecalciferol daily is a safe and well-tolerated therapy in patients with MS. Randomized controlled trials (RCT) are currently examining the effects of vitamin D supplementation on clinical and radiologic outcomes in MS, with dosages of 5000–10,000 IU per day [127, 128]. An open-label, 12 months, RCT of patients with MS treated with increasing doses of cholecalciferol (4000–40,000 IU/day) plus calcium (1200 mg/day), followed by maintenance with a lower intake (10,000 IU/day), showed that abnormal T cell reactions were suppressed in vivo by cholecalciferol at a serum 25(OH)D3 level above 100 nmol/L (i.e., >40 ng/mL) [129].
In their recent ancillary study of the vitamin D therapy in individuals at high risk of hypertension (DAYLIGHT), a randomized controlled trial where individuals with vitamin D deficiency and untreated pre- or early stage I hypertension had been randomized to receive either low-dose (400 IU/day) or high-dose (4000 IU/day) oral VD3 supplementation for 6 months, Konijeti et al. found that high-dose VD3 significantly reduced CD4+ T-cell activation compared to low-dose VD3. These data provide direct human evidence that vitamin D can influence cell-mediated immunity and high-dose VD3 supplementation could be more effective than low dose in immune-mediated disorders [130].
Interestingly, beneficial effects of high-dose VD3 therapy have also been described in autoimmune skin disorders. For instance, a Brazilian pilot study by Finamor et al. [131] on the efficacy and safety of prolonged high-dose VD3 therapy in patients with psoriasis and vitiligo suggested that an oral daily dose of 35,000 IU of vitamin D for 6 months, associated with preventive measures (low-calcium diet and a daily hydration of at least 2.5 L daily), is a safe and effective therapeutic strategy for reducing disease activity. All nine patients with psoriasis and 14 out of 16 with vitiligo recruited in the study received benefit from the treatment, showing a decreased disease activity with no side effects. Altogether these studies indicate that 25(OH)D3 blood levels below 100 ng/mL are safe. Nevertheless, there are groups of individuals in which high doses of vitamin D over a long period could be detrimental in terms of developing hypercalcemia and rapid deterioration of kidney function, such as patients with primitive hyperparathyroidism or those with chronic granulomatous diseases, which are prone to elevated extra-renal synthesis of 1,25(OH)2D3. Moreover, even healthy subjects with mutations in CYP24A1—the mythocondrial enzyme responsible for the deactivation of 1,25(OH)2D3—are susceptible to developing hypercalcemia, hypercalciuria, and kidney stones when exposed to high vitamin D doses. Therefore, to avoid toxicity, all patients eligible for a high-dose treatment should be previously screened for hypercalcemia and hypercalciuria, while serum and urinary calcium levels should be monitored during the vitamin D treatment itself. To date, it can be established that vitamin D dose up to 2000 IU/day, or more recently, 4000 IU/day [132] should be considered safe above the age of 9. This dose should be corrected according to non-pharmacological intake of the vitamin D. In other terms, if a dose of 2000 IU/day in elderly subjects with low solar exposure is considered safe, if not still insufficient, in other cases, the same dose might not be recommended, such as in young people with a regular body weight and frequent sun exposure. Thus, further studies are needed to establish optimal vitamin D dosing regimens in different groups of patients of different ages [133] and to better dissect the pathologies eligible for high-dose vitamin D therapies and the target serum levels for maximal clinical efficacy (Fig. 1).
Conclusions
The recent advances in vitamin D biology have broadened the interest of researchers and clinicians far beyond calcium-phosphorus metabolism. It is now clear that vitamin D plays a pivotal role in several extraskeletal tissues, such as skeletal muscle, immune cells, adipocytes, and pancreatic islets. The complex function of vitamin D in the modulation of immunity and inflammation confers a novel potential therapeutic role in several diseases, such as type 1 diabetes mellitus, multiple sclerosis, dermatological and thyroid autoimmune diseases, as well as in obesity and type 2 diabetes mellitus. The crucial issue of recommended doses, especially in these novel clinical settings, requires ad hoc interventional studies, given that most of the existing guidelines are only focused on the classical vitamin D target tissues (i.e., bone, kidney, and intestine). However, in extraskeletal target tissues, it seems necessary to consider higher vitamin D supplementation doses, compared to those suggested by most accredited scientific societies for skeletal effects, to gain full clinical benefit.
Abbreviations
- VD3 :
-
Vitamin D3
- 7-DHC:
-
7-Dehydrocholesterol
- 25(OH)D3 :
-
25-Hydroxyvitamin D3
- 1,25(OH)2D3 :
-
1,25-Dihydroxyvitamin D3
- DBP:
-
Vitamin D binding protein
- VDR:
-
Vitamin D receptor
- PTH:
-
Parathyroid hormone
- BMD:
-
Bone mineral density
References
Webb AR, Pilbeam C, Hanafin N, Holick MF (1990) An evaluation of the relative contributions of exposure to sunlight and of diet to the circulating concentrations of 25-hydroxyvitamin D in an elderly nursing home population in Boston. Am J Clin Nutr 51:1075–1081
MacLaughlin J, Holick MF (1985) Aging decreases the capacity of human skin to produce vitamin D3. J Clin Invest 76:1536–1538. doi:10.1172/JCI112134
Cheng JB, Levine MA, Bell NH, Mangelsdorf DJ, Russell DW (2004) Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase. Proc Natl Acad Sci USA 101:7711–7715. doi:10.1073/pnas.0402490101
Foresta C, Strapazzon G, De TL, Perilli L, Di MA, Muciaccia B et al (2011) Bone mineral density and testicular failure: evidence for a role of vitamin D 25-hydroxylase in human testis. J Clin Endocrinol Metab 96:E646–E652. doi:10.1210/jc.2010-1628
Henry HL (2011) Regulation of vitamin D metabolism. Best Pract Res Clin Endocrinol Metab 25:531–541. doi:10.1016/j.beem.2011.05.003
Jones G, Prosser DE, Kaufmann M (2014) Cytochrome P450-mediated metabolism of vitamin D. J Lipid Res 55:13–31. doi:10.1194/jlr.R031534
Razzaque MS, Sitara D, Taguchi T, St-Arnaud R, Lanske B (2006) Premature aging-like phenotype in fibroblast growth factor 23 null mice is a vitamin D-mediated process. FASEB J. 20:720–722. doi:10.1096/fj.05-5432fje
Murayama A, Takeyama K, Kitanaka S, Kodera Y, Hosoya T, Kato S (1998) The promoter of the human 25-hydroxyvitamin D3 1 alpha-hydroxylase gene confers positive and negative responsiveness to PTH, calcitonin, and 1 alpha,25(OH)2D3. Biochem Biophys Res Commun 249:11–16
Kato S, Fujiki R, Kim MS, Kitagawa H (2007) Ligand-induced transrepressive function of VDR requires a chromatin remodeling complex. WINAC J Steroid Biochem Mol Biol 103:372–380. doi:10.1016/j.jsbmb.2006.12.038
Fu GK, Lin D, Zhang MY, Bikle DD, Shackleton CH, Miller WL et al (1997) Cloning of human 25-hydroxyvitamin D-1 alpha-hydroxylase and mutations causing vitamin D-dependent rickets type 1. Mol Endocrinol 11:1961–1970. doi:10.1210/mend.11.13.0035
Overbergh L, Decallonne B, Valckx D, Verstuyf A, Depovere J, Laureys J et al (2000) Identification and immune regulation of 25-hydroxyvitamin D-1-alpha-hydroxylase in murine macrophages. Clin Exp Immunol 120:139–146
Bouillon R, Carmeliet G, Verlinden L, van EE, Luderer HF, Verstuyf A et al (2008) Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev 29:726–776. doi:10.1210/er.2008-0004
Merke J, Milde P, Lewicka S, Hugel U, Klaus G, Mangelsdorf DJ et al (1989) Identification and regulation of 1,25-dihydroxyvitamin D3 receptor activity and biosynthesis of 1,25-dihydroxyvitamin D3. Studies in cultured bovine aortic endothelial cells and human dermal capillaries. J Clin Invest 83:1903–1915. doi:10.1172/JCI114097
Caprio M, Mammi C, Rosano GM (2012) Vitamin D: a novel player in endothelial function and dysfunction. Arch Med Sci 8:4–5. doi:10.5114/aoms.2012.27271
Merke J, Hofmann W, Goldschmidt D, Ritz E (1987) Demonstration of 1,25(OH)2 vitamin D3 receptors and actions in vascular smooth muscle cells in vitro. Calcif Tissue Int 41:112–114
O’Connell TD, Berry JE, Jarvis AK, Somerman MJ, Simpson RU (1997) 1,25-Dihydroxyvitamin D3 regulation of cardiac myocyte proliferation and hypertrophy. Am J Physiol 272:H1751–H1758
Jensen SS, Madsen MW, Lukas J, Binderup L, Bartek J (2001) Inhibitory effects of 1alpha,25-dihydroxyvitamin D(3) on the G(1)-S phase-controlling machinery. Mol Endocrinol 15:1370–1380. doi:10.1210/mend.15.8.0673
Santoro D, Sebekova K, Teta D, De NL (2015) Extraskeletal Functions of Vitamin D. Biomed Res Int. doi:10.1155/2015/294719
Overbergh L, Stoffels K, Waer M, Verstuyf A, Bouillon R, Mathieu C (2006) Immune regulation of 25-hydroxyvitamin D-1alpha-hydroxylase in human monocytic THP1 cells: mechanisms of interferon-gamma-mediated induction. J Clin Endocrinol Metab 91:3566–3574. doi:10.1210/jc.2006-0678
Amado Diago CA (2016) Garcia-Unzueta MT, Farinas MD, Amado JA. Calcitriol-modulated human antibiotics: new pathophysiological aspects of vitamin D. Endocrinol Nutr 63:87–94. doi:10.1016/j.endonu.2015.09.005
Kankova M, Luini W, Pedrazzoni M, Riganti F, Sironi M, Bottazzi B et al (1991) Impairment of cytokine production in mice fed a vitamin D3-deficient diet. Immunology 73:466–471
Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR et al (2006) Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311:1770–1773. doi:10.1126/science.1123933
Lucisano S, Arena A, Stassi G, Iannello D, Montalto G, Romeo A et al (2015) Role of Paricalcitol in Modulating the Immune Response in Patients with Renal Disease. Int J Endocrinol 2015:765364. doi:10.1155/2015/765364
Bouillon R, Lieben L, Mathieu C, Verstuyf A, Carmeliet G (2013) Vitamin D action: lessons from VDR and Cyp27b1 null mice. Pediatr. Endocrinol. Rev. 10(Suppl 2):354–366
Armanini D, Andrisani A, Ambrosini G, Dona G, Camozzi V, Bordin L et al (2016) Interrelationship Between Vitamin D Insufficiency, Calcium Homeostasis, Hyperaldosteronism, and Autoimmunity. J Clin Hypertens (Greenwich). doi:10.1111/jch.12822
Chang SH, Chung Y, Dong C (2010) Vitamin D suppresses Th17 cytokine production by inducing C/EBP homologous protein (CHOP) expression. J Biol Chem 285:38751–38755. doi:10.1074/jbc.C110.185777
Herrada AA, Contreras FJ, Marini NP, Amador CA, Gonzalez PA, Cortes CM et al (2010) Aldosterone promotes autoimmune damage by enhancing Th17-mediated immunity. J Immunol 184:191–202. doi:10.4049/jimmunol.0802886
Marzolla V, Armani A, Feraco A, De Martino MU, Fabbri A, Rosano G et al (2014) Mineralocorticoid receptor in adipocytes and macrophages: a promising target to fight metabolic syndrome. Steroids 91:46–53. doi:10.1016/j.steroids.2014.05.001
Takiishi T, Van BT, Gysemans C, Mathieu C (2013) Effects of vitamin D on antigen-specific and non-antigen-specific immune modulation: relevance for type 1 diabetes. Pediatr Diabetes 14:81–89. doi:10.1111/j.1399-5448.2012.00923.x
Takiishi T, Ding L, Baeke F, Spagnuolo I, Sebastiani G, Laureys J et al (2014) Dietary supplementation with high doses of regular vitamin D3 safely reduces diabetes incidence in NOD mice when given early and long term. Diabetes 63:2026–2036. doi:10.2337/db13-1559
Treiber G, Prietl B, Frohlich-Reiterer E, Lechner E, Ribitsch A, Fritsch M et al (2015) Cholecalciferol supplementation improves suppressive capacity of regulatory T-cells in young patients with new-onset type 1 diabetes mellitus—a randomized clinical trial. Clin Immunol 161:217–224. doi:10.1016/j.clim.2015.08.002
Giulietti A, Gysemans C, Stoffels K, van EE, Decallonne B, Overbergh L et al (2004) Vitamin D deficiency in early life accelerates Type 1 diabetes in non-obese diabetic mice. Diabetologia 47:451–462. doi:10.1007/s00125-004-1329-3
Zella JB, DeLuca HF (2003) Vitamin D and autoimmune diabetes. J Cell Biochem 88:216–222. doi:10.1002/jcb.10347
Cooper JD, Smyth DJ, Walker NM, Stevens H, Burren OS, Wallace C et al (2011) Inherited variation in vitamin D genes is associated with predisposition to autoimmune disease type 1 diabetes. Diabetes 60:1624–1631. doi:10.2337/db10-1656
Guo SW, Magnuson VL, Schiller JJ, Wang X, Wu Y, Ghosh S (2006) Meta-analysis of vitamin D receptor polymorphisms and type 1 diabetes: a HuGE review of genetic association studies. Am J Epidemiol 164:711–724. doi:10.1093/aje/kwj278
Mathieu C, Gysemans C, Giulietti A, Bouillon R (2005) Vitamin D and diabetes. Diabetologia 48:1247–1257. doi:10.1007/s00125-005-1802-7
Karvonen M, Jantti V, Muntoni S, Stabilini M, Stabilini L, Muntoni S et al (1998) Comparison of the seasonal pattern in the clinical onset of IDDM in Finland and Sardinia. Diabetes Care 21:1101–1109
Gabbay MA, Sato MN, Finazzo C, Duarte AJ, Dib SA (2012) Effect of cholecalciferol as adjunctive therapy with insulin on protective immunologic profile and decline of residual beta-cell function in new-onset type 1 diabetes mellitus. Arch Pediatr Adolesc Med 166:601–607. doi:10.1001/archpediatrics.2012.164
Baidal DA, Ricordi C, Garcia-Contreras M, Sonnino A, Fabbri A (2016) Combination high-dose omega-3 fatty acids and high-dose cholecalciferol in new onset type 1 diabetes: a potential role in preservation of beta-cell mass. Eur Rev Med Pharmacol Sci 20:3313–3318
Mokry LE, Ross S, Ahmad OS, Forgetta V, Smith GD, Leong A et al (2015) Vitamin D and risk of multiple sclerosis: a mendelian randomization study. PLoS Med 12:e1001866 doi:10.1371/journal.pmed.1001866
Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A (2006) Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 296:2832–2838. doi:10.1001/jama.296.23.2832
Soilu-Hanninen M, Aivo J, Lindstrom BM, Elovaara I, Sumelahti ML, Farkkila M et al (2012) A randomised, double blind, placebo controlled trial with vitamin D3 as an add on treatment to interferon beta-1b in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 83:565–571. doi:10.1136/jnnp-2011-301876
Muscogiuri G, Tirabassi G, Bizzaro G, Orio F, Paschou SA, Vryonidou A et al (2015) Vitamin D and thyroid disease: to D or not to D? Eur J Clin Nutr 69:291–296. doi:10.1038/ejcn.2014.265
Vondra K, Starka L, Hampl R (2015) Vitamin D and thyroid diseases. Physiol Res 64(Suppl 2):S95–S100
Unal AD, Tarcin O, Parildar H, Cigerli O, Eroglu H, Demirag NG (2014) Vitamin D deficiency is related to thyroid antibodies in autoimmune thyroiditis. Cent Eur J Immunol 39:493–497. doi:10.5114/ceji.2014.47735
Evliyaoglu O, Acar M, Ozcabi B, Erginoz E, Bucak F, Ercan O et al (2015) Vitamin D deficiency and hashimoto’s thyroiditis in children and adolescents: a critical vitamin D level for this association? J Clin Res Pediatr Endocrinol 7:128–133. doi:10.4274/jcrpe.2011
Mazokopakis EE, Papadomanolaki MG, Tsekouras KC, Evangelopoulos AD, Kotsiris DA, Tzortzinis AA (2015) Is vitamin D related to pathogenesis and treatment of Hashimoto’s thyroiditis? Hell J Nucl Med 18:222–227
Raman M, Milestone AN, Walters JR, Hart AL, Ghosh S (2011) Vitamin D and gastrointestinal diseases: inflammatory bowel disease and colorectal cancer. Therap Adv Gastroenterol 4:49–62. doi:10.1177/1756283X10377820
Hypponen E, Boucher BJ, Berry DJ, Power C (2008) 25-hydroxyvitamin D, IGF-1, and metabolic syndrome at 45 years of age: a cross-sectional study in the 1958 British Birth Cohort. Diabetes 57:298–305. doi:10.2337/db07-1122
Stokic E, Kupusinac A, Tomic-Naglic D, Smiljenic D, Kovacev-Zavisic B, Srdic-Galic B et al (2015) Vitamin D and dysfunctional adipose tissue in obesity. Angiology 66:613–618. doi:10.1177/0003319714543512
Bellia A, Garcovich C, D’Adamo M, Lombardo M, Tesauro M, Donadel G et al (2013) Serum 25-hydroxyvitamin D levels are inversely associated with systemic inflammation in severe obese subjects. Intern Emerg Med 8:33–40. doi:10.1007/s11739-011-0559-x
Marcotorchino J, Tourniaire F, Astier J, Karkeni E, Canault M, Amiot MJ et al (2014) Vitamin D protects against diet-induced obesity by enhancing fatty acid oxidation. J Nutr Biochem 25:1077–1083. doi:10.1016/j.jnutbio.2014.05.010
Sergeev IN, Song Q (2014) High vitamin D and calcium intakes reduce diet-induced obesity in mice by increasing adipose tissue apoptosis. Mol Nutr Food Res 58:1342–1348. doi:10.1002/mnfr.201300503
Vimaleswaran KS, Berry DJ, Lu C, Tikkanen E, Pilz S, Hiraki LT et al (2013) Causal relationship between obesity and vitamin D status: bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med 10:e1001383 doi:10.1371/journal.pmed.1001383
Davidson MB, Duran P, Lee ML, Friedman TC (2013) High-dose vitamin D supplementation in people with prediabetes and hypovitaminosis D. Diabetes Care 36:260–266. doi:10.2337/dc12-1204
Yin X, Yan L, Lu Y, Jiang Q, Pu Y, Sun Q (2016) Correction of hypovitaminosis D does not improve the metabolic syndrome risk profile in a Chinese population: a randomized controlled trial for 1 year. Asia Pac J Clin Nutr 25:71–77. doi:10.6133/apjcn.2016.25.1.06
Wood AD, Secombes KR, Thies F, Aucott L, Black AJ, Mavroeidi A et al (2012) Vitamin D3 supplementation has no effect on conventional cardiovascular risk factors: a parallel-group, double-blind, placebo-controlled RCT. J Clin Endocrinol Metab 97:3557–3568. doi:10.1210/jc.2012-2126
Mammi C, Calanchini M, Antelmi A, Feraco A, Gnessi L, Falcone S, Quintarelli F, Rosano GM, Fabbri A, Caprio M (2013) Bisphosphonates and adipogenesis: evidence for alendronate inhibition of adipocyte differentiation in 3T3-L1 preadipocytes through a vitamin D receptor mediated effect. Nat Sci 5(8) doi:10.4236/ns.2013.58116
Armani A, Mammi C, Marzolla V, Calanchini M, Antelmi A, Rosano GM et al (2010) Cellular models for understanding adipogenesis, adipose dysfunction, and obesity. J Cell Biochem 110:564–572. doi:10.1002/jcb.22598
Milner RD, Hales CN (1967) The role of calcium and magnesium in insulin secretion from rabbit pancreas studied in vitro. Diabetologia 3:47–49
Sergeev IN, Rhoten WB (1995) 1,25-Dihydroxyvitamin D3 evokes oscillations of intracellular calcium in a pancreatic beta-cell line. Endocrinology 136:2852–2861. doi:10.1210/endo.136.7.7789310
Johnson JA, Grande JP, Roche PC, Kumar R (1994) Immunohistochemical localization of the 1,25(OH)2D3 receptor and calbindin D28 k in human and rat pancreas. Am J Physiol 267:E356–E360
Zeitz U, Weber K, Soegiarto DW, Wolf E, Balling R, Erben RG (2003) Impaired insulin secretory capacity in mice lacking a functional vitamin D receptor. FASEB J. 17:509–511. doi:10.1096/fj.02-0424fje
Maestro B, Davila N, Carranza MC, Calle C (2003) Identification of a Vitamin D response element in the human insulin receptor gene promoter. J Steroid Biochem Mol Biol 84:223–230
Dunlop TW, Vaisanen S, Frank C, Molnar F, Sinkkonen L, Carlberg C (2005) The human peroxisome proliferator-activated receptor delta gene is a primary target of 1alpha,25-dihydroxyvitamin D3 and its nuclear receptor. J Mol Biol 349:248–260. doi:10.1016/j.jmb.2005.03.060
Wright DC, Hucker KA, Holloszy JO, Han DH (2004) Ca2+ and AMPK both mediate stimulation of glucose transport by muscle contractions. Diabetes 53:330–335
Gilsanz V, Kremer A, Mo AO, Wren TA, Kremer R (2010) Vitamin D status and its relation to muscle mass and muscle fat in young women. J Clin Endocrinol Metab 95:1595–1600. doi:10.1210/jc.2009-2309
Chiu KC, Chuang LM, Lee NP, Ryu JM, McGullam JL, Tsai GP et al (2000) Insulin sensitivity is inversely correlated with plasma intact parathyroid hormone level. Metabolism 49:1501–1505. doi:10.1053/meta.2000.17708
Pittas AG, Lau J, Hu FB, Dawson-Hughes B (2007) The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab 92:2017–2029. doi:10.1210/jc.2007-0298
George PS, Pearson ER, Witham MD (2012) Effect of vitamin D supplementation on glycaemic control and insulin resistance: a systematic review and meta-analysis. Diabet Med 29:e142–e150. doi:10.1111/j.1464-5491.2012.03672.x
Chandler PD, Giovannucci EL, Scott JB, Bennett GG, Ng K, Chan AT et al (2015) Effects of vitamin D supplementation on C-peptide and 25-hydroxyvitamin D concentrations at 3 and 6 months. Sci. Rep 5:10411. doi:10.1038/srep10411
Calvo-Romero JM, Ramiro-Lozano JM (2016) Metabolic effects of supplementation with vitamin D in type 2 diabetic patients with vitamin D deficiency. Diabetes Metab Syndr 10:72–74. doi:10.1016/j.dsx.2015.09.008
Forouhi NG, Luan J, Cooper A, Boucher BJ, Wareham NJ (2008) Baseline serum 25-hydroxy vitamin d is predictive of future glycemic status and insulin resistance: the Medical Research Council Ely Prospective Study 1990–2000. Diabetes 57:2619–2625. doi:10.2337/db08-0593
Salekzamani S, Mehralizadeh H, Ghezel A, Salekzamani Y, Jafarabadi MA, Bavil AS et al (2016) Effect of high-dose vitamin D supplementation on cardiometabolic risk factors in subjects with metabolic syndrome: a randomized controlled double-blind clinical trial. J Endocrinol Invest. doi:10.1007/s40618-016-0507-8
Jafari T, Fallah AA, Barani A (2016) Effects of vitamin D on serum lipid profile in patients with type 2 diabetes: a meta-analysis of randomized controlled trials. Clin Nutr. doi:10.1016/j.clnu.2016.03.001
Boland R (1986) Role of vitamin D in skeletal muscle function. Endocr Rev 7:434–448. doi:10.1210/edrv-7-4-434
Houston DK, Cesari M, Ferrucci L, Cherubini A, Maggio D, Bartali B et al (2007) Association between vitamin D status and physical performance: the InCHIANTI study. J Gerontol A Biol Sci Med Sci 62:440–446
Yoshikawa S, Nakamura T, Tanabe H, Imamura T (1979) Osteomalacic myopathy. Endocrinol. Jpn. 26:65–72
Bouillon R, Van Schoor NM, Gielen E, Boonen S, Mathieu C, Vanderschueren D et al (2013) Optimal vitamin D status: a critical analysis on the basis of evidence-based medicine. J Clin Endocrinol Metab 98:E1283–E1304. doi:10.1210/jc.2013-1195
Bischoff-Ferrari HA, Dawson-Hughes B, Baron JA, Burckhardt P, Li R, Spiegelman D et al (2007) Calcium intake and hip fracture risk in men and women: a meta-analysis of prospective cohort studies and randomized controlled trials. Am J Clin Nutr 86:1780–1790
Bischoff-Ferrari HA (2012) Relevance of vitamin D in muscle health. Rev Endocr Metab Disord 13:71–77. doi:10.1007/s11154-011-9200-6
Fornari R, Francomano D, Greco EA, Marocco C, Lubrano C, Wannenes F et al (2015) Lean mass in obese adult subjects correlates with higher levels of vitamin D, insulin sensitivity and lower inflammation. J Endocrinol Invest 38:367–372. doi:10.1007/s40618-014-0189-z
Rondanelli M, Klersy C, Terracol G, Talluri J, Maugeri R, Guido D et al (2016) Whey protein, amino acids, and vitamin D supplementation with physical activity increases fat-free mass and strength, functionality, and quality of life and decreases inflammation in sarcopenic elderly. Am J Clin Nutr 103:830–840. doi:10.3945/ajcn.115.113357
Stockton KA, Mengersen K, Paratz JD, Kandiah D, Bennell KL (2011) Effect of vitamin D supplementation on muscle strength: a systematic review and meta-analysis. Osteoporos Int 22:859–871. doi:10.1007/s00198-010-1407-y
Wang Y, DeLuca HF (2011) Is the vitamin d receptor found in muscle? Endocrinology 152:354–363. doi:10.1210/en.2010-1109
Chen S, Law CS, Grigsby CL, Olsen K, Hong TT, Zhang Y et al (2011) Cardiomyocyte-specific deletion of the vitamin D receptor gene results in cardiac hypertrophy. Circulation 124:1838–1847. doi:10.1161/CIRCULATIONAHA.111.032680
Endo I, Inoue D, Mitsui T, Umaki Y, Akaike M, Yoshizawa T et al (2003) Deletion of vitamin D receptor gene in mice results in abnormal skeletal muscle development with deregulated expression of myoregulatory transcription factors. Endocrinology 144:5138–5144. doi:10.1210/en.2003-0502
Szulc P, Schoppet M, Goettsch C, Rauner M, Dschietzig T, Chapurlat R et al (2012) Endocrine and clinical correlates of myostatin serum concentration in men–the STRAMBO study. J Clin Endocrinol Metab 97:3700–3708. doi:10.1210/jc.2012-1273
Pike JW (2016) Closing in on vitamin D action in skeletal muscle: early activity in muscle stem cells? Endocrinology 157:48–51. doi:10.1210/en.2015-2009
Teng K (2011) Premenopausal osteoporosis, an overlooked consequence of anorexia nervosa. Cleve Clin J Med 78:50–58. doi:10.3949/ccjm.78a.10023
Umhau JC, George DT, Heaney RP, Lewis MD, Ursano RJ, Heilig M et al (2013) Low vitamin D status and suicide: a case-control study of active duty military service members. PLoS One 8:e51543 doi:10.1371/journal.pone.0051543
Favaro A, Ferrara S, Santonastaso P (2007) Self-injurious behavior in a community sample of young women: relationship with childhood abuse and other types of self-damaging behaviors. J Clin Psychiatry 68:122–131
Divasta AD, Feldman HA, Brown JN, Giancaterino C, Holick MF, Gordon CM (2011) Bioavailability of vitamin D in malnourished adolescents with anorexia nervosa. J Clin Endocrinol Metab 96:2575–2580. doi:10.1210/jc.2011-0243
Turner JM, Bulsara MK, McDermott BM, Byrne GC, Prince RL, Forbes DA (2001) Predictors of low bone density in young adolescent females with anorexia nervosa and other dieting disorders. Int J Eat Disord 30:245–251
Trombetti A, Richert L, Herrmann FR, Chevalley T, Graf JD, Rizzoli R (2013) Selective determinants of low bone mineral mass in adult women with anorexia nervosa. Int J Endocrinol 2013:897193. doi:10.1155/2013/897193
Veronese N, Solmi M, Rizza W, Manzato E, Sergi G, Santonastaso P et al (2015) Vitamin D status in anorexia nervosa: a meta-analysis. Int J Eat Disord 48:803–813. doi:10.1002/eat.22370
Giel KE, Kullmann S, Preissl H, Bischoff SC, Thiel A, Schmidt U et al (2013) Understanding the reward system functioning in anorexia nervosa: crucial role of physical activity. Biol Psychol 94:575–581. doi:10.1016/j.biopsycho.2013.10.004
Haagensen AL, Feldman HA, Ringelheim J, Gordon CM (2008) Low prevalence of vitamin D deficiency among adolescents with anorexia nervosa. Osteoporos Int 19:289–294. doi:10.1007/s00198-007-0476-z
Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF (2000) Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 72:690–693
Schebendach JE, Porter KJ, Wolper C, Walsh BT, Mayer LE (2012) Accuracy of self-reported energy intake in weight-restored patients with anorexia nervosa compared with obese and normal weight individuals. Int J Eat Disord 45:570–574. doi:10.1002/eat.20973
De RM, Toffanello ED, Veronese N, Zambon S, Bolzetta F, Sartori L et al (2014) Vitamin D deficiency and leisure time activities in the elderly: are all pastimes the same? PLoS One 9:e94805 doi:10.1371/journal.pone.0094805
Goss K, Allan S (2009) Shame, pride and eating disorders. Clin Psychol Psychother 16:303–316. doi:10.1016/j.clnu.2016.03.001
Helou M, Ning Y, Yang S, Irvine P, Bachmann LM, Godder K et al (2014) Vitamin d deficiency in children with cancer. J Pediatr Hematol Oncol 36:212–217. doi:10.1097/MPH.0b013e31829f3754
Gatti D, El GM, Viapiana O, Ruocco A, Chignola E, Rossini M et al (2015) Strong relationship between vitamin D status and bone mineral density in anorexia nervosa. Bone 78:212–215. doi:10.1016/j.bone.2015.05.014
Tasegian A, Curcio F, Dalla RL, Rossetti F, Cataldi S, Codini M et al (2016) Hypovitaminosis D3, leukopenia, and human serotonin transporter polymorphism in anorexia nervosa and bulimia nervosa. Mediators Inflamm 2016:8046479. doi:10.1155/2016/8046479
Amaya-Mejia AS, O’Farrill-Romanillos PM, Galindo-Pacheco LV, Vargas-Ortega G, Mendoza-Zubieta V, Del Rivero-Hernandez LG et al (2013) Vitamin D deficiency in patients with common variable immunodeficiency, with autoimmune diseases and bronchiectasis. Rev Alerg Mex 60:110–116
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP et al (2011) Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 96:1911–1930. doi:10.1210/jc.2011-0385
Adami S, Romagnoli E, Carnevale V, Scillitani A, Giusti A, Rossini M et.al (2011) [Guidelines on prevention and treatment of vitamin D deficiency. Italian Society for Osteoporosis, Mineral Metabolism and Bone Diseases (SIOMMMS)]. Reumatismo 63:129–147 doi:10.4081/reumatismo.2011.129
Mitri J, Pittas AG (2014) Vitamin D and diabetes. Endocrinol Metab Clin North Am 43:205–232. doi:10.1016/j.ecl.2013.09.010
Institute of Medicine Dietary. Dietary Reference Intakes for Calcium and Vitamin D. 2011. (GENERIC)
Rosen CJ, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA et al (2012) IOM committee members respond to Endocrine Society vitamin D guideline. J Clin Endocrinol Metab 97:1146–1152. doi:10.1210/jc.2011-2218
Rosen CJ, Adams JS, Bikle DD, Black DM, Demay MB, Manson JE et al (2012) The nonskeletal effects of vitamin D: an Endocrine Society scientific statement. Endocr Rev 33:456–492. doi:10.1210/er.2012-1000
Alvarez JA, Law J, Coakley KE, Zughaier SM, Hao L, Shahid SK et al (2012) High-dose cholecalciferol reduces parathyroid hormone in patients with early chronic kidney disease: a pilot, randomized, double-blind, placebo-controlled trial. Am J Clin Nutr 96:672–679. doi:10.3945/ajcn.112.040642
Kearns MD, Alvarez JA, Seidel N, Tangpricha V (2015) Impact of vitamin D on infectious disease. Am J Med Sci 349:245–262. doi:10.1097/MAJ.0000000000000360
Masood MQ, Khan A, Awan S, Dar F, Naz S, Naureen G et al (2015) Comparison of vitamin D replacement strategies with high-dose intramuscular or oral cholecalciferol: a prospective intervention study. Endocr Pract 21:1125–1133. doi:10.4158/EP15680.OR
Bacchetta J, Zaritsky JJ, Sea JL, Chun RF, Lisse TS, Zavala K et al (2014) Suppression of iron-regulatory hepcidin by vitamin D. J Am Soc Nephrol 25:564–572. doi:10.1681/ASN.2013040355
Adams JS, Ren S, Liu PT, Chun RF, Lagishetty V, Gombart AF et al (2009) Vitamin d-directed rheostatic regulation of monocyte antibacterial responses. J. Immunol. 182:4289–4295. doi:10.4049/jimmunol.0803736
Murad MH, Elamin KB, Abu Elnour NO, Elamin MB, Alkatib AA, Fatourechi MM et al (2011) Clinical review: the effect of vitamin D on falls: a systematic review and meta-analysis. J Clin Endocrinol Metab 96:2997–3006. doi:10.1210/jc.2011-1193
Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, Orav JE, Stuck AE, Theiler R et al (2009) Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ 339:b3692. doi:10.1136/bmj.b3692
American Geriatrics Society (2014) Recommendations abstracted from the American Geriatrics Society Consensus Statement on vitamin D for Prevention of Falls and Their Consequences. J Am Geriatr Soc 62:147–152. doi:10.1111/jgs.12631
Kearns MD, Alvarez JA, Tangpricha V (2014) Large, single-dose, oral vitamin d supplementation in adult populations: a systematic review. Endocr Pract 20:341–351. doi:10.4158/EP13265.RA
Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Young D et al (2010) Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA 303:1815–1822. doi:10.1001/jama.2010.594
Bischoff-Ferrari HA, Dawson-Hughes B, Orav EJ, Staehelin HB, Meyer OW, Theiler R et al (2016) Monthly high-dose vitamin D treatment for the prevention of functional decline: a randomized clinical trial. JAMA Intern Med 176:175–183. doi:10.1001/jamainternmed.2015.7148
Holick MF (2007) Vitamin D deficiency. N Engl J Med 357:266–281. doi:10.1056/NEJMra070553
Heaney RP (2007) The case for improving vitamin D status. J Steroid Biochem Mol Biol 103:635–641. doi:10.1016/j.jsbmb.2006.12.006
Sotirchos ES, Bhargava P, Eckstein C, Van HK, Baynes M, Ntranos A et al (2016) Safety and immunologic effects of high- vs low-dose cholecalciferol in multiple sclerosis. Neurology 86:382–390. doi:10.1212/WNL.0000000000002316
Bhargava P, Cassard S, Steele SU, Azevedo C, Pelletier D, Sugar EA et al (2014) The vitamin D to ameliorate multiple sclerosis (VIDAMS) trial: study design for a multicenter, randomized, double-blind controlled trial of vitamin D in multiple sclerosis. Contemp Clin Trials 39:288–293. doi:10.1016/j.cct.2014.10.004
Dorr J, Ohlraun S, Skarabis H, Paul F (2012) Efficacy of vitamin D supplementation in multiple sclerosis (EVIDIMS Trial): study protocol for a randomized controlled trial. Trials 13:15. doi:10.1186/1745-6215-13-15
Kimball S, Vieth R, Dosch HM, Bar-Or A, Cheung R, Gagne D et al (2011) Cholecalciferol plus calcium suppresses abnormal PBMC reactivity in patients with multiple sclerosis. J Clin Endocrinol Metab 96:2826–2834. doi:10.1210/jc.2011-0325
Konijeti GG, Arora P, Boylan MR, Song Y, Huang S, Harrell F et al (2016) Vitamin D Supplementation Modulates T Cell-Mediated Immunity in Humans: results from a Randomized Control Trial. J Clin Endocrinol Metab 101:533–538. doi:10.1210/jc.2015-3599
Finamor DC, Sinigaglia-Coimbra R, Neves LC, Gutierrez M, Silva JJ, Torres LD et al (2013) A pilot study assessing the effect of prolonged administration of high daily doses of vitamin D on the clinical course of vitiligo and psoriasis. Dermatoendocrinology 5:222–234. doi:10.4161/derm.24808
Rosen CJ (2011) Clinical practice. Vitamin D insufficiency. N Engl J Med 364:248–254. doi:10.1056/NEJMcp1009570
Pepper KJ, Judd SE, Nanes MS, Tangpricha V (2009) Evaluation of vitamin D repletion regimens to correct vitamin D status in adults. Endocr Pract 15:95–103. doi:10.4158/EP.15.2.95
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
For this type of study Informed consent is not required.
Rights and permissions
About this article
Cite this article
Caprio, M., Infante, M., Calanchini, M. et al. Vitamin D: not just the bone. Evidence for beneficial pleiotropic extraskeletal effects. Eat Weight Disord 22, 27–41 (2017). https://doi.org/10.1007/s40519-016-0312-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40519-016-0312-6