Abstract
Background
Insulin resistance (IR) is not limited to obese individuals. Normal weight individuals may also be insulin resistant. The aim of this study was to determine the association of lifestyle and diet patterns with IR in normal weight Iranian men.
Methods
This cross-sectional study was conducted in 232 men with a body mass index lower than 25 kg/m2 (aged 20–72 years old) between September 2010 and April 2011 in Qazvin, Iran. Metabolically obese normal weight (MONW) was defined as IR using the homeostatic model assessment (HOMA). The optimal cut point to diagnose IR was the 80th percentile of HOMA-IR values in normal subjects. The HOMA-IR cut point was 2.48. Dietary pattern was assessed by a semi-quantitative food frequency questionnaire. Data were analyzed using backward logistic regression and ANCOVA.
Results
Fat and meat consumption and energy intake in subjects with MONW were more than subjects without MONW. Each serving of meat consumption was associated with three times increased risk of MONW (OR: 3.06), while each serving of dairy consumption was associated with 56 % lower risk of MONW with borderline significance (OR: 0.64). Adjusted mean of HOMA-IR in the first tertile of dairy consumption was significantly higher than other tertiles. Adjusted HOMA-IR value in the third tertile of meat consumption was significantly higher than the second tertile.
Conclusion
Higher meat consumption was associated with MONW in men. Higher meat consumption and lower dairy consumption were associated with higher means of HOMA-IR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insulin resistance (IR) plays a key role in the pathophysiology of type 2 diabetes and cardiovascular disease. Both genetic and lifestyle factors contribute to complex etiology of IR [1]. Obesity is the most important and the most common cause of IR. Body mass index (BMI), waist circumference (WC), hip circumference, and/or skinfold caliper are of non-invasive anthropometric measures of obesity. The most widely used classification of obesity is expressed in terms of BMI.
IR is not limited to obese individuals. Normal weight individuals may also be insulin resistant [2], while an individual may exhibit an obese phenotype in the absence of any metabolic abnormalities [3]. In the 1980s, Ruderman et al. introduced the term “metabolically obese but normal weight” (MONW) for individuals who are not obese or even overweight by standard weight tables, but who have metabolic abnormalities that are associated with obesity [4]. In addition to metabolic derangement, various complications including increased cardiovascular events and shortened 10 years survival have been reported in these subjects [5, 6].
BMI is not sufficient as anthropometric evaluation to define body composition and may be inaccurate, because BMI does not measure body fat directly and poorly distinguishes between fat mass and lean or bone mass. Direct measurement of body fat percentage would be a better tool for diagnosis of obesity [7]. Increased adipose tissue, especially visceral adipose tissue, leads to an increase in free fatty acids and inflammatory mediators that may result in post-receptor IR and its related complications [8].
Several studies have been performed to clarify contributing factors to IR other than BMI. Physical activity [9], sleep quality, sleep quantity [10], and diet patterns [5, 11] were significantly associated with the MONW phenotype in the previous studies. Protective effect of Mediterranean diet against metabolic obesity has also been reported [11].
Association of meat consumption in men with IR has been reported in some studies, but participants were not restricted to normal weight subjects, so other factors related to overweight or obesity could be interfered with the results [12, 13]. The aim of this study was to determine the association of lifestyle and diet patterns, especially meat consumption with IR in normal weight Iranian men.
Methods
This cross-sectional study was conducted in men with normal body mass index (BMI) that was identified in the Qazvin Metabolic Diseases Study (QMDS), Iran. The study protocol was approved by the ethics committee of Qazvin University of Medical Sciences (code: 28/20/11141).
In the QMDS, 1107 subjects aged ≥20 years were selected by multistage cluster random sampling method in Minoodar district of Qazvin, from September 2010 to April 2011. All households had health profiles and contact information at the Minoodar health center. Subjects were invited by telephone to the study and after face to face explanation of the study details they were free to participate. All subjects gave their written informed consent. Social and demographic information was self-reported. Two practitioners recorded their medical history, family history, drug history, and physical examination, using an organized questionnaire. Weight, height, and WC were measured after 12–14 h overnight fasting. The WC was measured midway between the costal margin and the iliac crest at the end of normal expiration. BMI was calculated as weight (kg) divided by the squared height (m). After a 15 min of rest, blood pressure (BP) was measured three times in a seated position and on a single occasion using a mercury sphygmomanometer. A venous blood sample was taken after 12–14 h overnight fasting to measure levels of glucose, insulin, triglycerides (TGs), total cholesterol, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C). Complete details of the QMDS have been reported elsewhere [14].
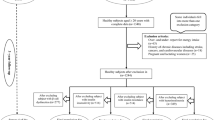
Subjects with a BMI >25 and/or subjects with diabetes were excluded from the present study. The flow chart on the selection of subjects is shown in Fig. 1. MONW was defined as IR using the homeostatic model assessment (HOMA). HOMA-IR was calculated as fasting serum insulin (μIU/ml) × fasting plasma glucose (mg/dL)/405 [15]. The optimal cut point of HOMA to diagnose IR was the 80th percentile of HOMA-IR values in normal subjects [16, 17]. Normal subjects were defined according to the following criteria: BMI ≤25 kg/m2; systolic blood pressure <130 mmHg; diastolic blood pressure <85 mmHg; total cholesterol ≤200 mg/dL; HDL-C ≥40 mg/dL in males, ≥50 mg/dL in females; fasting plasma glucose (FPG) <100 mg/dL; and TGs <150 mg/dL. Therefore, the HOMA cut point for IR was 2.48 and subjects with HOMA-IR above 2.48 were considered as MONW. Metabolic syndrome was defined based on the diagnostic criteria proposed by national cholesterol education program third Adult treatment panel (ATPIII 2004) [18].
Dietary pattern was assessed by a 168-item semi-quantitative food frequency questionnaire (FFQ) in Persian, in which its validity and reliability have been confirmed previously [19]. A list of foods has been developed in this questionnaire with a standard serving size. Food consumption frequency was self-reported on a daily, weekly or monthly basis. Physical activity was assessed by the Global Physical Activity Questionnaire (GPAQ) [20]. The World Health Organization has developed this questionnaire that consists of 16 questions about physical activity in a typical week. The GPAQ assesses three domains of physical activity including work, transportation, and recreational activities. The total physical activity (TPA) was calculated as the sum of the metabolic equivalents (MET) minutes of activity computed for three domains. Self-reported sleep duration was assessed by questions about bedtime and wake time.
Statistical analysis
T test and Mann–Whitney U test were used for analysis of continuous variables where appropriate. The relationship between biochemical and anthropometric parameters was assessed by Pearson’s correlation coefficients. Linear regression analysis was performed to examine the association of meat and dairy consumption, anthropometric parameters, and metabolic syndrome components (except FPG) with HOMA-IR. Backward logistic regression test was performed to investigate the association between MONW and each risk factor including factors with P value less than 0.2 by univariate analysis and known predictors of IR (such as age and physical activity). An ANCOVA test was used to adjust HOMA-IR for the effect of age and BMI. P values less than 0.05 were considered statistically significant.
Results
The study was conducted in 232 men with a BMI lower than 25 kg/m2 aged 20–72 years old. The mean age of participants was 38.4 ± 11.9 years. Seventy-eight subjects were insulin resistant and 19 had metabolic syndrome. The clinical and biochemical characteristics, physical activity level, sleep duration and dietary patterns of the study subjects are reported in Table 1. In subjects with MONW, fat and meat consumption and energy intake were more than subjects without MONW, while the physical activity and sleep duration were not significantly different between two groups.
The relationship between biochemical and anthropometric parameters was assessed by Pearson’s correlation coefficients. BMI had positive and significant correlation with weight, WC, and TGs, while it had negative and significant correlation with height and HDL-C. There was no significant correlation between FPG and other parameters. There was negative and significant correlation between HDL-C and other parameters except height and FPG.
Linear regression analysis was performed for association of meat and dairy consumption, anthropometric parameters, and metabolic syndrome components with HOMA-IR. Only dairy consumption was significantly associated with HOMA-IR after age adjustment (β = −0.192, P: 0.048) and meat consumption was not associated with HOMA-IR. In logistic regression analysis, WC was associated with a higher risk of MONW (Table 2). Daily meat consumption was associated with three times increased risk of MONW, while daily dairy consumption was associated with 56 % lower risk of MONW with borderline significance. The association of energy intake and fat with MONW was not confirmed in multivariate analysis.
Age and BMI-adjusted means of HOMA-IR for different tertiles of dairy products and meat consumption are reported in Table 3. Adjusted mean of HOMA-IR in the second and third tertiles of dairy consumption was significantly lower than the first tertile. Adjusted mean of HOMA-IR in the third tertile of meat consumption was higher than other tertiles; however, the difference was only statistically significant in comparison with the second tertile.
Discussion
In the present study, fat and meat consumption in subjects with MONW was more than other subjects. Meat consumption was the independent predictor of insulin resistance. The more the meat intake, the higher is the mean HOMA-IR value while the more the dairy consumption, the less is the mean HOMA-IR value.
Several studies have been investigated the association of meat consumption and IR. In Snowdon et al. study, meat consumption was associated with higher risk of diabetes within the male population as an underlying or contributing cause of death during 21 years of follow-up [21]. Van Dam et al. identified that frequent consumption of processed meat (≥5/week) was associated with 1.46 times higher risk for type two diabetes during 12 years of follow-up. In a cross-sectional study among Brazilian middle-aged men, the subjects with the highest tertile of red meat consumption had higher prevalence of central obesity and metabolic syndrome and higher values of HOMA-IR [12]. A meta-analysis on three cohorts of US adults has revealed that red meat consumption, especially processed red meat, is associated with an increased risk of type 2 diabetes [22].
Several mechanisms may explain the association of meat consumption and insulin resistance. Meat consumption is associated with a significant higher plasma concentration of free iron that may lead to increased oxidative stress and subsequent increase in inflammatory mediators [23]. Meat contains a high level of advanced glycation end products that form during the cooking process of foods. Advanced glycation end products have significant pro-inflammatory actions [24, 25]. Increased inflammatory mediators can increase IR [26].
In the present study, higher consumption of dairy products was associated with improved insulin sensitivity. In most studies, the inverse association between dairy consumption and IR has been reported [27–29]. Fumeron et al. reported that a higher consumption of dairy products was associated with a lower 9-year incidence of metabolic syndrome and type 2 diabetes [27]. In Lutsey et al. study, the last quintile of dairy consumption was associated with lower incidence of metabolic syndrome compared to the first quintile [28]. Elwood et al. in a meta-analysis of four prospective cohort studies reported that the relative risk of diabetes in the subjects with the highest consumption of milk or dairy products was 0.92 [29]. In the same study, the meta-analysis of 15 prospective cohort studies revealed that the relative risk of cardiovascular disease and cerebrovascular accident in subjects with high milk consumption was 0.79 and 0.84, respectively [29]. However, the results of different studies are not consistent. In Lawlor et al. study, avoiding milk was associated with lower HOMA-IR values and a reduced risk of IR and the metabolic syndrome in women [30]. These differences may be attributable to gender, age or ethnicity.
Several pathophysiologic reasons may explain the increased insulin sensitivity with higher dairy consumption. Dairy products contain high levels of calcium and magnesium. Reduced adiposity and increased thermogenesis due to high calcium diets have been reported previously [31]. In Melanson et al. study, higher calcium intake was associated with higher rates of fat oxidation [32]. High magnesium diets were also associated with a lower risk of type 2 diabetes among US black women [33]. Dairy products contain medium-chain triglycerides. Although medium-chain fatty acids induce steatosis and IR in the liver, but reduce adipose accumulation and preserve insulin action in the tissues [34]. This is another reason for IR in lower dairy consumption.
There was linear association between dairy consumption and HOMA-IR in the present study, but there was not such association between meat consumption and HOMA-IR. In ANCOVA, only the third tertile of meat consumption was associated with IR. These findings indicate that only higher consumption of meat can cause IR.
In the present study, univariate analysis showed that fat consumption was associated with MONW. However, this association was not confirmed in multivariate analysis. In Shab-Bidar et al. study, fatty acid consumption was significantly associated with risk of the metabolic syndrome among the Iranian population [35]. It has been shown that a dietary intervention based on a typical Italian Mediterranean Diet reduces the cardiovascular risk factors in subjects with metabolic syndrome [36]. On the other hand, reducing saturated fatty acids has no effect on insulin sensitivity in obese subjects with metabolic syndrome [37].
Obesity has an important role in cardiometabolic disturbances, but BMI is not an accurate measurement for classification of obesity [7]. Not only a high degree of metabolic dysregulation may be present in individuals with normal BMI [2], but also any metabolic abnormalities may be absent in individuals with high body weight by BMI [3].
The present study had some limitations including its cross-sectional design and the absence of visceral fat and total fat mass measurements. Using BMI to define obesity and to assess body composition is also a limitation of this study. There are limited data about contributing factors in normal weight obesity and the strength of our study was investigating normal weight participants.
In conclusion, higher meat consumption was associated with IR in normal weight men. Higher meat consumption and lower dairy products consumption were associated with higher means of HOMA-IR.
References
Risérus U, Arnlöv J, Berglund L (2007) Long-term predictors of insulin resistance: role of lifestyle and metabolic factors in middle-aged men. Diabetes Care 30:2928–2933
Oliveros E, Somers VK, Sochor O et al (2014) The concept of normal weight obesity. Prog Cardiovasc Dis 56:426–433. doi:10.1016/j.pcad.2013.10.003
Seo MH, Rhee EJ (2014) Metabolic and cardiovascular implications of a metabolically healthy obesity phenotype. Endocrinol Metab (Seoul) 29:427–434. doi:10.3803/EnM.2014.29.4.427
Ruderman NB, Schneider SH, Berchtold P (1981) The “metabolically-obese,” normal-weight individual. Am J Clin Nutr 34:1617–1621. doi:10.2337/diabetes.47.5.699
Choi KM, Cho HJ, Choi HY et al (2013) Higher mortality in metabolically obese normal-weight people than in metabolically healthy obese subjects in elderly Koreans. Clin Endocrinol (Oxf) 79:364–370. doi:10.1111/cen.12154
Kwon BJ, Kim DW, Her SH et al (2013) Metabolically obese status with normal weight is associated with both the prevalence and severity of angiographic coronary artery disease. Metabolism 62:952–960. doi:10.1016/j.metabol.2013.01.006
De Lorenzo A, Bianchi A, Maroni P et al (2013) Adiposity rather than BMI determines metabolic risk. Int J Cardiol 166:111–117. doi:10.1016/j.ijcard.2011.10.006
McLaughlin T, Lamendola C, Liu A, Abbasi F (2011) Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J Clin Endocrinol Metab 96:E1756–E1760. doi:10.1210/jc.2011-0615
Bradshaw PT, Monda KL, Stevens J (2013) Metabolic syndrome in healthy obese, overweight, and normal weight individuals: the Atherosclerosis Risk in Communities Study. Obesity (Silver Spring) 21:203–209. doi:10.1002/oby.20248
Liu R, Zee PC, Chervin RD et al (2011) Short sleep duration is associated with insulin resistance independent of adiposity in Chinese adult twins. Sleep Med 12:914–919. doi:10.1016/j.sleep.2011.04.006
Buscemi S, Nicolucci A, Mattina A et al (2013) Association of dietary patterns with insulin resistance and clinically silent carotid atherosclerosis in apparently healthy people. Eur J Clin Nutr 67:1284–1290. doi:10.1038/ejcn.2013.172
van Dam RM, Willett WC, Rimm EB et al (2002) Dietary fat and meat intake in relation to risk of type 2 diabetes in men. Diabetes Care 25:417–424
Cocate PG, Natali AJ, de Oliveira A et al (2015) Red but not white meat consumption is associated with metabolic syndrome, insulin resistance and lipid peroxidation in Brazilian middle-aged men. Eur J Prev Cardiol 22:223–230. doi:10.1177/2047487313507684
Ziaee A, Esmailzadehha N, Ghorbani A (2012) Association between uric acid and metabolic syndrome in Qazvin Metabolic Diseases Study (QMDS), Iran. Glob J Health Sci 5:155–165. doi:10.5539/gjhs.v5n1p155
Matthews DR, Hosker JP, Rudenski AS (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419. doi:10.1007/BF00280883
Esteghamati A, Ashraf H, Esteghamati AR et al (2009) Optimal threshold of homeostasis model assessment for insulin resistance in an Iranian population: the implication of metabolic syndrome to detect insulin resistance. Diabetes Res Clin Pract 84:279–287. doi:10.1016/j.diabres.2009.03.005
Ziaee A, Esmailzadehha N, Oveisi S et al (2015) The threshold value of homeostasis model assessment for insulin resistance in Qazvin Metabolic Diseases Study (QMDS): assessment of metabolic syndrome. J Res Health Sci 15:94–100
Grundy SM, Hansen B, Smith SC Jr et al (2004) Clinical management of metabolic syndrome: report of the American Heart Association/National Heart, Lung, and Blood Institute/American Diabetes Association conference on scientific issues related to management. Arterioscler Thromb Vasc Biol 24:e19–e24
Azadbakht L, Mirmiran P, Esmaillzadeh A, Azizi F (2005) Dairy consumption is inversely associated with the prevalence of the metabolic syndrome in Tehranian adults. Am J Clin Nutr 82:523–530
WHO. Global physical activity questionnaire (GPAQ). Geneva (Switzerland), World Health Organization. Retrieved from: http://www.who.int/entity/chp/steps/resources/GPAQ_Analysis_Guide.pdf
Snowdon DA, Phillips RL (1985) Does a vegetarian diet reduce the occurrence of diabetes? Am J Public Health 75:507–512. doi:10.2105/AJPH.75.5.507
Pan A, Sun Q, Bernstein AM et al (2011) Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. Am J Clin Nutr 94:1088–1096. doi:10.3945/ajcn.111.018978
Fernández-Real JM, López-Bermejo A, Ricart W (2002) Cross-talk between iron metabolism and diabetes. Diabetes 51:2348–2354
Uribarri J, Woodruff S, Goodman S et al (2010) Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc 110(911–16):e12. doi:10.1016/j.jada.2010.03.018
Uribarri J, Cai W, Sandu O et al (2005) Diet-derived advanced glycation end products are major contributors to the body’s AGE pool and induce inflammation in healthy subjects. Ann N Y Acad Sci 1043:461–466. doi:10.1196/annals.1333.052
Lee CC, Adler AI, Sandhu MS et al (2009) Association of C-reactive protein with type 2 diabetes: prospective analysis and meta-analysis. Diabetologia 52:1040–1047. doi:10.1007/s00125-009-1338-3
Fumeron F, Lamri A, Abi Khalil C et al (2011) Data from the Epidemiological Study on the Insulin Resistance Syndrome (DESIR) Study Group. Dairy consumption and the incidence of hyperglycemia and the metabolic syndrome: results from a French prospective study, Data from the Epidemiological Study on the Insulin Resistance Syndrome (DESIR). Diabetes Care 34:813–817. doi:10.2337/dc10-1772
Lutsey PL, Steffen LM, Stevens J (2008) Dietary intake and the development of the metabolic syndrome: the Atherosclerosis Risk in Communities study. Circulation 117:754–761. doi:10.1161/CIRCULATIONAHA.107.716159
Elwood PC, Givens DI, Beswick AD et al (2008) The survival advantage of milk and dairy consumption: an overview of evidence from cohort studies of vascular diseases, diabetes and cancer. J Am Coll Nutr 27:723S–734S. doi:10.1080/07315724.2008.10719750
Lawlor DA, Ebrahim S, Timpson N, Davey Smith G (2005) Avoiding milk is associated with a reduced risk of insulin resistance and the metabolic syndrome: findings from the British Women’s Heart and Health Study. Diabet Med 22:808–811. doi:10.1111/j.1464-5491.2005.01537.x
Zemel MB (2002) Regulation of adiposity and obesity risk by dietary calcium: mechanisms and implications. J Am Coll Nutr 21:146S–151S. doi:10.1080/07315724.2002.10719212
Melanson EL, Sharp TA, Schneider J et al (2003) Relation between calcium intake and fat oxidation in adult humans. Int J Obes Relat Metab Disord 27:196–203. doi:10.1038/sj.ijo.802202
van Dam RM, Hu FB, Rosenberg L et al (2006) Dietary calcium and magnesium, major food sources, and risk of type 2 diabetes in U.S. black women. Diabetes Care 29:2238–2243. doi:10.2337/dc06-1014
Turner N, Hariharan K, TidAng J et al (2009) Enhancement of muscle mitochondrial oxidative capacity and alterations in insulin action are lipid species dependent: potent tissue-specific effects of medium-chain fatty acids. Diabetes 58:2547–2554. doi:10.2337/db09-0784
Shab-Bidar S, Hosseini-Esfahani F, Mirmiran P et al (2014) Metabolic syndrome profiles, obesity measures and intake of dietary fatty acids in adults: Tehran Lipid and Glucose Study. J Hum Nutr Diet 27(Suppl 2):98–108. doi:10.1111/jhn.12117
Di Daniele N, Petramala L, Di Renzo L et al (2013) Body composition changes and cardiometabolic benefits of a balanced Italian Mediterranean Diet in obese patients with metabolic syndrome. Acta Diabetol 50:409–416. doi:10.1007/s00592-012-0445-7
Tierney AC, McMonagle J, Shaw DI et al (2011) Effects of dietary fat modification on insulin sensitivity and on other risk factors of the metabolic syndrome–LIPGENE: a European randomized dietary intervention study. Int J Obes (Lond) 35:800–809. doi:10.1038/ijo.2010.209
Acknowledgments
This research was officially registered as internal medicine specialty thesis No. 403 at the School of Medicine, Qazvin University of Medical Sciences. The authors would like to thank the participants involved in this study and the Department of Research of Qazvin University of Medical Science for endorsing the project. The authors would also like to thank Dr Asghar Mohammadpoorasl, Mrs Zahra Mohammadi, Mrs Mahsa khoshpanjeh and the staff of the Centre for Clinical Research at Qazvin Children Hospital, affiliated to Qazvin University of Medical Sciences for their help in preparing this paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Nothing to declare.
Ethical standards
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the ethics committee of Qazvin University of medical sciences.
Informed consent
Written informed consent was obtained from all subjects.
Rights and permissions
About this article
Cite this article
Hashemipour, S., Esmailzadehha, N., Mohammadzadeh, M. et al. Association of meat and dairy consumption with normal weight metabolic obesity in men: the Qazvin Metabolic Diseases Study. Eat Weight Disord 21, 419–425 (2016). https://doi.org/10.1007/s40519-015-0250-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40519-015-0250-8